Identification and Pathogenicity of Pestalotiod Fungi Associated with Woody Oil Plants in Sichuan Province, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection, Examination, and Fungal Isolation
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Sequence Alignment and Phylogenetic Analyses
2.4. PHI Analysis
2.5. Pathogenicity Tests on Olive Leaves
2.6. Statistical Analysis
3. Results
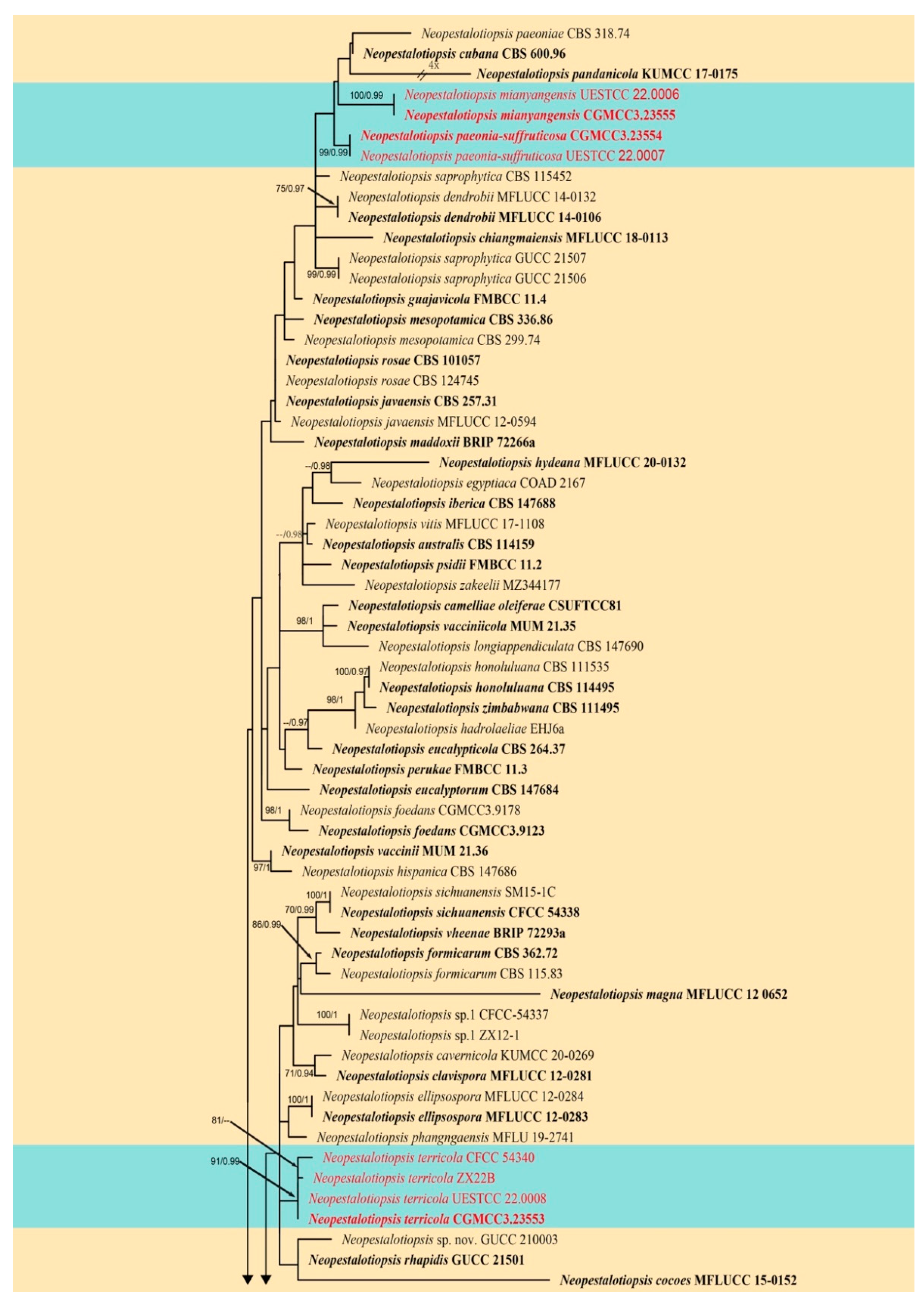
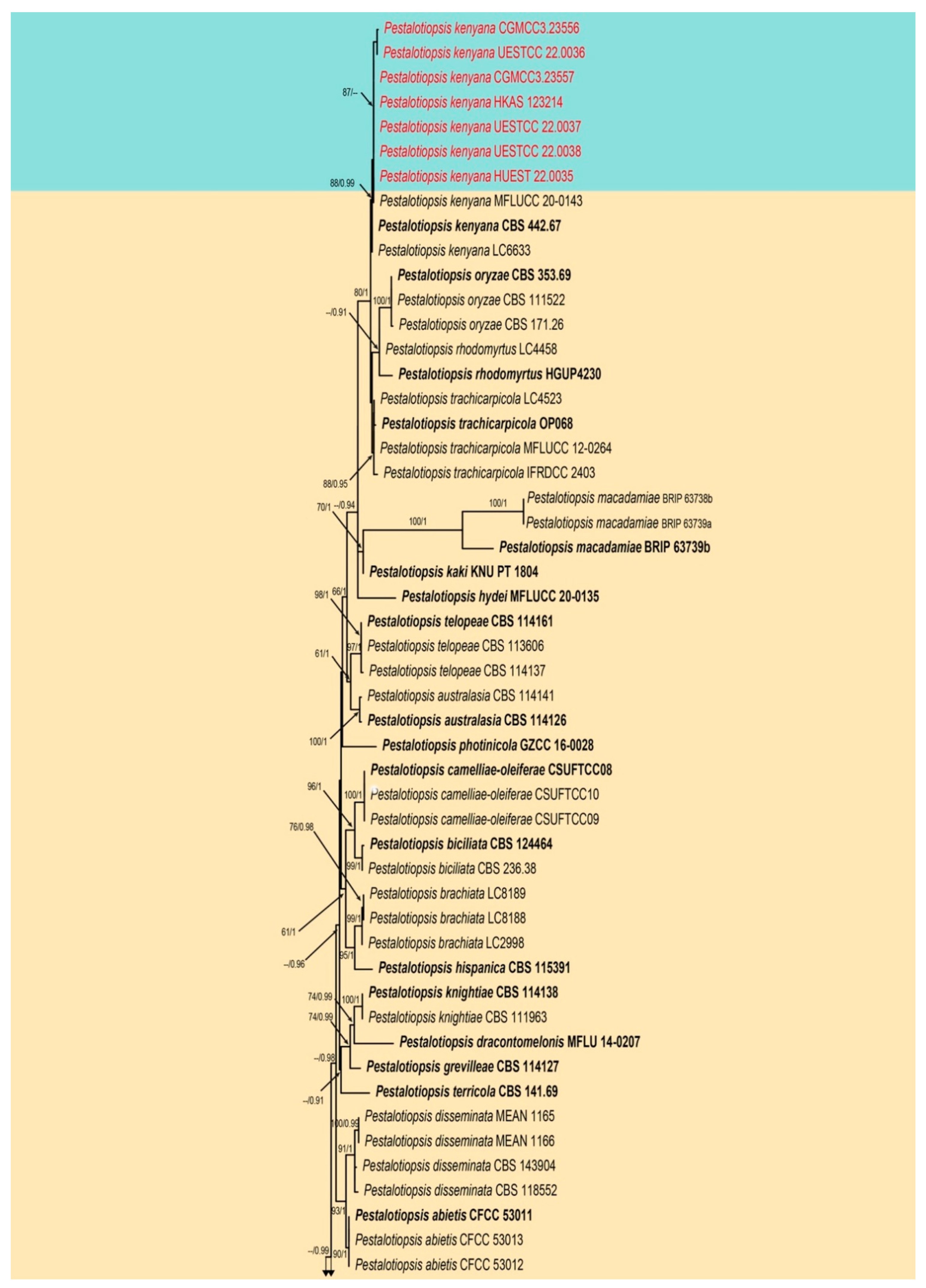
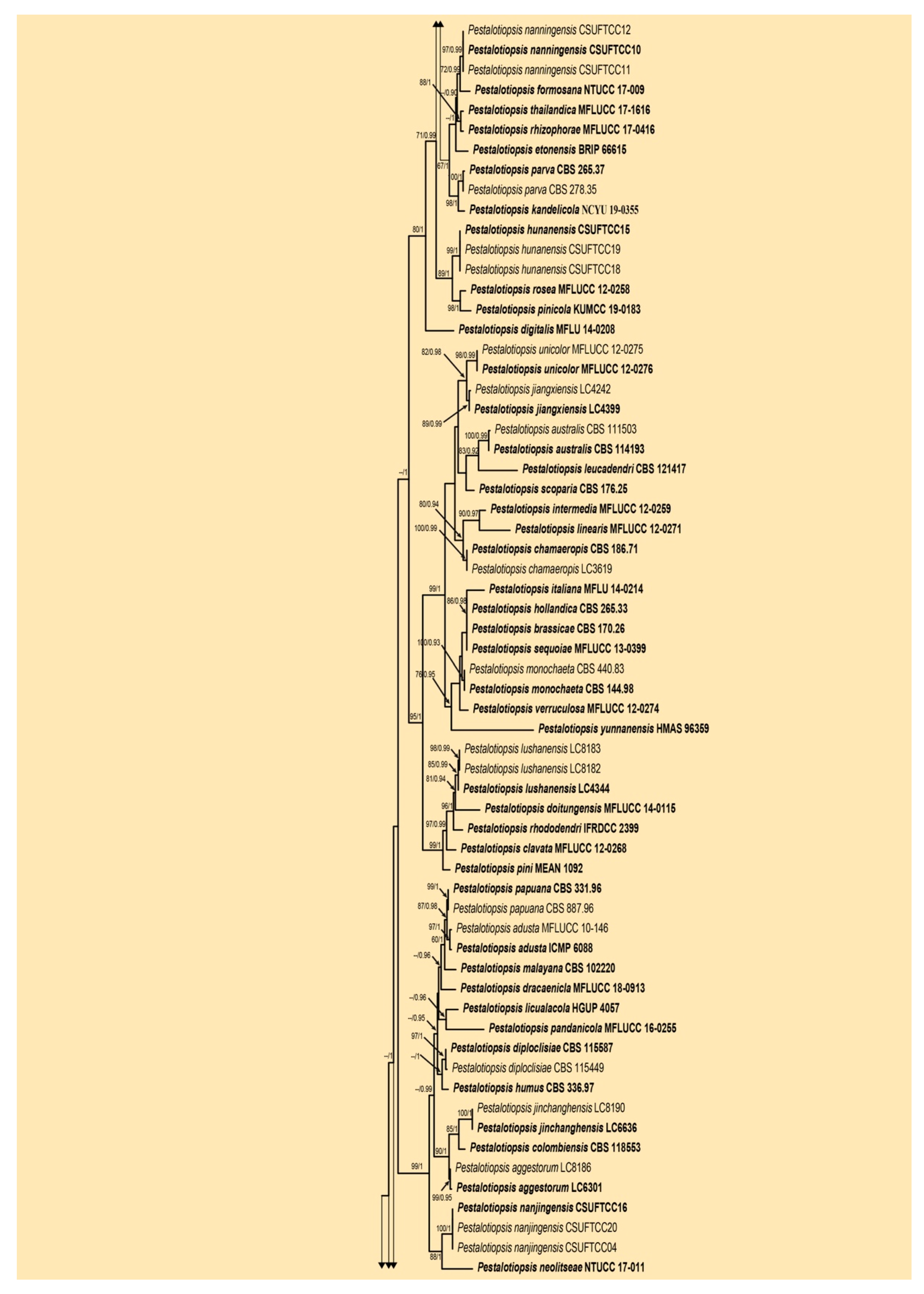
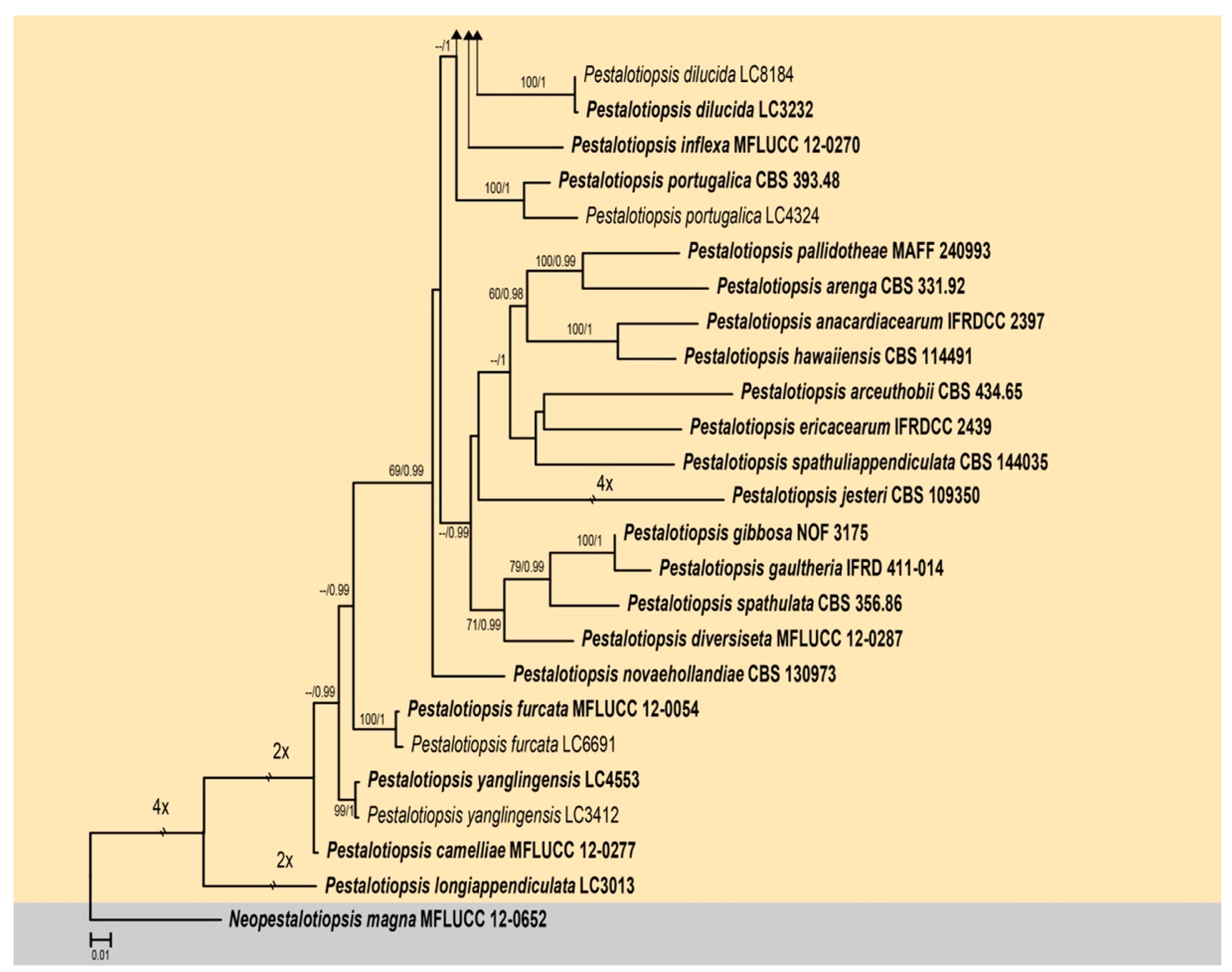
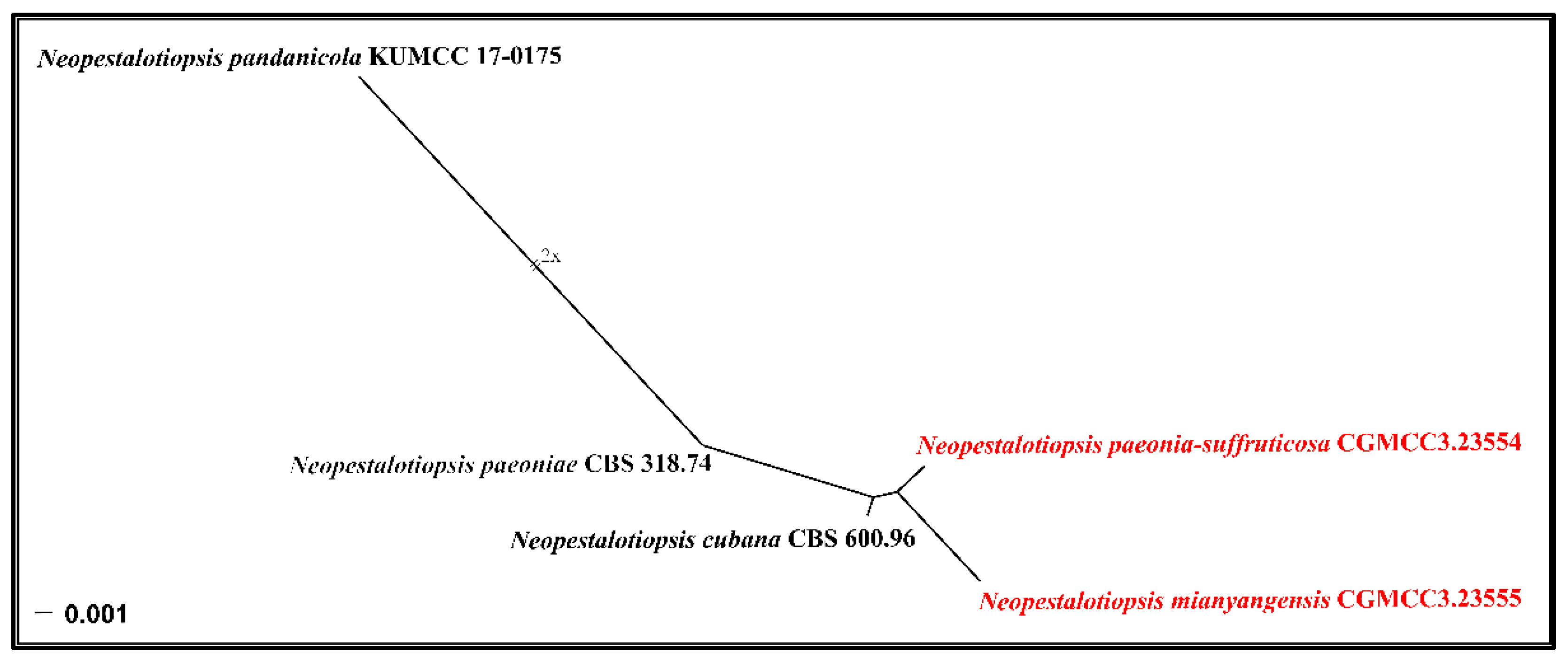
3.1. Phylogenetic Analyses
3.2. Taxonomy
3.2.1. Neopestalotiopsis mianyangensis W.L. Li and Jian K. Liu sp. nov. (Figure 5)
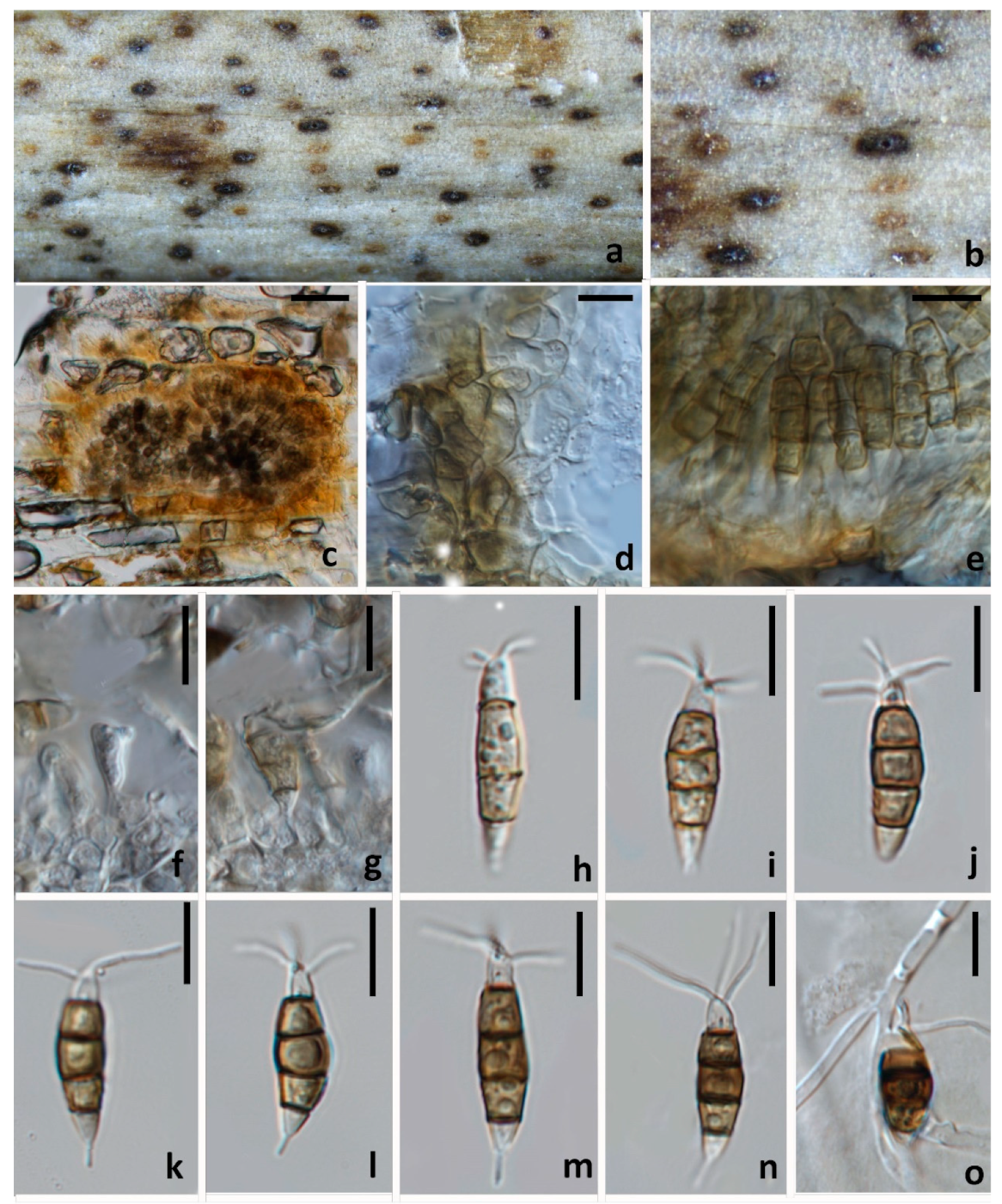
3.2.2. Neopestalotiopsis paeonia-suffruticosa W.L. Li and Jian K. Liu., sp. nov. (Figure 7)
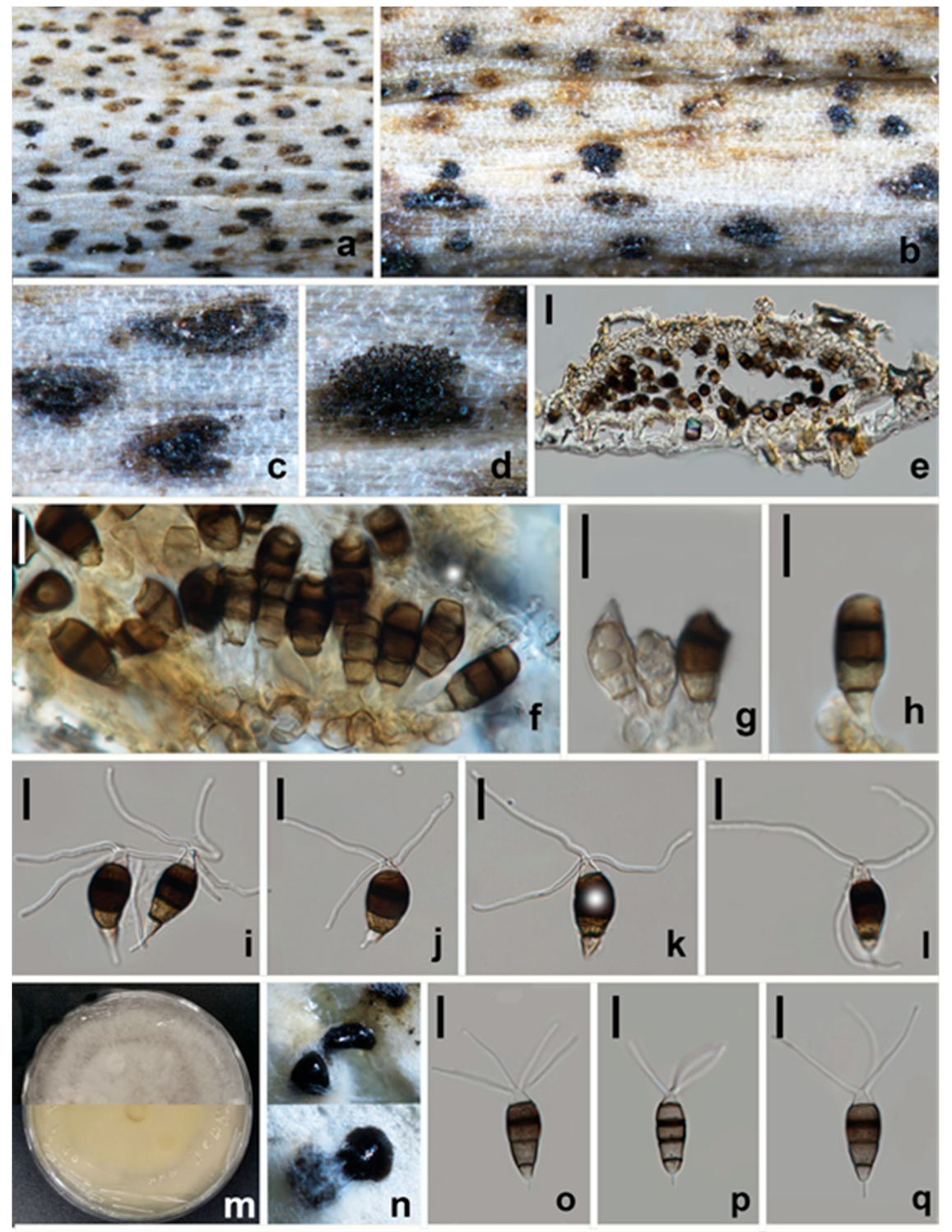
3.2.3. Neopestalotiopsis terricola W.L. Li and Jian K. Liu, sp. nov. (Figure 8 and Figure 9)
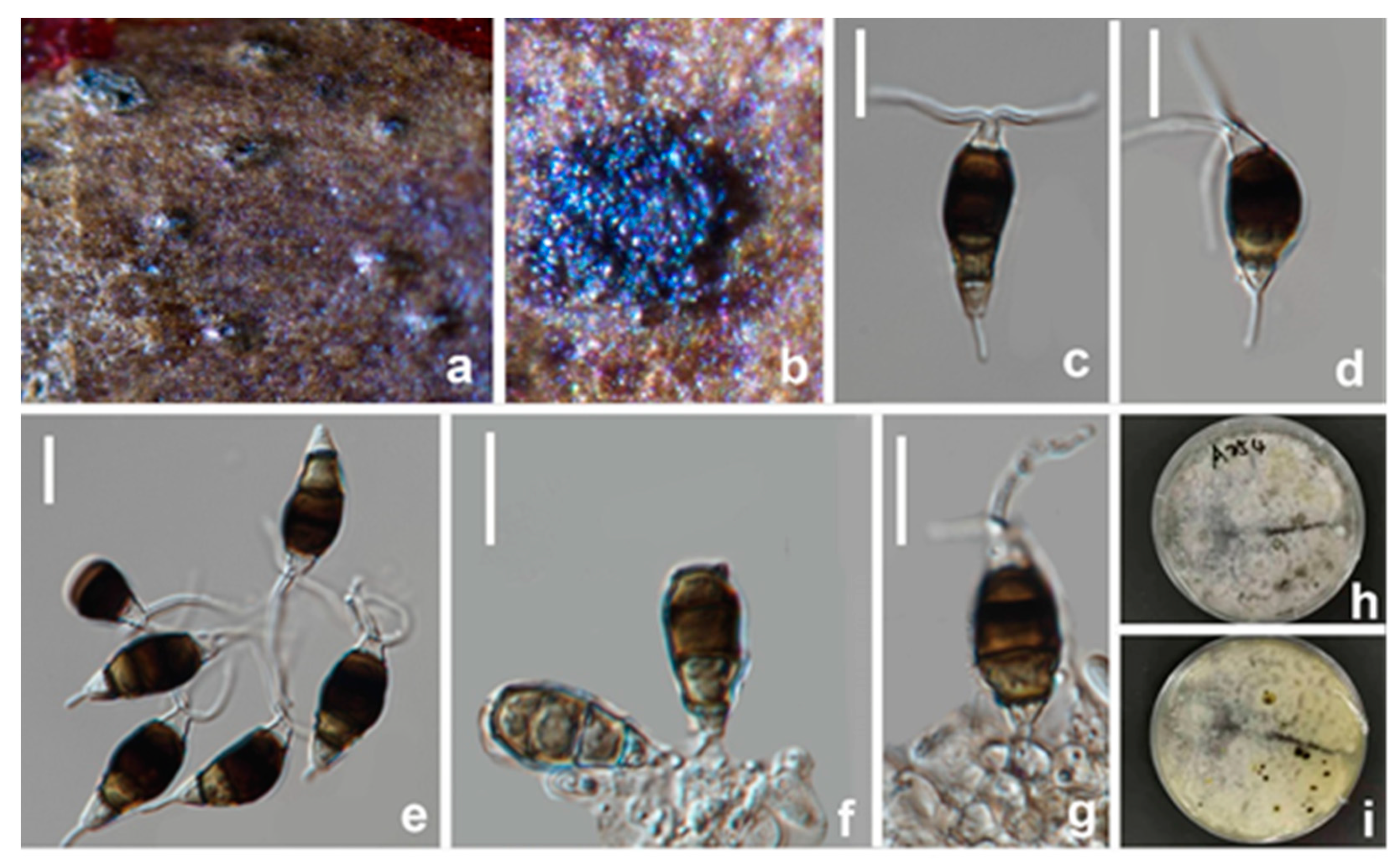

3.2.4. Pestalotiopsis kenyana Maharachch., K.D. Hyde and Crous, Studies in Mycology, 79:121–186 (Figure 10 and Figure 11)


3.2.5. Seiridium ceratosporum De Notaris, G. Memorie della Reale Accademia delle Scienze di Torino. Ser. 2. 3: 55–68 (1841) (Figure 12)

3.2.6. Seiridium guangyuanum W.L. Li and Jian K. Liu, sp. nov. (Figure 13)

3.2.7. Seiridium oleae W.L. Li and Jian K. Liu, sp. nov. (Figure 14)

3.2.8. Seiridium rosarum Wanas., Camporesi, E.B.G. Jones and K.D. Hyde, Fungal Diversity 89: 199 (2018) (Figure 15)
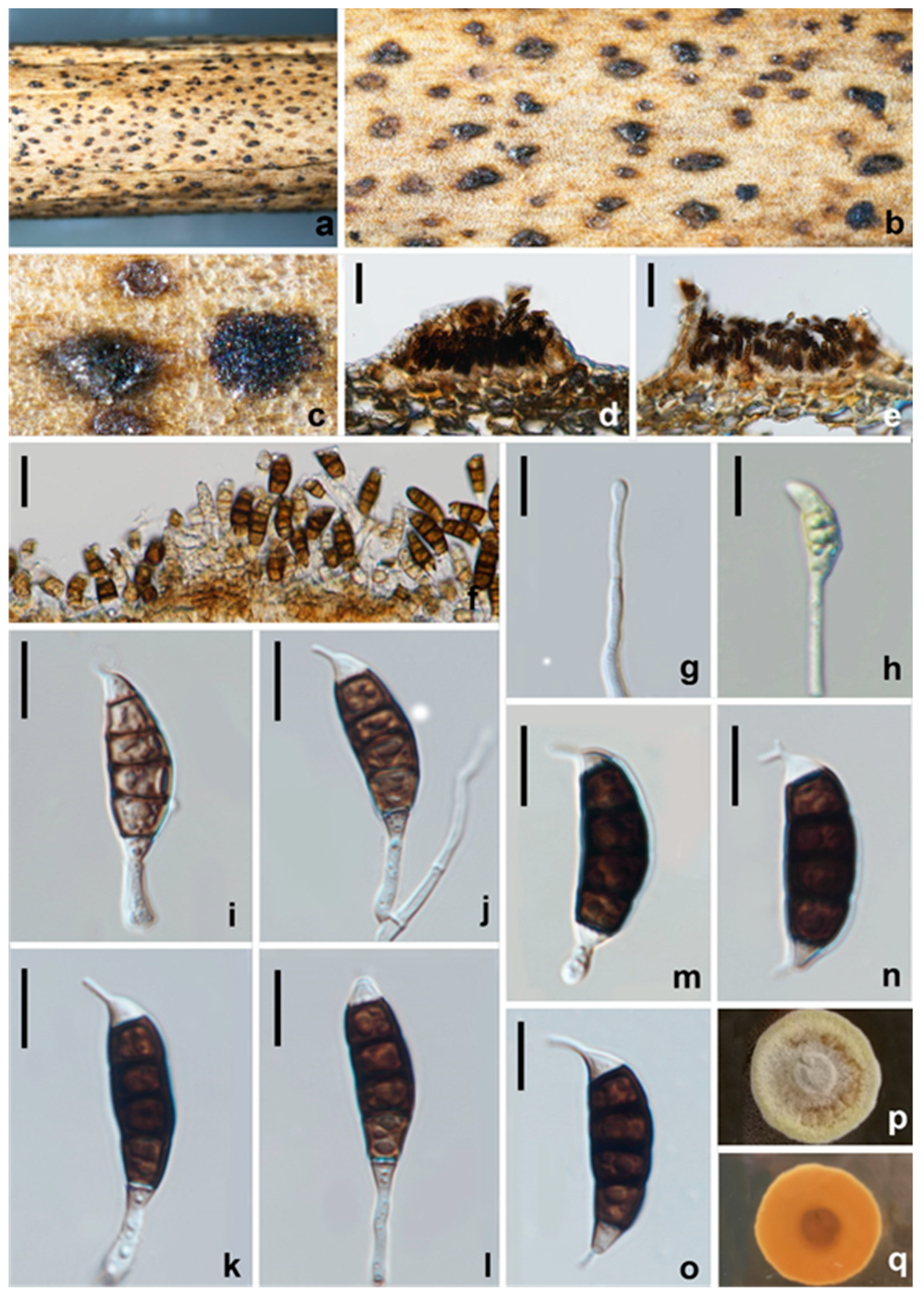
3.2.9. Seiridium vernicola W.L. Li and Jian K. Liu, sp. nov. (Figure 16)

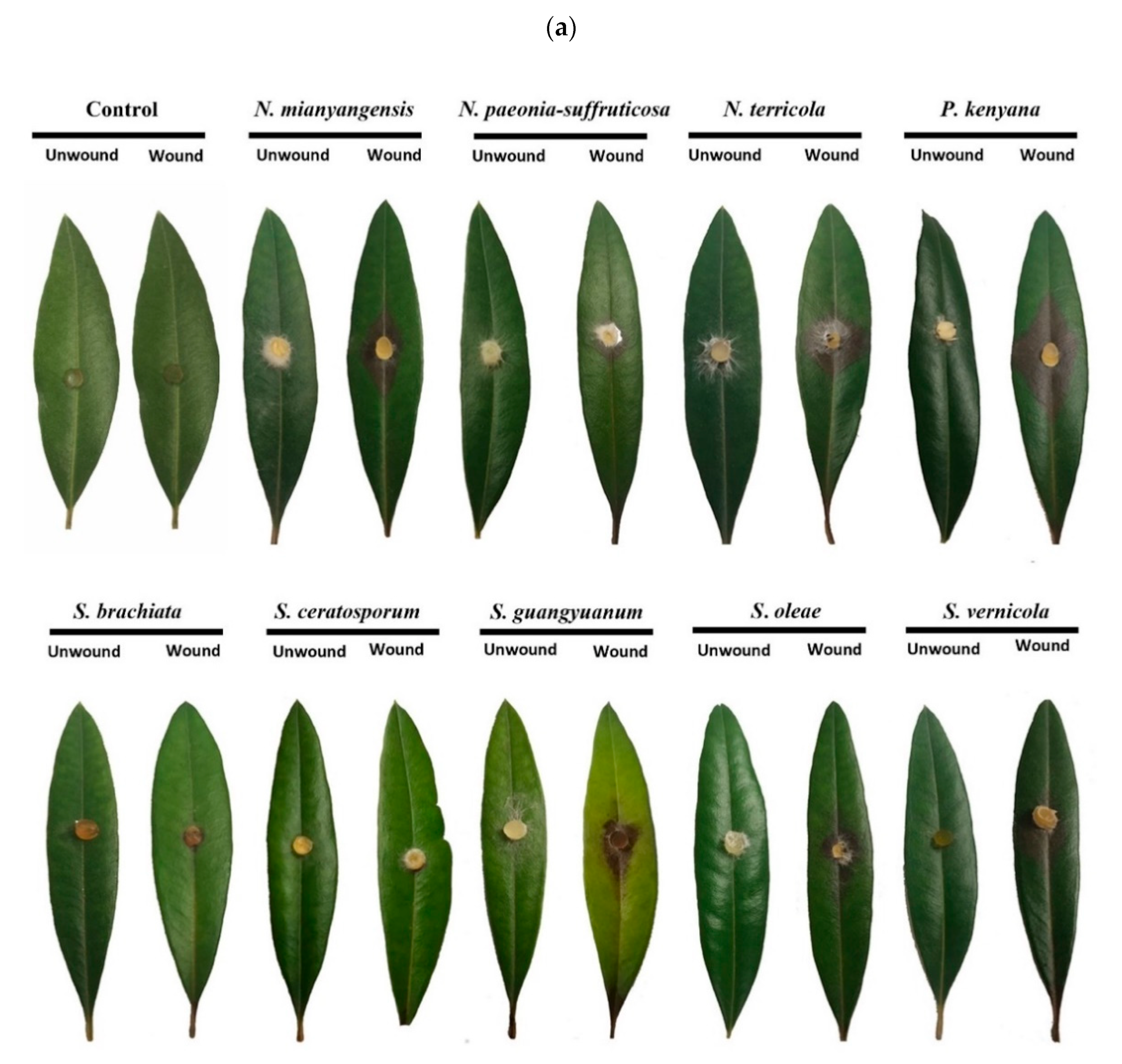
3.3. Pathogenicity Assay
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, Y.; Sun, D.; Yang, X. Research on the status and development proposals for the wood oil industry in China. Cereals Oils 2015, 28, 17–19. [Google Scholar]
- Wang, Z.W. Analysis and strategies on woody oil industry develoment in China. For. Resour. Manag. 2012, 1, 11–16. [Google Scholar]
- Ma, X.Y.; Maharachchikumbura, S.S.N.; Chen, B.W.; Hyde, K.D.; Mckenzie, E.H.; Chomnunti, P.; Kang, J.C. Endophytic pestalotiod taxa in Dendrobium orchids. Phytotaxa 2019, 419, 268–286. [Google Scholar] [CrossRef]
- Li, L.L.; Yang, Q.; Li, H. Morphology, Phylogeny, and Pathogenicity of Pestalotioid Species on Camellia oleifera in China. J. Fungi 2021, 7, 1080. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yuan, Y.; Zhang, S.; Zhou, X. First Report of Anthracnose on Camellia japonica Caused by Colletotrichum siamense in Zhejiang Province, China. Plant Dis. 2022, 106, 768. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, R.; Sellami, H.; Gharbi, Y.; Krid, S.; Cheffi, M.; Kammoun, S.; Dammak, M.; Mseddi, A.; Gdoura, R.; Triki, M.A. Morphological and molecular characterization of Fusarium spp. associated with olive trees dieback in Tunisia. 3 Biotech 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Yan, W.R.; Xiao, M.; Xiao, T.B.; Lei, F. Molecular Identification of Pathogens Causing Root Rot of Camellia oleifera in Tropical. Mol. Pathog. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Liu, F.; Hou, L.; Raza, M.; Cai, L. Pestalotiopsis and allied genera from Camellia, with description of 11 new species from China. Sci. Rep. 2017, 7, 866. [Google Scholar] [CrossRef]
- Crous, P.W.; Summerell, B.A.; Swart, L.; Denman, S.; Taylor, J.; Bezuidenhout, C.; Palm, M.E.; Marincowitz, S.; Groenewald, J.Z. Fungal pathogens of Proteaceae. Pers.-Mol. Phylogeny Evol. Fungi 2011, 27, 20–45. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Guo, L.D.; Cai, L.; Chukeatirote, E.; Wu, W.P.; Sun, X.; Crous, P.W.; Bhat, D.J.; McKenzie, E.H.C.; Bahkali, A.H.; et al. A multi-locus backbone tree for Pestalotiopsis, with a polyphasic characterization of 14 new species. Fungal Divers. 2012, 56, 95–129. [Google Scholar] [CrossRef]
- Zhang, Y.; Maharachchikumbura, S.S.N.; Mckenzie, E.H.; Hyde, K.D. A novel species of Pestalotiopsis causing leaf spots of Trachycarpus fortunei. Cryptogam. Mycol. 2012, 33, 311–318. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Guo, L.D.; Chukeatirote, E.; Hyde, K.D. Improving the backbone tree for the genus Pestalotiopsis; addition of P. steyaertii and P. magna sp. nov. Mycol. Prog. 2014, 13, 617–624. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Hyde, K.D.; Groenewald, J.Z.; Xu, J.; Crous, P.W. Pestalotiopsis revisited. Stud. Mycol. 2014, 79, 121–186. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, T. Yield loss of new tea shoots due to grey blight caused by Pestalotia longiseta Spegazzini. Bull. Shizuoka Tea Exp. Stn. 1986, 12, 1–8. [Google Scholar]
- Yan, M.Z.; Maharachchikumbura, S.S.N.; Tian, Q.; Hyde, K.D. Pestalotiopsis species on ornamental plants in Yunnan Province, China. Sydowia-Horn 2013, 65, 113–128. [Google Scholar]
- Chen, Y.J.; Zeng, L.; Shu, N.; Jiang, M.Y.; Wang, H.; Huang, Y.J.; Tong, H.R. Pestalotiopsis-Like Species Causing Gray Blight Disease on Camellia sinensis in China. Plant Dis. 2018, 102, 98–106. [Google Scholar] [CrossRef]
- Wang, S.; Mi, X.; Wu, Z.; Zhang, L.; Wei, C. Characterization and pathogenicity of Pestalotiopsis-like species associated with gray blight disease on Camellia sinensis in Anhui province, China. Plant Dis. 2019, 103, 2786–2797. [Google Scholar] [CrossRef]
- Jaklitsch, W.M.; Gardiennet, A.; Voglmayr, H. Resolution of morphology-based taxonomic delusions: Acrocordiella, Basiseptospora, Blogiascospora, Clypeosphaeria, Hymenopleella, Lepteutypa, Pseudapiospora, Requienella, Seiridium and Strickeria. Persoonia 2016, 37, 82–105. [Google Scholar] [CrossRef]
- Liu, F.; Bonthond, G.; Groenewald, J.Z.; Cai, L.; Crous, P.W. Sporocadaceae, a family of coelomycetous fungi with appendage-bearing conidia. Stud. Mycol. 2019, 92, 287–415. [Google Scholar] [CrossRef]
- Esenbeck, V.; Nees, C.G. Das System der Pilze und Schwämme: Ein Versuch; In der Stahelschen buchhandlung, 1817. [Google Scholar]
- Bonthond, G.; Sandoval-Denis, M.; Groenewald, J.Z.; Crous, P.W. Seiridium (Sporocadaceae): An important genus of plant pathogenic fungi. Persoonia 2018, 40, 96–118. [Google Scholar] [CrossRef]
- Jiang, N.; Fan, X.; Tian, C. Identification and Characterization of Leaf-Inhabiting Fungi from Castanea Plantations in China. J. Fungi 2021, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Huanaluek, N.; Jayawardena, R.S.; Maharachchikumbura, S.S.; Harishchandra, D.L. Additions to pestalotioid fungi in Thailand: Neopestalotiopsis hydeana sp. nov. and Pestalotiopsis hydei sp. nov. Phytotaxa 2021, 479, 23–43. [Google Scholar] [CrossRef]
- Diogo, E.; Gonçalves, C.I.; Silva, A.C.; Valente, C.; Bragança, H.; Phillips, A.J.L. Five new species of Neopestalotiopsis associated with diseased Eucalyptus spp. in Portugal. Mycol. Prog. 2021, 20, 1441–1456. [Google Scholar] [CrossRef]
- Rayner, R.W. A mycological colour chart. In A Mycological Colour Chart; Commonwealth Mycological Institute & British Mycological Society: Ferry Lane, UK, 1970. [Google Scholar]
- Jeewon, R.; Hyde, K.D. Establishing species boundaries and new taxa among fungi: Recommendations to resolve taxonomic ambiguities. Mycosphere 2016, 7, 1669–1677. [Google Scholar] [CrossRef]
- Jayasiri, S.C.; Hyde, K.D.; Ariyawansa, H.A.; Bhat, J.; Buyck, B.; Cai, L.; Dai, Y.C.; Abd-Elsalam, K.A.; Ertz, D.; Hidayat, I.; et al. The Faces of Fungi database: Fungal names linked with morphology, phylogeny and human impacts. Fungal Divers. 2015, 74, 3–18. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus fusarium are nonorthologous. Mol. Phylogenetics Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Sung, G.H.; Sung, J.M.; Hywel-Jones, N.L.; Spatafora, J.W. A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Mol. Phylogenetics Evol. 2007, 44, 1204–1223. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Dissanayake, A.J.; Bhunjun, C.S.; Maharachchikumbura, S.S.N.; Liu, J.K. Applied aspects of methods to infer phylogenetic relationships amongst fungi. Mycosphere 2020, 11, 2653–2677. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v1.4: Tree Figure Drawing Tool. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 15 May 2022).
- De Notaris, G. Micromycetes Italici novi vel minus cogniti, decas 2. Mem. Della R. Accad. Delle Sci. Di Torino Ser. 1841, 2, 69–82. [Google Scholar]
- Nag Raj, T.R. Coelomycetous Anamorphs with Appendage-Bearing Conidia; Mycologue Publications: Waterloo, ON, Canada, 1993; pp. 1–1101. [Google Scholar]
- Liu, A.R.; Xu, T.; Guo, L.D. Molecular and morphological description of Pestalotiopsis hainanensis sp. nov., a new endophyte from a tropical region of China. Fungal Divers. 2007, 24, 23–36. [Google Scholar]
- Brunetti, A.; Matere, A.; Lumia, V.; Pasciuta, V.; Fusco, V.; Sansone, D.; Marangi, P.; Cristella, N.; Faggioli, F.; Scortichini, M.; et al. Neofusicoccum mediterraneum Is Involved in a Twig and Branch Dieback of Olive Trees Observed in Salento (Apulia, Italy). Pathogens 2022, 11, 53. [Google Scholar] [CrossRef]
- Garrido, A.; Fernández-González, M.; Cortiñas Rodríguez, J.A.; Carrera, L.; González-Fernández, E.; Almaguer-Chávez, M.; Rodríguez-Rajo, F.J. Fungal Phytopathogenic Spore First Assessment in an Olive Orchard of Northwestern Spain. Agronomy 2022, 12, 246. [Google Scholar] [CrossRef]
- López-Moral, A.; Lovera, M.; Antón Domínguez, B.I.; Gámiz, A.M.; Michailides, T.; Arquero, O.; Trapero-Casas, A.; Agustí-Brisach, C. Effects of Cultivar Susceptibility, Branch Age, and Temperature on the Infection by Botryosphaeriaceae and Diaporthe Fungi on English Walnut (Juglans regia L.). Plant Dis. 2022, 106, 2920–2926. [Google Scholar] [CrossRef]
- Crous, P.; Verkley, G.; Christensen, M.; Castañeda-Ruiz, R.; Groenewald, J. How important are conidial appendages? Pers. Mol. Phylogeny Evol. Fungi 2012, 28, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Shang, Z.; Su, Q.; Feng, L.; An, L. First report of a Pestalotiopsis sp. causing leaf spot of blueberry in China. Plant Dis. 2008, 92, 171. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.; Yao, K.; Chen, C.; Lin, C. First report of gray leaf spot of mango (Mangifera indica) caused by Pestalotiopsis mangiferae in Taiwan. Plant Dis. 2007, 91, 1684. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, M.; Aguado, A.; De los Santos, B. First report of root and crown rot caused by Pestalotiopsis clavispora (Neopestalotiopsis clavispora) on strawberry in Spain. Plant Dis. 2016, 100, 1495. [Google Scholar] [CrossRef]
- Karakaya, A. First report of infection of kiwifruit by Pestalotiopsis sp. in Turkey. Plant Dis. 2001, 85, 1028. [Google Scholar] [CrossRef]




| Species | Culture Accession No | Host/Substrate | GenBank Accession | ||||
|---|---|---|---|---|---|---|---|
| LSU | ITS | rpb2 | tef1-α | tub2 | |||
| Neopestalotiopsis mianyangensis | UESTCC 22.0006 | Paeonia suffruticosa | N/A | OP082291 | N/A | OP204793 | OP235979 |
| Neopestalotiopsis mianyangensis | CGMCC 3.23554 * | Paeonia suffruticosa | N/A | OP546681 | N/A | OP723490 | OP672161 |
| N. paeonia-suffruticosa | CGMCC 3.23555 * | Paeonia suffruticosa | N/A | OP082292 | N/A | OP204794 | OP235980 |
| N. paeonia-suffruticosa | UESTCC 22.0033 | Paeonia suffruticosa | N/A | OP082293 | N/A | OP204795 | OP235981 |
| N. terricola | CGMCC 3.23553 * | Paeonia suffruticosa | N/A | OP082294 | N/A | OP204796 | OP235982 |
| N. terricola | UESTCC 22.0034 | Paeonia suffruticosa | N/A | OP082295 | N/A | OP204797 | OP235983 |
| Pestalotiopsis kenyana | CGMCC 3.23557 | Olea europaea | N/A | OP082296 | N/A | OP204798 | OP235984 |
| P. kenyana | UESTCC 22.0035 | Olea europaea | N/A | OP082297 | N/A | OP204799 | OP235985 |
| P. kenyana | HUEST 22.0035 | Paeonia suffruticosa | N/A | OP729296 | N/A | OP204801 | OP235987 |
| P. kenyana | HKAS 123214 | Camellia oleifera | N/A | OP082298 | N/A | OP204800 | OP235986 |
| P. kenyana | CGMCC 3.23556 | Camellia oleifera | N/A | OP082299 | N/A | OP204802 | OP235988 |
| P. kenyana | UESTCC 22.0036 | Paeonia suffruticosa | N/A | OP082301 | N/A | OP204805 | OP235991 |
| P. kenyana | UESTCC 22.0037 | Paeonia suffruticosa | N/A | OP082300 | N/A | OP204803 | OP235989 |
| Seiridium ceratosporum | CGMCC 3.23559 | Vernicia fordii | OP082278 | OP082304 | OP204825 | OP204808 | OP235994 |
| S. ceratosporum | UESTCC 22.0039 | Vernicia fordii | OP082277 | OP082303 | OP204824 | OP204807 | OP235993 |
| S. ceratosporum | UESTCC 22.0040 | Vernicia fordii | OP082276 | OP082302 | OP723486 | OP204806 | OP235992 |
| S. guangyuanum | CGMCC 3.23561 * | Vernicia fordii | OP082279 | OP082305 | OP204826 | OP204809 | OP235995 |
| S. guangyuanum | UESTCC 22.0041 | Vernicia fordii | OP082280 | OP082306 | OP204827 | OP204810 | OP235996 |
| S. guangyuanum | UESTCC 22.0042 | Vernicia fordii | OP082281 | OP082307 | OP204828 | OP204811 | OP235997 |
| S. guangyuanum | UESTCC 22.0043 | Olea europaea | OP082282 | OP082308 | OP204829 | OP204812 | OP235998 |
| S. guangyuanum | UESTCC 22.0044 | Vernicia fordii | OP082283 | OP082309 | OP204830 | OP204813 | OP235999 |
| S. guangyuanum | UESTCC 22.0045 | Camellia oleifera | OP714449 | OP082310 | OP723487 | OP204814 | OP236000 |
| S. guangyuanum | UESTCC 22.0046 | Camellia oleifera | OP714450 | OP082311 | OP204831 | OP204815 | OP236001 |
| S. guangyuanum | UESTCC 22.0047 | Vernicia fordii | OP082284 | OP082312 | OP204832 | OP204816 | OP236002 |
| S. oleae | CGMCC 3.23558 * | Olea europaea | OP082285 | OP082313 | OP204833 | OP204817 | OP236003 |
| S. oleae | UESTCC 22.0051 | Olea europaea | OP082286 | OP082314 | OP204834 | OP204818 | OP723489 |
| S. rosarum | CGMCC 3.23562 | Paeonia suffruticosa | OP082287 | OP082315 | OP204835 | OP204819 | OP236004 |
| S. rosarum | UESTCC 22.0048 | Paeonia suffruticosa | OP714451 | OP082316 | OP723488 | OP204820 | OP236005 |
| S. vernicola | UESTCC 22.0049 | Sapium sebiferum | OP082288 | OP082317 | OP204836 | OP204821 | OP236006 |
| S. vernicola | UESTCC 22.0050 | Sapium sebiferum | OP082289 | OP082318 | OP204837 | OP204822 | OP236007 |
| S. vernicola | CGMCC 3.23560 * | Vernicia fordii | OP082290 | OP082319 | OP204838 | OP204823 | OP236008 |
| Species | Isolate Number | Conidial Size (μm) | Apical Appendages (μm) | Basal Appendage | |
|---|---|---|---|---|---|
| Number | Length | ||||
| Neopestalotiopsis chiangmaiensis | MFLUCC 18-0113 | 18–22 × 8–11 | (2–)3 | 4–28 | 3–5 |
| N. cubana | CBS 600.96 | (19–)20–25(–27) × (7.5–)8–9.5(–10) | 2–4 | (19–)21–27(–28) | 4–7 |
| N. dendrobii | MFLUCC 14- | (19–)20.5–23(–24.5) × (6–)6.5–7.5 (–8) | 2–3(2) | (4–)5–6.5(–6.6) | NA |
| N. mianyangensis | UESTCC 22.0006 | 19–23 × 5.5–7 | 3 | 5.5–11 | 3–4 |
| N. paeonia-suffruticosa | CGMCC3.23554 | 20–23 × 9–11 | 3–4 | 22.5–34 | 3.5–7.5 |
| N. pandanicola | KUMCC 17-0175 | 27–35 × 7.5–11 | 2(–3) | 9.5–26 | 3–6 |
| N. saprophyta | MFLUCC 12-0282 | 22–30 × 5–6 | 2–4(3) | 4–5 | 4–7 |
| Isolates | ITS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 54 | 59 | 60 | 63 | 73 | 76 | 77 | 90 | 108 | 112 | 355 | 430 | |
| Seiridium ceratosporum PHSI2001Pathcw07 | G | A | C | T | T | G | G | A | T | C | A | T |
| S. ceratosporum UESTCC 22.0040 | G | A | C | T | T | G | G | A | T | C | A | T |
| S. ceratosporum UESTCC 22.0039 | – | A | C | T | T | G | G | A | T | C | A | T |
| S. ceratosporum CGMCC3.23559 | G | A | C | T | T | G | G | A | T | C | A | T |
| S. guangyuan UESTCC 22.0043 | T | C | G | C | G | A | C | G | C | A | G | C |
| S. guangyuan CGMCC3.23561 | T | C | G | C | G | A | C | G | C | A | G | C |
| S. guangyuan UESTCC 22.0044 | T | C | G | C | G | A | C | G | C | A | G | C |
| S. guangyuan UESTCC 22.0047 | T | C | G | C | G | A | C | G | C | A | G | C |
| S. guangyuan UESTCC 22.0037 | T | C | G | C | G | A | C | G | C | A | G | C |
| S. guangyuan UESTCC 22.0046 | T | C | G | C | G | A | C | G | C | A | G | C |
| S. guangyuan UESTCC 22.0045 | T | C | G | C | G | A | C | G | C | A | G | C |
| S. guangyuan UESTCC 22.0041 | T | C | G | C | G | A | C | G | C | A | G | C |
| Species | Isolated Number | Conidial Size | Apical Appendages (μm) | Basal Appendage | |
|---|---|---|---|---|---|
| Number | Length | ||||
| Seiridium aquaticum | MFLUCC 17-0474 | 29–35 × 12–14 | NA | NA | NA |
| S. ceratosporum | Unknown | 29–35 × 10–12(–12.5) | 2 | 4–8(–11) | 1–5(–6) |
| S. ceratosporum | CGMCC3.23559 | 26–32 × 8.5–10.5 | 2 | 4–6 | 2.5–3.5 |
| S. chinense | CFCC 53031 | (24–)25.5–28(–29.5) × (8–)8.5–9.5(–11) | 2 | 4.5–15 | 6–18 |
| S. guangyuanum | CGMCC3.23561 | 27–30 × 8–9 | 2 | 2.5–5.5 | 3–4.5 |
| S. marginatum | CBS 140403 | 32–42(–47) × 7–9.5 | 2 | 32–52 | 22–44 |
| S. oleae | CGMCC3.23558 | 20–26 × 7.5–9 | 2 | 3–5 | 2.5–3.5 |
| S. papillatum | CBS 340.97 | 26.5–34.5 × 10–15 | 2 | 1.25–2.5 | 2 |
| S. rosarum | MFLUCC 17-0654 | 22–28 × 7–9 | 1 | 3.5–4 | up to 12 |
| S. rosarum | CGMCC3.23562 | 24–28 × 8.5–10 | 2 | 2.5–5 | 3–4.5 |
| S. venetum | MFLU 14-0265 | 20–30 × 6.5–8.5 | 2 | 10–35 | 2–5 |
| S. vernicola | CGMCC3.23560 | 25–32 × 8–10 | 2 | 3–4.5 | 2.5–5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.-L.; Dissanayake, A.J.; Zhang, T.; Maharachchikumbura, S.S.N.; Liu, J.-K. Identification and Pathogenicity of Pestalotiod Fungi Associated with Woody Oil Plants in Sichuan Province, China. J. Fungi 2022, 8, 1175. https://doi.org/10.3390/jof8111175
Li W-L, Dissanayake AJ, Zhang T, Maharachchikumbura SSN, Liu J-K. Identification and Pathogenicity of Pestalotiod Fungi Associated with Woody Oil Plants in Sichuan Province, China. Journal of Fungi. 2022; 8(11):1175. https://doi.org/10.3390/jof8111175
Chicago/Turabian StyleLi, Wen-Li, Asha J. Dissanayake, Tian Zhang, Sajeewa S. N. Maharachchikumbura, and Jian-Kui Liu. 2022. "Identification and Pathogenicity of Pestalotiod Fungi Associated with Woody Oil Plants in Sichuan Province, China" Journal of Fungi 8, no. 11: 1175. https://doi.org/10.3390/jof8111175
APA StyleLi, W.-L., Dissanayake, A. J., Zhang, T., Maharachchikumbura, S. S. N., & Liu, J.-K. (2022). Identification and Pathogenicity of Pestalotiod Fungi Associated with Woody Oil Plants in Sichuan Province, China. Journal of Fungi, 8(11), 1175. https://doi.org/10.3390/jof8111175








