Abstract
Plants harbor a variety of fungal symbionts both above- and belowground, yet little is known about how these fungi interact within hosts, especially in a world where resource availability is changing due to human activities. Systemic vertically transmitted endophytes such as Epichloë spp. may have particularly strong effects on the diversity and composition of later-colonizing symbionts such as root fungal endophytes, especially in primary successional systems. We made use of a long-term field experiment in Great Lakes sand dunes to test whether Epichloë colonization of the dune-building grass, Ammophila breviligulata, could alter fungal root endophyte species richness or community composition in host plants. We also tested whether nitrogen addition intensified the effects of Epichlöe on the root endophyte community. We found that Epichloë increased richness of root endophytes in Ammophila by 17% overall, but only shifted community composition of root endophytes under nitrogen-enriched conditions. These results indicate that Epichlöe acts as a key species within Ammophila, changing richness and composition of the root mycobiome and integrating above- and belowground mycobiome interactions. Further, effects of Epichloë on root endophyte communities were enhanced by N addition, indicating that this fungal species may become even more important in future environments.
1. Introduction
The plant mycobiome includes a wide variety of fungal symbionts located within the above- and belowground tissues of host plants. Shifts in the mycobiome due to biotic interactions among fungal taxa can alter host plant growth, stress tolerance, and nutrient uptake [1]. Vertically transmitted, systemic Clavicipitaceous endophytes such as Epichlöe spp. may have particularly strong effects on the mycobiome of their host plants, as they have priority effects within a plant host and may influence assembly of later-colonizing, horizontally transmitted fungi in both above- and belowground tissues [2]. However, effects of Epichlöe on the plant mycobiome are not consistent. For example, Epichlöe has been shown both to reduce [3] and increase colonization of roots by arbuscular mycorrhizal fungi (AMF) [4,5,6,7]. Epichlöe has also had mixed effects on leaf endophytes in a variety of host plant species [8,9,10]. Epichlöe effects on root endophyte (non-AMF) communities are not well-understood, but seem minimal in the few studies that have examined them [11,12,13]. However, the magnitude of the effect of Epichlöe on mycobiome composition may depend on environmental conditions and resource availability (e.g., [14,15]), both of which are shifting in the Anthropocene [16,17].
Anthropogenic nitrogen (N) enrichment may be a particularly influential aspect of global change on fungal interactions and mycobiome community composition, as resource availability directly impacts plant-fungal associations [18]. Activities associated with agriculture, industry, wastewater, and fossil fuel combustion has more than doubled rates of nitrogen input into the terrestrial nitrogen cycle [19], and atmospheric N deposition is expected to increase globally by 250% over the next century [19,20]. Nitrogen enrichment is known to directly influence mycobiome composition within plant organs, for example by reducing root mycobiome diversity [21,22,23]. Aboveground, N addition is associated with decreased foliar endophyte diversity [24,25]. The few studies that have explored N enrichment effects on Epichlöe showed that N addition enhanced Epichlöe benefits to hosts [16,26]. For example, increasing soil N improved the alleviation of drought stress provided by endophytes to some grasses [27]. However, it is unclear whether N availability alters above-belowground fungal interactions within host plants.
Changes in N availability may be particularly impactful on mycobiomes of plants in low nutrient primary successional ecosystems such as Great Lakes sand dunes. The U.S. Great Lakes coastal region has high N enrichment due to agricultural, atmospheric, and point-source inputs [28]. For example, atmospheric N deposition concentration levels, especially of NH4+, into Great Lakes ecosystems have increased 400% from historic levels [29,30] while dissolved inorganic N in Great Lakes coastal wetlands has risen as a direct result of row-crop agriculture in the region [28]. The dominant dune-building grass in this region, Ammophila breviligulata (hereafter Ammophila) harbors a variety of fungal symbionts including the systemic endophyte, Epichlöe amarillans (hereafter Epichlöe) [31], which is found in approximately one third of natural Ammophila populations in the Great Lakes [32,33,34,35] and in almost all nursery stock used for dune restoration work [36]. This provides an ideal system to examine effects of Epichlöe and N enrichment on other aspects of the plant mycobiome.
Here, we evaluated the effects of Epichlöe and N addition on community composition of fungal root endophytes associated with Ammophila in a long-term experiment within the Great Lakes dunes. Specifically, we asked: Does colonization of the host grass by Epichlöe alter root fungal endophyte species richness or community composition? Furthermore, if so, does N addition intensify the effects of Epichlöe on the root endophyte community? While very little is known about how Epichlöe interacts with root endophyte communities in general, earlier work in this dune system showed that Epichlöe reduced diversity of other root-associated fungal communities (AMF) in Ammophila [14]. AMF and non-mycorrhizal root endophyte communities often show opposing responses to changing conditions [37,38,39,40] (although also see [41]), and so we expected that root endophyte richness would increase in response to Epichlöe presence. By enhancing the plant-Epichlöe symbiosis, we also expected that N addition would strengthen the effects of Epichlöe on root endophyte diversity and composition. Alternatively, N enrichment could act as an environmental filter that limits which fungal species can colonize plant hosts, or could enhance dominance of certain taxa at the expense of mycobiome biodiversity, independent of Epichlöe [21,22,23,42]. Our findings provide some of the first evidence that Epichlöe can increase root endophyte richness in host plants, and that nitrogen enrichment strengthens the effects of Epichlöe on root endophyte community composition.
2. Methods
2.1. Experimental Design
In May 2010, we established a factorial field experiment on a bare dune blowout approximately 200 m from the shoreline of Lake Michigan in Leelanau State Park, Michigan, USA (45.8109640 N, 85.8345780 W). To manipulate the presence or absence of Epichloë in Ammophila, we used endophyte-free seeds collected from nearby dunes, germinated seedlings of Ammophila in the lab, and either artificially inoculated seedlings with Epichloë (E+) or sham inoculated them with sterile water (E−) (see details in [34]). Plants were clonally propagated in a greenhouse and then transported to the field. Into 90 2 m × 2 m plots, we transplanted 25 E+ or 25 E− Ammophila plants and monitored plots yearly from 2010–2020. Because plants can sometimes lose Epichloë symbionts over time [35,43], we used commercial immunoblot kits (Phytoscreen: Agrinostics, Watkinsville, GA, USA) to assess treatment reliability in two tillers per plot in 2019. Any inconclusive assay results were followed up with microscopy to confirm presence or absence of hyphae within leaf tissue. 91% of E+ treatment plot tillers maintained evidence of Epichloë infection, while 89% of E− plot tillers still lacked Epichloë.
In 2016 we introduced N addition treatments to a subset of 60 plots in the long-term experiment (30 E+ and 30 E− plots). One third of plots (10 each E+ and E−) received a low level of N (0.5 g NH4+ m−2), corresponding to N deposition rates in the nearby urban centers of the Midwest [30], another third received high levels of N (10 g NH4+ m−2), comparable to global N addition experiments [44], and the last set of plots received no added fertilizer (control). We added N as urea slow-release fertilizer (ESN Urea coated fertilizer: Nutrient Ltd., New Madrid MO, USA), applied twice yearly: once in May at the beginning of the growing season, and again mid-season in July. Due to COVID-19 travel restrictions, we were unable to apply fertilizer in May 2020, but treatments resumed as planned in July 2020.
2.2. Root Endophyte Isolation & Identification
In July 2020, we excavated roots from 2 randomly chosen visibly healthy Ammophila tiller clumps per plot in the N addition experiment (n = 60 plots). These tillers with attached roots were kept at 4 °C and transported on ice back to the lab at the University of Louisville for processing. One root segment per tiller was cut from each Ammophila plant and surface-sterilized using standard methods. In brief, roots were soaked in 95% ethanol for one minute then in a 1% bleach solution for two minutes. Root samples were then washed with 70% ethanol for two minutes, then rinsed with autoclaved water 3 times. After surface-sterilization of each plant root, five 3–5 mm root segments were chosen haphazardly and plated tip first into Petri dishes containing 2% malt extract agar (MEA) + 10 mL Penicillin-Streptomycin to decrease risk of bacterial contamination. Plates were then sealed with parafilm and stored in a cabinet at room temperature. Plates were monitored for approximately two weeks for fungal growth. A subset of plates was stored at 4 °C to reduce spread of fast-growing endophytes and encourage growth of slower-emerging fungi. There were no differences in taxa seen between room temperature vs. refrigerated plates, so room temperature fungal cultures were used for this study. Emergent hyphae were sub-cultured onto new MEA plates to isolate individual taxa. Isolates were allowed to grow until the colony covered the agar plate. Isolates were then grouped into morphotypes based on color and mycelial growth patterns. Once morphotypes were determined, at least one isolate from each morphotype group was identified using Sanger sequencing of the fungal ITS region (see below). For common morphotypes, multiple representative fungal isolates were sequenced. Fungal isolates were also vouchered in sterile water for long-term storage.
To verify that our morphotype designations were accurately grouped and to taxonomically identify fungal isolates, we extracted DNA and conducted polymerase chain reaction (PCR) using Sigma Aldrich Extract-N-Amp™ Plant Tissue PCR Kits [45]. We used ITS1F and LR3 primers to amplify the nuclear ribosomal internal transcribed spacer region and 5.8S gene (ITSrDNA) and approximately 600 bp of the adjacent large ribosomal subunit (LSUrDNA) [46]. Each 20 μL reaction included 4 mL molecular-grade water, 10 µL PCR Ready Mix (includes buffer, salts, dNTPs, and Taq polymerase), 1 µL ITS1F primer, 1 µL LR3 primer, and 4 µL template DNA. Amplification was done using BioRad T100 Thermal Cycler. The thermocycler settings were conducted as follows: initial three minutes of denaturation at 95 °C, followed by 37 cycles (30 s of denaturation at 95 °C, 30 s of annealing at 55 °C, and two minutes of elongation at 72 °C. and 10 min final extension at 72 °C). To confirm successful gene amplification, gel electrophoresis using SYBR Safe (Invitrogen) produced single bands for all products as well as no bands for negative controls. PCR products were enzymatically cleaned using Illustra ExoProStar (Cytiva Life Sciences) following the manufacturer’s protocol. Purified PCR samples were then sent to Eurofins Genomics (Louisville, Kentucky) for Sanger sequencing of both forward (ITS1F) and reverse (LR3) reads.
We used Sequencher v. 5.4 (Gene Codes Corporation, Ann Arbor, MI, USA) to assemble sequences into operational taxonomic units (OTUs) according to 97% ITS sequence similarity, with a minimum of 40% overlap. We used the NCBI Basic Local Alignment Search Tool (BLAST) [47] and the Ribosomal Database Project (RDP) Bayesian Classifier with both the Warcup [48] and UNITE [48] ITS training sets to obtain best match taxonomic names for each OTU (Table S1). Sequence data are archived at GenBank under accession numbers OP679885-OP679925.
2.3. Statistical Analysis
To analyze shifts in root fungal endophyte species richness in response to Epichloë presence and N addition, we conducted treatment level comparisons using a general linear mixed-effects model, with nitrogen addition treatment, Epichloë presence and their interaction as fixed effects and spatial plot level factors (row and column) as random effects. Neither row, column, nor their interactions with plot level factors were significant (data not shown), so they were removed from the model for subsequent analyses to better identify main treatment effects. These analyses were performed using R version 1.4.1106.
We used a two-factor PERMANOVA to analyze changes in root fungal endophyte community composition [49], with N addition treatment and Epichloë presence or absence, and the interaction between the two treatments as fixed effects. Using a binary community matrix comprised of presence and absence of OTUs, we performed a PERMANOVA using the Bray–Curtis distance metric with 9999 permutations under a reduced model with Type III sum of squares. Singleton taxa were excluded from this analysis to improve resolution. Heterogeneity of the community in response to the different treatment factors was tested using PERMDISP [50]. To visualize differences in root endophyte communities, we then conducted a nonmetric multidimensional scaling (NMDS) ordination [51] with Bray–Curtis dissimilarity measures. Finally, a SIMPER (similarity percentages species contribution) analysis was used to determine which fungal species contributed most to differences in community composition among treatment combinations [52]. The PERMANOVA and NMDS ordination were conducted in R using the VEGAN package [53]. PERMDISP and SIMPER analyses were conducted in Primer v. 6 [54].
3. Results
We identified a total of 23 OTUs and 11 singleton taxa in our study (Figure S1). Root endophyte species richness increased by 17% when Epichloë was present within the host grass (Table 1, Figure 1). However, N addition had no significant main or interactive effects on root endophyte richness (Table 1).

Table 1.
Statistical results from analyses of root fungal endophyte species richness (GLM), community composition (PERMANOVA), and community heterogeneity (PERMDISP). Statistically significant results are indicated in bold and by an asterisk (* p < 0.10, ** p < 0.05).
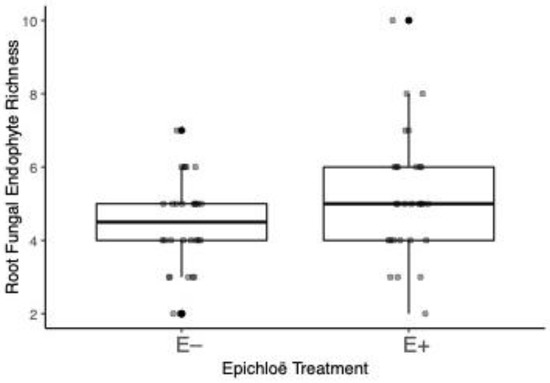
Figure 1.
Root fungal endophyte species richness per plot from Ammophila plants where Epichloë was either present or absent. Treatment medians and quartiles shown, with points jittered to aid with viewing. Darker circles indicate overlapping data points.
In contrast, root endophyte community composition was affected by N addition, especially where Epichloë was present, as indicated by the presence or absence of overlap in treatments within the ordination (Table 1, Figure 2). Pairwise comparisons showed significant differences between all N treatments for E+ plants, and no significant differences between N treatments for E− plants (Table S2).
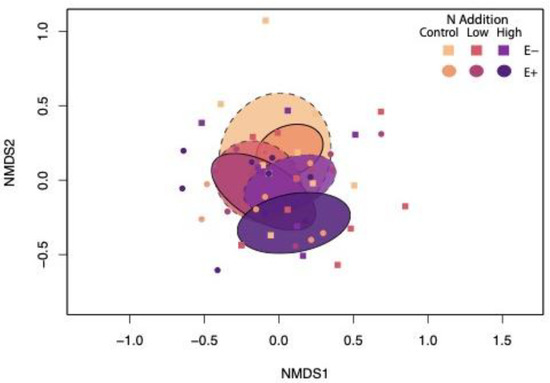
Figure 2.
Nonmetric multidimensional scaling plot displaying differences in root endophyte community composition among Epichloë and N addition treatments. 2D stress = 0.2. Ellipses represent standard deviation of point scores for each treatment interaction. Dashed lines represent E− plots.
Epichloë and N addition also interacted to affect heterogeneity among root endophyte communities within treatments, as shown by the PERMDISP analysis (Table 1) and NMDS ordination, where decrease in oval size corresponds to decreased heterogeneity (Figure 2). Pairwise contrasts (Table S2) indicated that low and high levels of N addition reduced root endophyte community heterogeneity in Ammophila roots by 32–36% compared to plants in the control treatment, but only when Epichloë was absent. N addition had no effects on heterogeneity when Epichloë was present. In control N treatments, Epichloë presence reduced heterogeneity of root endophyte communities by 43% compared to plants lacking Epichloë.
Finally, The SIMPER analysis indicated that two taxa, Microdochium bolleyi and Fusarium sp., were common taxa within root endophyte communities across all treatments (Table S3). Fusarium sp., along with Leptosphaeria sp., Cadophora sp., and Sarocladium strictum showed reduced occurrences in response to N addition, while a different Fusarium sp. and Acremonium sp. increased in frequency in response to N addition (Table S4).
4. Discussion
Epichloë influenced belowground mycobiome diversity and composition in this dune grass system, especially under N-enriched conditions, providing moderate evidence that Epichloë may act as a key species restructuring the above- and belowground mycobiome of host plants. Several potential explanations exist for why root fungal endophyte communities increased in richness in response to Epichloë infection. It might be expected that Epichloë would suppress root endophyte diversity due to its ability to produce systemic alkaloids [55,56,57] which directly inhibit growth of other fungal species, including pathogens [4,5,57,58,59,60]. However, indirect effects of Epichloë on its plant host may override direct mycobiome interactions to improve conditions for root endophyte communities. Within the harsh sand dune environment specific to our study, Epichloë acts as a mutualist, increasing Ammophila survival, vegetative growth, and belowground biomass [14,34,61]. In other systems, Epichloë can improve rhizosphere characteristics including soil fertility, root morphology, soil nutrients, and organic carbon [62,63]. By increasing habitat space in roots and improving belowground conditions, Epichloë may indirectly facilitate root endophyte diversity. The other studies that have demonstrated no effects of Epichloë on root endophyte communities were conducted in less extreme habitats such as agricultural fields and prairies [11,12], where indirect effects of Epichloë on host plants may be less important.
Epichloë infection also altered root endophyte community composition in Ammophila, especially under N-enriched conditions; while under ambient conditions, the presence of Epichloë caused root fungal endophyte communities to converge across plots. These findings suggest that Epichloë acts a filter to restructure the endophyte communities that colonize Ammophila roots, possibly by altering the physical or chemical environment of host plants, such as root exudate chemistry [64,65]. Plant mycobiome composition can be strongly influenced by plant secondary metabolites [66,67], and Epichloë is known for enhancing alkaloid production inside host plants [68]. Alkaloids are nitrogen-rich secondary metabolites, so adding nitrogen could alter Epichloë’s ability to produce these chemicals within hosts, either positively [69,70,71] or negatively [72] (but see [73] where no effect was found), which could induce a strong filter on root endophyte species composition.
We were able to identify several fungal taxa responsible for the shifts in root endophyte community composition. Both Microdochium bolleyi and Fusarium sp. were common taxa within root endophyte communities across all treatments. This Fusarium sp. best matched with Fusarium fujikuroi in the BLAST database (99.82% match), though we recognize that species designations within Fusarium usually require a tef1 sequence, which we did not have. Microdochium bolleyi is a common dark septate endophyte, primarily of grasses [74,75,76], including dune grasses of the Pacific Northwest [77]. A recent study found no effects of Epichloë exudates on M. bolleyi growth in an in vitro assay [6], which our field results support. However, F. fujikuroi (putative) was suppressed by Epichloë presence in N-enriched conditions, along with Sarocladium strictum, Leptosphaeria sp., and Cadophora sp. While the functions of many root endophytes are unknown, Fusarium fujikuroi has been classified in other systems as an asymptomatic nonobligate root symbiont best known for its gibberellin production [78,79] and has been found in other marine and coastal systems, along with S. strictum [80]. Since Epichloë is known to stimulate gibberellin production in both seeds and plants [81], any benefits that F. fujikuroi provides to hosts may become redundant. Sarocladium, Leptosphaeria and Cadophora are common root endophyte genera [82,83] with functions ranging from commensal to parasitic to saprophytic, making generalizations difficult.
Two taxa increased in abundance in response to N addition for Epichloë colonized plants: Fusarium sporotrichioides (putative) and Acremonium sp. Fusarium sporotrichioides is a known pathogen of maize and an opportunistic pathogen of other cereal crops [84,85]. In agricultural systems, N fertilization often increases abundance of this and related Fusarium spp., possibly due to changes in plant N-metabolism (e.g., [86]). This may explain why increased N availability increased the occurrence of this species in Ammophila roots. Acremonium species may act as potential mutualists by increasing root growth in host plants [87], and so may hold a functionally similar role to Epichloë. While very little is known about root endophyte biology, especially in non-agricultural systems, these species-specific responses provide some insights into how Epichloë may filter mycobiome communities.
In conclusion, by manipulating the presence of Epichloë in a long-term experiment, we found moderate evidence that this systemic endophyte is acting as a key species within Ammophila, changing diversity and composition of the root mycobiome and integrating above- and belowground mycobiome interactions. Further, effects of Epichloë on root endophyte communities were enhanced by N addition, indicating that this fungal species may become even more important in future environments. Ammophila is widely used in coastal dune restorations, and the importance of plant-fungal symbioses for restoration efforts are starting to be recognized [88]. Future work should address the consequences of such shifts in mycobiome communities for host plants, as intentional manipulation of mycobiome interactions may improve future restoration efforts in changing environments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8111142/s1, Table S1. Comparison of different fungal databases (UNITE, Warcup, BLAST) to identify fungal root endophyte OTUs detected within the study to taxon. Percentages listed in the table are indicative of close matches for designated taxa. For UNITE and Warcup, the percentages given in brackets indicates confidence thresholds whereas BLAST displays the percent identity compared to its best match. OTUs beginning with S indicate singleton taxa. Table S2. Pairwise comparisons of community composition (PERMANOVA) and heterogeneity (PERMDISP) between N-addition treatments within each Epichloë treatment. Table S3. Similarity Percentages (SIMPER) Analysis results, showing taxon contributions to community composition similarity within each Epichloë × N addition treatment, up to 90% cumulative composition. Table S4. Similarity Percentages (SIMPER) Analysis results, showing root endophyte taxon average frequencies and their contribution to community differences among N addition treatments when Epichloë was present. Figure S1. Heatmap displaying presence/absence of fungal root endophyte OTUs within each Epichloë and N-addition treatment.
Author Contributions
Conceptualization, K.R.G. and S.M.E. Methodology K.R.G., H.E.S., N.C. and S.M.E. Formal analysis, K.R.G., H.E.S., N.C. and S.M.E. Resources, K.R.G., N.C. and S.M.E. Data Curation, K.R.G., N.C. and S.M.E. Writing—original draft preparation, K.R.G. Writing—review and editing, H.E.S., N.C. and S.M.E. Visualization, K.R.G., N.C. and S.M.E. Supervision, K.R.G., N.C. and S.M.E. Project Administration, K.R.G., N.C. and S.M.E. Funding acquisition, K.R.G. and H.E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been primarily funded by an NSF Graduate Research Fellowship awarded to K.R.G. Additional funding support was provided by the University of Louisville’s Summer Research Opportunity Program (SROP) awarded to H.E.S. The original field experiment was funded by NSF#0918267 to S.M.E. and a National Parks Ecological Research Post-Doctoral Fellowship to S.M.E.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request from the authors.
Acknowledgments
We thank Susanne Cramer, Brad Kimbrough, and Josh Cramer for field assistance in 2020. A special thanks to Elizabeth Rodgers, Marissa Huber, and Griffin McHugh for lab assistance. Jennifer Rudgers and Lukas Bell-Dereske were instrumental in initial experiment design and setup. Additionally, we thank Connor Morozumi for encouragement and feedback on this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Porras-Alfaro, A.; Bayman, P. Hidden Fungi, Emergent Properties: Endophytes and Microbiomes. Annu. Rev. Phytopathol. 2011, 49, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Jumpponen, A.; Trappe, J. Dark Septate Endophytes: A Review of Facultative Biotrophic Root-Colonizing Fungi. New Phytol. 1998, 140, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Mack, K.M.L.; Rudgers, J.A. Balancing Multiple Mutualists: Asymmetric Interactions among Plants, Arbuscular Mycorrhizal Fungi, and Fungal Endophytes. Oikos 2008, 117, 310–320. [Google Scholar] [CrossRef]
- Victoria Novas, M.; Iannone, L.J.; Godeas, A.M.; Cabral, D. Positive Association between Mycorrhiza and Foliar Endophytes in Poa Bonariensis, a Native Grass. Mycol. Prog. 2008, 8, 75. [Google Scholar] [CrossRef]
- Vignale, M.V.; Iannone, L.J.; Scervino, J.M.; Novas, M.V. Epichloë Exudates Promote in Vitro and in Vivo Arbuscular Mycorrhizal Fungi Development and Plant Growth. Plant Soil 2018, 422, 267–281. [Google Scholar] [CrossRef]
- Terlizzi, N.L.; Rodríguez, M.A.; Iannone, L.J.; Lanari, E.; Novas, M.V. Epichloë Endophyte Affects the Root Colonization Pattern of Belowground Symbionts in a Wild Grass. Fungal Ecol. 2022, 57–58, 101143. [Google Scholar] [CrossRef]
- Novas, M.V.; Cabral, D.; Godeas, A.M. Interaction between Grass Endophytes and Mycorrhizas in Bromus Setifolius from Patagonia, Argentina. Symbiosis 2005, 40, 23–30. [Google Scholar]
- Konig, J.; Guerreiro, M.; Persoh, D.; Begerow, D.; Krauss, J. Knowing Your Neighbourhood- the Effects of Epichloe Endophytes on Foliar Fungal Assemblages in Perennial Ryegrass in Dependence of Season and Land-Use Intensity. PeerJ 2018, 6, e4660. [Google Scholar] [CrossRef]
- Zabalgogeazcoa, I.; Gundel, P.E.; Helander, M.; Saikkonen, K. Non-Systemic Fungal Endophytes in Festuca Rubra Plants Infected by Epichloë Festucae in Subarctic Habitats. Fungal Divers. 2013, 60, 25–32. [Google Scholar] [CrossRef]
- Nissinen, R.; Helander, M.; Kumar, M.; Saikkonen, K. Heritable Epichloë Symbiosis Shapes Fungal but Not Bacterial Communities of Plant Leaves. Sci. Rep. 2019, 9, 5253. [Google Scholar] [CrossRef]
- Vandergrift, R.; Roy, B.; Pfeifer-Meister, L.; Johnson, B.; Bridgham, S. The Herbaceous Landlord: Integrating the Effects of Symbiont Consortia within a Single Host. PeerJ 2015, 3, e1379. [Google Scholar] [CrossRef]
- Slaughter, L.C.; McCulley, R.L. Aboveground Epichloë Coenophiala–Grass Associations Do Not Affect Belowground Fungal Symbionts or Associated Plant, Soil Parameters. Microb. Ecol. 2016, 72, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.; Aldana, B.; San Emeterio, L.; Zabalgogeazcoa, I. A Survey of Culturable Fungal Endophytes From Festuca Rubra Subsp. Pruinosa, a Grass From Marine Cliffs, Reveals a Core Microbiome. Front. Microbiol. 2019, 9, 3321. [Google Scholar] [CrossRef] [PubMed]
- Bell-Dereske, L.; Gao, X.; Masiello, C.A.; Sinsabaugh, R.L.; Emery, S.M.; Rudgers, J.A. Plant–Fungal Symbiosis Affects Litter Decomposition during Primary Succession. Oikos 2017, 126, 801–811. [Google Scholar] [CrossRef]
- Zhong, R.; Xia, C.; Ju, Y.; Zhang, X.; Duan, T.; Nan, Z.; Li, C. A Foliar Epichloë Endophyte and Soil Moisture Modified Belowground Arbuscular Mycorrhizal Fungal Biodiversity Associated with Achnatherum Inebrians. Plant Soil 2021, 458, 105–122. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Emery, S.M.; Rudgers, J.A. Fungal Symbionts Alter Plant Responses to Global Change. Am. J. Bot. 2013, 100, 1445–1457. [Google Scholar] [CrossRef]
- Classen, A.T.; Sundqvist, M.K.; Henning, J.A.; Newman, G.S.; Moore, J.A.M.; Cregger, M.A.; Moorhead, L.C.; Patterson, C.M. Direct and Indirect Effects of Climate Change on Soil Microbial and Soil Microbial-Plant Interactions: What Lies Ahead? Ecosphere 2015, 6, art130. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The Unseen Majority: Soil Microbes as Drivers of Plant Diversity and Productivity in Terrestrial Ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D.G. Human Alteration of the Global Nitrogen Cycle: Sources and Consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef]
- Lamarque, J.F.; Dentener, F.; McConnell, J.; Ro, C.U.; Shaw, M.; Vet, R.; Bergmann, D.; Cameron-Smith, P.; Dalsoren, S.; Doherty, R.; et al. Multi-Model Mean Nitrogen and Sulfur Deposition from the Atmospheric Chemistry and Climate Model Intercomparison Project (ACCMIP): Evaluation of Historical and Projected Future Changes. Atmos. Chem. Phys. 2013, 13, 7997–8018. [Google Scholar] [CrossRef]
- Treseder, K.K. A Meta-Analysis of Mycorrhizal Responses to Nitrogen, Phosphorus, and Atmospheric CO2 in Field Studies. New Phytol. 2004, 164, 347–355. [Google Scholar] [CrossRef]
- Emery, S.M.; Reid, M.L.; Bell-Dereske, L.; Gross, K.L. Soil Mycorrhizal and Nematode Diversity Vary in Response to Bioenergy Crop Identity and Fertilization. GCB Bioenergy 2017, 9, 1644–1656. [Google Scholar] [CrossRef]
- Ma, X.; Geng, Q.; Zhang, H.; Bian, C.; Chen, H.Y.H.; Jiang, D.; Xu, X. Global Negative Effects of Nutrient Enrichment on Arbuscular Mycorrhizal Fungi, Plant Diversity and Ecosystem Multifunctionality. New Phytol. 2021, 229, 2957–2969. [Google Scholar] [CrossRef] [PubMed]
- Oono, R.; Black, D.; Slessarev, E.; Sickler, B.; Strom, A.; Apigo, A. Species Diversity of Fungal Endophytes across a Stress Gradient for Plants. New Phytol. 2020, 228, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Henning, J.A.; Kinkel, L.; May, G.; Lumibao, C.Y.; Seabloom, E.W.; Borer, E.T. Plant Diversity and Litter Accumulation Mediate the Loss of Foliar Endophyte Fungal Richness Following Nutrient Addition. Ecology 2021, 102, e03210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Deng, Y.; Ge, X.; Shi, X.; Fan, X.; Dong, K.; Chen, L.; Zhao, N.; Gao, Y.; Ren, A. The Beneficial Effect of Epichloë Endophytes on the Growth of Host Grasses Was Affected by Arbuscular Mycorrhizal Fungi, Pathogenic Fungi and Nitrogen Addition. Environ. Exp. Bot. 2022, 201, 104979. [Google Scholar] [CrossRef]
- Ren, A.Z.; Li, X.; Han, R.; Yin, L.J.; Wei, M.Y.; Gao, Y.B. Benefits of a Symbiotic Association with Endophytic Fungi Are Subject to Water and Nutrient Availability in Achnatherum Sibiricum. Plant Soil 2011, 346, 363. [Google Scholar] [CrossRef]
- Morrice, J.A.; Danz, N.P.; Regal, R.R.; Kelly, J.R.; Niemi, G.J.; Reavie, E.D.; Hollenhorst, T.; Axler, R.P.; Trebitz, A.S.; Cotter, A.M.; et al. Human Influences on Water Quality in Great Lakes Coastal Wetlands. Environ. Manag. 2008, 41, 347–357. [Google Scholar] [CrossRef]
- Foley, T.A.; Betterton, E.A. Nitrogen Dry Deposition to Lake Superior and Lake Michigan. J. Great Lakes Res. 2019, 45, 224–239. [Google Scholar] [CrossRef]
- Du, E.; de Vries, W.; Galloway, J.N.; Hu, X.; Fang, J. Changes in Wet Nitrogen Deposition in the United States between 1985 and 2012. Environ. Res. Lett. 2014, 9, 095004. [Google Scholar] [CrossRef]
- Drake, I.; White, J.F.; Belanger, F.C. Identification of the Fungal Endophyte of Ammophila Breviligulata (American Beachgrass) as Epichloë Amarillans. PeerJ 2018, 2018, e4300. [Google Scholar] [CrossRef] [PubMed]
- Emery, S.M.; Rudgers, J.A. Biotic and Abiotic Predictors of Ecosystem Engineering Traits of the Dune Building Grass, Ammophila Breviligulata. Ecosphere 2014, 5, 1–18. [Google Scholar] [CrossRef]
- Rudgers, J.A.; Bell-Dereske, L.; Crawford, K.M.; Emery, S.M. Fungal Symbiont Effects on Dune Plant Diversity Depend on Precipitation. J. Ecol. 2015, 103, 219–230. [Google Scholar] [CrossRef]
- Emery, S.M.; Bell-Dereske, L.; Rudgers, J.A. Fungal Symbiosis and Precipitation Alter Traits and Dune Building by the Ecosystem Engineer, Ammophila Breviligulata. Ecology 2015, 96, 927–935. [Google Scholar] [CrossRef] [PubMed]
- David, A.S.; Bell-Dereske, L.P.; Emery, S.M.; McCormick, B.M.; Seabloom, E.W.; Rudgers, J.A. Testing for Loss of Epichloë and Non-Epichloid Symbionts under Altered Rainfall Regimes. Am. J. Bot. 2019, 106, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Emery, S.M.; Rudgers, J.A. Ecological Assessment of Dune Restorations in the Great Lakes Region. Restor. Ecol. 2010, 18, 184–194. [Google Scholar] [CrossRef]
- Gehring, C.; Sevanto, S.; Patterson, A.; Marias Ulrich, D.; Kuske, C. Ectomycorrhizal and Dark Septate Fungal Associations of Pinyon Pine Are Differentially Affected by Experimental Drought and Warming. Front. Plant Sci. 2020, 11, 582574. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.L.; French, K. Soil Nutrients Differentially Influence Root Colonisation Patterns of AMF and DSE in Australian Plant Species. Symbiosis 2021, 83, 209–223. [Google Scholar] [CrossRef]
- Gibert, A.; Hazard, L. Endophyte Infection of Festuca Eskia Enhances Seedling Survival to Drought and Cutting at the Expense of Clonal Expansion. J. Plant Ecol. 2011, 4, 201–208. [Google Scholar] [CrossRef]
- Bueno de Mesquita, C.P.; Sartwell, S.A.; Ordemann, E.V.; Porazinska, D.L.; Farrer, E.C.; King, A.J.; Spasojevic, M.J.; Smith, J.G.; Suding, K.N.; Schmidt, S.K. Patterns of Root Colonization by Arbuscular Mycorrhizal Fungi and Dark Septate Endophytes across a Mostly-Unvegetated, High-Elevation Landscape. Fungal Ecol. 2018, 36, 63–74. [Google Scholar] [CrossRef]
- Menoyo, E.; Teste, F.P.; Ferrero, M.A.; Lugo, M.A. Associations between Fungal Root Endophytes and Grass Dominance in Arid Highlands. Fungal Ecol. 2020, 45, 100924. [Google Scholar] [CrossRef]
- Sternhagen, E.C.; Black, K.L.; Hartmann, E.D.L.; Shivega, W.G.; Johnson, P.G.; McGlynn, R.D.; Schmaltz, L.C.; Asheim Keller, R.J.; Vink, S.N.; Aldrich-Wolfe, L. Contrasting Patterns of Functional Diversity in Coffee Root Fungal Communities Associated with Organic and Conventionally Managed Fields. Appl. Environ. Microbiol. 2020, 86, e00052-20. [Google Scholar] [CrossRef] [PubMed]
- Rudgers, J.A.; Afkhami, M.E.; Rúa, M.A.; Davitt, A.J.; Hammer, S.; Huguet, V.M. A Fungus among Us: Broad Patterns of Endophyte Distribution in the Grasses. Ecology 2009, 90, 1531–1539. [Google Scholar] [CrossRef]
- Borer, E.T.; Harpole, W.S.; Adler, P.B.; Lind, E.M.; Orrock, J.L.; Seabloom, E.W.; Smith, M.D. Finding Generality in Ecology: A Model for Globally Distributed Experiments. Methods Ecol. Evol. 2014, 5, 65–73. [Google Scholar] [CrossRef]
- U’Ren, J.M.; Miadlikowska, J.; Zimmerman, N.B.; Lutzoni, F.; Stajich, J.E.; Arnold, A.E. Contributions of North American Endophytes to the Phylogeny, Ecology, and Taxonomy of Xylariaceae (Sordariomycetes, Ascomycota). Mol. Phylogenet. Evol. 2016, 98, 210–232. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.E.; Henk, D.A.; Eells, R.L.; Lutzoni, F.; Vilgalys, R. Diversity and Phylogenetic Affinities of Foliar Fungal Endophytes in Loblolly Pine Inferred by Culturing and Environmental PCR. Mycologia 2007, 99, 185–206. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Qiong, W.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of RRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Anderson, M.J. A New Method for Non-parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Anderson, M.J. Distance-Based Tests for Homogeneity of Multivariate Dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef]
- McCune, B.P.; Grace, J. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. Primer, Version 6.1. 10: User Manual and Tutorial; Primer-E: Plymouth, UK, 2009. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 29 September 2022).
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; Primer-E: Plymouth, UK, 2008; pp. 1–214. [Google Scholar]
- Guerre, P. Ergot Alkaloids Produced by Endophytic Fungi of the Genus Epichloë. Toxins (Basel) 2015, 7, 773–790. [Google Scholar] [CrossRef] [PubMed]
- Saikkonen, K.; Gundel, P.E.; Helander, M. Chemical Ecology Mediated by Fungal Endophytes in Grasses. J. Chem. Ecol. 2013, 39, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Young, C.A.; Pan, J.; Florea, S.; Takach, J.E.; Panaccione, D.G.; Farman, M.L.; Webb, J.S.; Jaromczyk, J.; Charlton, N.D.; et al. Currencies of Mutualisms: Sources of Alkaloid Genes in Vertically Transmitted Epichloae. Toxins (Basel) 2013, 5, 1064–1088. [Google Scholar] [CrossRef] [PubMed]
- Card, S.D.; Bastias, D.; Caradus, J.R. Antagonism to Plant Pathogens by Epichloë Fungal Endophytes—A Review. Plants 2021, 10, 1997. [Google Scholar] [CrossRef]
- Pérez, L.I.; Gundel, P.E.; Zabalgogeazcoa, I.; Omacini, M. An Ecological Framework for Understanding the Roles of Epichloë Endophytes on Plant Defenses against Fungal Diseases. Fungal Biol. Rev. 2020, 34, 115–125. [Google Scholar] [CrossRef]
- Roberts, E.L.; Ferraro, A. Rhizosphere Microbiome Selection by Epichloë Endophytes of Festuca Arundinacea. Plant Soil 2015, 396, 229–239. [Google Scholar] [CrossRef]
- Emery, S.M.; Doran, P.J.; Legge, J.T.; Kleitch, M.; Howard, S. Aboveground and Belowground Impacts Following Removal of the Invasive Species Baby’s Breath (Gypsophila Paniculata) on Lake Michigan Sand Dunes. Restor. Ecol. 2013, 21, 506–514. [Google Scholar] [CrossRef]
- Wang, J.; Hou, W.; Christensen, M.J.; Li, X.; Xia, C.; Li, C.; Nan, Z. Role of Epichloë Endophytes in Improving Host Grass Resistance Ability and Soil Properties. J. Agric. Food Chem. 2020, 68, 6944–6955. [Google Scholar] [CrossRef]
- Lee, K.; Missaoui, A.; Mahmud, K.; Presley, H.; Lonnee, M. Interaction between Grasses and Epichloë Endophytes and Its Significance to Biotic and Abiotic Stress Tolerance and the Rhizosphere. Microorganisms 2021, 9, 2186. [Google Scholar] [CrossRef]
- Patchett, A.; Newman, J.A. Comparison of Plant Metabolites in Root Exudates of Lolium Perenne Infected with Different Strains of the Fungal Endophyte Epichloë Festucae Var. Lolii. J. Fungi 2021, 7, 148. [Google Scholar] [CrossRef]
- Rasmussen, S.; Parsons, A.J.; Newman, J.A. Metabolomics Analysis of the Lolium Perenne–Neotyphodium Lolii Symbiosis: More than Just Alkaloids? Phytochem. Rev. 2009, 8, 535–550. [Google Scholar] [CrossRef]
- Pang, Z.; Chen, J.; Wang, T.; Gao, C.; Li, Z.; Guo, L.; Xu, J.; Cheng, Y. Linking Plant Secondary Metabolites and Plant Microbiomes: A Review. Front. Plant Sci. 2021, 12, 621276. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Plants and Endophytes: Equal Partners in Secondary Metabolite Production? Biotechnol. Lett. 2015, 37, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Grossman, R.B.; Nagabhyru, P.; Faulkner, J.R.; Mallik, U.P. Loline Alkaloids: Currencies of Mutualism. Phytochemistry 2007, 68, 980–996. [Google Scholar] [CrossRef] [PubMed]
- Rottinghaus, G.E.; Garner, G.B.; Cornell, C.N.; Ellis, J.L. HPLC Method for Quantitating Ergovaline in Endophyte-Infested Tall Fescue: Seasonal Variation of Ergovaline Levels in Stems with Leaf Sheaths, Leaf Blades, and Seed Heads. J. Agric. Food Chem. 1991, 39, 112–115. [Google Scholar] [CrossRef]
- Krauss, J.; Harri, S.A.; Bush, L.; Husi, R.; Bigler, L.; Power, S.A.; Muller, C.B. Effects of Fertilizer, Fungal Endophytes and Plant Cultivar on the Performance of Insect Herbivores and Their Natural Enemies. Funct. Ecol. 2007, 21, 107–116. [Google Scholar] [CrossRef]
- Lane, G.; Tapper, B.; Davies, E.; Hume, D.; Latch, G.; Barker, D.; Easton, H.; Rolston, P. Effect of Growth Conditions on Alkaloid Concentrations in Perennial Ryegrass Naturally Infected with Endophyte; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Rasmussen, S.; Parsons, A.J.; Bassett, S.; Christensen, M.J.; Hume, D.E.; Johnson, L.J.; Johnson, R.D.; Simpson, W.R.; Stacke, C.; Voisey, C.R.; et al. High Nitrogen Supply and Carbohydrate Content Reduce Fungal Endophyte and Alkaloid Concentration in Lolium Perenne. New Phytol. 2007, 173, 787–797. [Google Scholar] [CrossRef]
- Bylin, A.G.; Hume, D.E.; Card, S.D.; Mace, W.J.; Lloyd-West, C.M.; Huss-Danell, K. Influence of Nitrogen Fertilization on Growth and Loline Alkaloid Production of Meadow Fescue (Festuca Pratensis) Associated with the Fungal Symbiont Neotyphodium Uncinatum. Botany 2014, 92, 370–376. [Google Scholar] [CrossRef]
- David, A.S.; Sajeet, H.; Kurt, L.; Joanne, L.; Anna, L.; Mei, W.; Kerrie, B.; Igor, G.V.; Joseph, W.S.; Georgiana, M. Draft Genome Sequence of Microdochium Bolleyi, a Dark Septate Fungal Endophyte of Beach Grass. Genome Announc. 2016, 4, e00270-16. [Google Scholar] [CrossRef]
- Mandyam, K.; Loughin, T.; Jumpponen, A. Isolation and Morphological and Metabolic Characterization of Common Endophytes in Annually Burned Tallgrass Prairie. Mycologia 2010, 102, 813–821. [Google Scholar] [CrossRef]
- Knapp, D.G.; Imrefi, I.; Boldpurev, E.; Csíkos, S.; Akhmetova, G.; Berek-Nagy, P.J.; Otgonsuren, B.; Kovács, G.M. Root-Colonizing Endophytic Fungi of the Dominant Grass Stipa Krylovii From a Mongolian Steppe Grassland. Front. Microbiol. 2019, 10, 2565. [Google Scholar] [CrossRef]
- David, A.S.; Seabloom, E.W.; May, G. Plant Host Species and Geographic Distance Affect the Structure of Aboveground Fungal Symbiont Communities, and Environmental Filtering Affects Belowground Communities in a Coastal Dune Ecosystem. Microb. Ecol. 2016, 71, 912–926. [Google Scholar] [CrossRef] [PubMed]
- Avalos, J.; Cerda-Olmedo, E.; Reyes, F.; Barrero, F.A. Gibberellins and Other Metabolites of Fusarium Fujikuroi and Related Fungi. Curr. Org. Chem. 2007, 11, 721–737. [Google Scholar] [CrossRef]
- Bacon, C.W.; Hinton, D.M. Symptomless Endophytic Colonization of Maize by Fusarium Moniliforme. Can. J. Bot. 1996, 74, 1195–1202. [Google Scholar] [CrossRef]
- Fuentes, M.; Quinones, R. Carbon Utilization Profile of the Filamentous Fungal Species Fusarium Fujikuroi, Penicillium Decumbens and Sarocladium Strictum Isolated from Marine Coastal Environments. Mycologia 2016, 108, 1069–1081. [Google Scholar] [CrossRef]
- Bastías, D.A.; Gianoli, E.; Gundel, P.E. Fungal Endophytes Can Eliminate the Plant Growth–Defence Trade-Off. New Phytol. 2021, 230, 2105–2113. [Google Scholar] [CrossRef]
- Junker, C.; Draeger, S.; Schulz, B. A Fine Line—Endophytes or Pathogens in Arabidopsis Thaliana. Fungal Ecol. 2012, 5, 657–662. [Google Scholar] [CrossRef]
- Jallow, M.F.A.; Dugassa-Gobena, D.; Vidal, S. Influence of an Endophytic Fungus on Host Plant Selection by a Polyphagous Moth via Volatile Spectrum Changes. Arthropod. Plant. Interact. 2008, 2, 53–62. [Google Scholar] [CrossRef]
- Yli-Mattila, T. Ecology and Evolution of Toxigenic Fusarium Species in Cereals in Northern Europe and Asia. J. Plant Pathol. 2010, 92, 7–18. [Google Scholar]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium Species and Mycotoxins Associated with Head Blight in Small-Grain Cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Martin, R.A.; Macleod, J.A.; Caldwell, C.D. Influences of Production Inputs on Incidence of Infection by Fusarium Species on Cereal Seed. Plant Dis. 1991, 75, 784–788. [Google Scholar] [CrossRef]
- Ndinga-Muniania, C.; Mueller, R.C.; Kuske, C.R.; Porras-Alfaro, A. Seasonal Variation and Potential Roles of Dark Septate Fungi in an Arid Grassland. Mycologia 2021, 113, 1181–1198. [Google Scholar] [CrossRef] [PubMed]
- Farrer, E.C.; Van Bael, S.A.; Clay, K.; Smith, M.K.H. Plant-Microbial Symbioses in Coastal Systems: Their Ecological Importance and Role in Coastal Restoration. Estuaries Coasts 2022, 45, 1805–1822. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).