Assessing the Interplay between Weather and Septoria Leaf Blotch Severity on Lower Leaves on the Disease Risk on Upper Leaves in Winter Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fields Data and Disease Monitoring
2.2. Data Analyses
3. Results
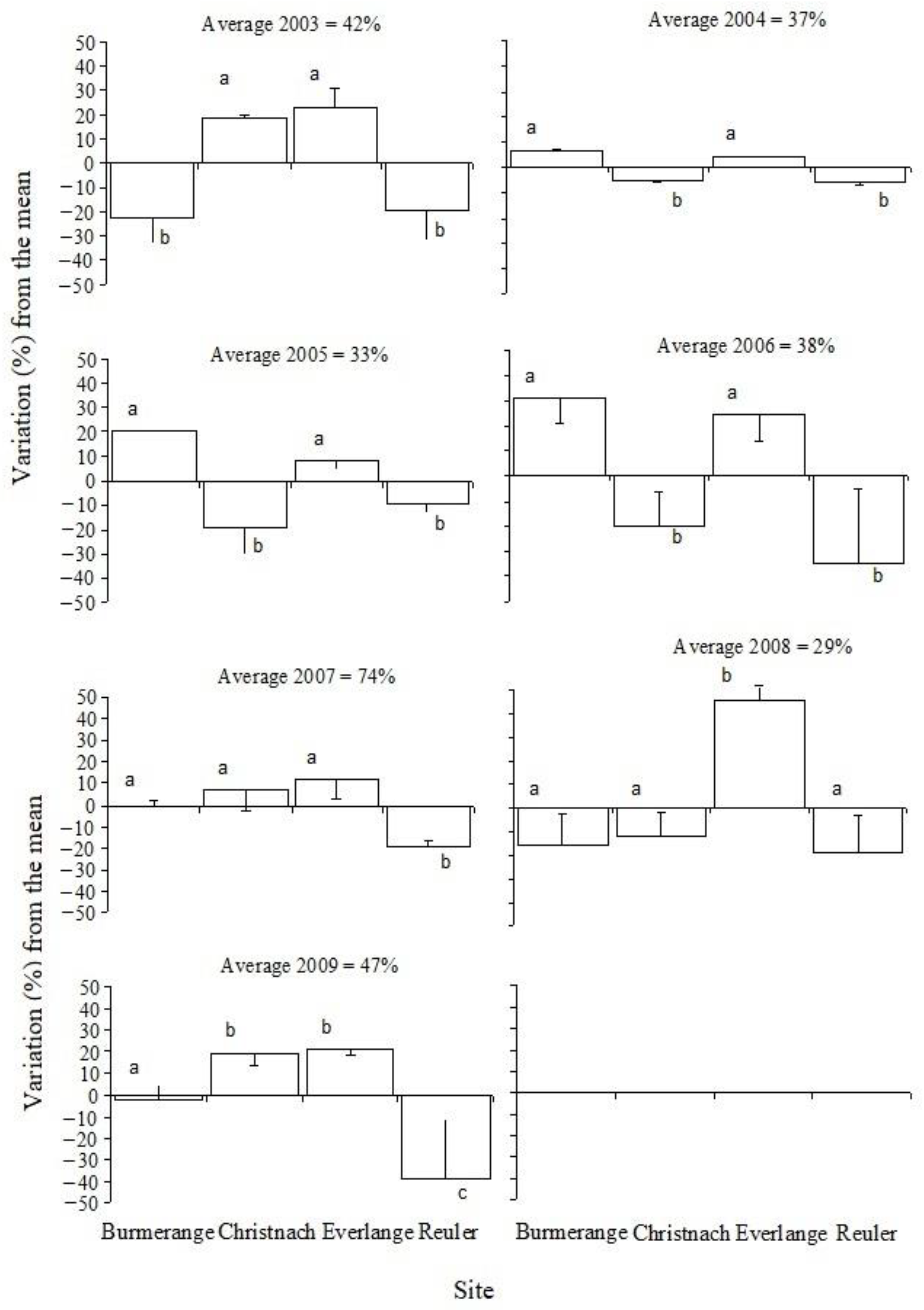
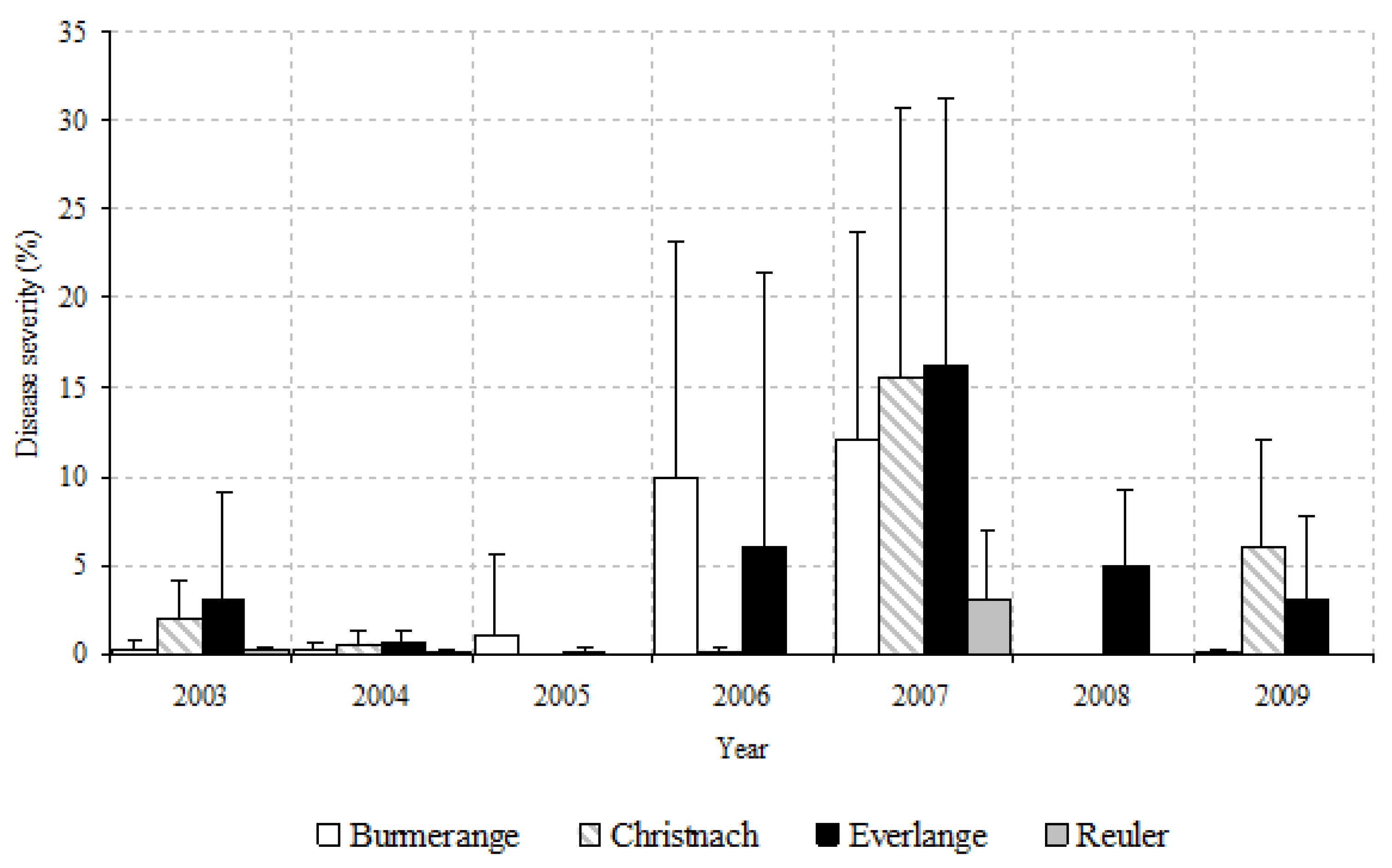
3.1. Septoria Leaf Blotch Severity during the Study Period
3.2. Weather Factors Influencing SLB Severity at The study Sites
3.3. Relationship between Disease Severity on the Upper Leaves at GS 85 and Disease Severity on L5 at L3 Emergence and Simulated Potential Daily Infection Events
3.4. Modeling SLB Risk Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coakley, S.M.; McDaniel, L.R.; Shaner, G. Model for predicting severity of Septoria tritici blotch on winter wheat. Phytopathology 1985, 75, 1245–1251. [Google Scholar] [CrossRef]
- Eyal, Z. The Septoria tritici and Stagonospora nodorum blotch diseases of wheat. Eur. J. Plant Pathol. 1999, 105, 629–641. [Google Scholar] [CrossRef]
- Eyal, Z.; Ziv, O. The relationship between epidemics of Septoria leaf blotch and yield losses in spring wheat. Phytopathology 1974, 64, 1385–1389. [Google Scholar] [CrossRef]
- Shaw, M.W.; Royle, D.J. Airborne inoculum as a major source of Septoria tritici (Mycosphaerella graminicola) infections in winter wheat crops in the U.K. Plant Pathol. 1989, 38, 35–43. [Google Scholar] [CrossRef]
- Beyer, M.; Pallez-Barthel, M.; Dam, D.; Hoffmann, L.; El Jarroudi, M. Enhancing septoria leaf blotch forecasts in winter wheat I: The effect of temperature on the temporal distance between critical rainfall periods and the breaking of the control threshold. J. Plant Dis. Protect. 2022, 129, 37–44. [Google Scholar] [CrossRef]
- Shaw, M.W.; Royle, D.J. Estimation and validation of a function describing the rate at which Mycosphaerella graminicola causes yield loss in winter wheat. Ann. Appl. Biol. 1989, 115, 425–442. [Google Scholar] [CrossRef]
- El Jarroudi, M.; Kouadio, L.; Bertrand, M.; Curnel, Y.; Giraud, F.; Delfosse, P.; Hoffmann, L.; Oger, R.; Tychon, B. Integrating the impact of wheat fungal diseases in the Belgian crop yield forecasting system (B-CYFS). Eur. J. Agron. 2012, 40, 8–17. [Google Scholar] [CrossRef]
- Shtienberg, D. Effect of foliar diseases of wheat on the physiological processes affecting yield under semi-arid conditions. Plant Pathol. 1991, 40, 533–541. [Google Scholar] [CrossRef]
- Fones, H.; Gurr, S. The impact of Septoria tritici Blotch disease on wheat: An EU perspective. Fungal Genet. Biol. 2015, 79, 3–7. [Google Scholar] [CrossRef]
- Shtienberg, D. Effects of moisture and septoria tritici blotch stresses on wheat yields under semi-arid conditions: A simulation study. Phytoparasitica 1991, 19, 301–310. [Google Scholar] [CrossRef]
- Arraiano, L.S.; Brading, P.A.; Brown, J.K.M. A detached seedling leaf technique to study resistance to Mycosphaerella graminicola (anamorph Septoria tritici) in wheat. Plant Pathol. 2001, 50, 339–346. [Google Scholar] [CrossRef]
- Cornish, P.S.; Baker, G.R.; Murray, G.M. Physiological responses of wheat (Triticum aestivum) to infection with Mycosphaerella graminicolla causing Septoria tritici blotch. Austr. J. Agr. Res. 1990, 41, 317–327. [Google Scholar] [CrossRef]
- Oerke, E.-C.; Dehne, H.-W. Global crop production and the efficacy of crop protection—Current situation and future trends. Eur. J. Plant Pathol. 1997, 103, 203–215. [Google Scholar] [CrossRef]
- Ficke, A.; Cowger, C.; Bergstrom, G.; Brodal, G. Understanding yield loss and pathogen biology to improve disease management: Septoria nodorum blotch—A case study in wheat. Plant Dis. 2018, 102, 696–707. [Google Scholar] [CrossRef] [PubMed]
- El Jarroudi, M.; Delfosse, P.; Maraite, H.; Hoffmann, L.; Tychon, B. Assessing the accuracy of simulation model for Septoria leaf blotch disease progress on winter wheat. Plant Dis. 2009, 93, 983–992. [Google Scholar] [CrossRef] [PubMed]
- El Jarroudi, M.; Kouadio, L.; Bock, C.H.; El Jarroudi, M.; Junk, J.; Pasquali, M.; Maraite, H.; Delfosse, P. A threshold-based weather model for predicting stripe rust infection in winter wheat. Plant Dis. 2017, 101, 693–703. [Google Scholar] [CrossRef]
- El Jarroudi, M.; Kouadio, L.; Delfosse, P.; Tychon, B. Brown rust disease control in winter wheat: I. Exploring an approach for disease progression based on night weather conditions. Environ. Sci. Pollut. Res. 2014, 21, 4797–4808. [Google Scholar] [CrossRef]
- El Jarroudi, M.; Kouadio, L.; Giraud, F.; Delfosse, P.; Tychon, B. Brown rust disease control in winter wheat: II. Exploring the optimization of fungicide sprays through a decision support system. Environ. Sci. Pollut. Res. 2014, 21, 4809–4818. [Google Scholar] [CrossRef][Green Version]
- Moreau, J.M.; Maraite, H. Integration of knowledge on wheat phenology and septoria tritici epidemiology into a disease risk simulation model validated in Belgium. Asp. Appl. Biol. 1999, 55, 1–6. [Google Scholar]
- Moreau, J.M.; Maraite, H. Development of an interaction decision-support system on a web site for control of Mycosphaerella graminicola in winter wheat. Bull. OEPV/EPPO 2000, 30, 161–163. [Google Scholar] [CrossRef]
- Pietravalle, S.; Shaw, M.W.; Parker, S.R.; Van Den Bosch, F. Modeling of relationships between weather and Septoria tritici epidemics on winter wheat: A critical approach. Phytopathology 2003, 93, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.R.; Cook, R.J.; King, J.E. Factors affecting development of Septoria tritici in winter wheat and its affect on yield. Plant Pathol. 1989, 38, 246–257. [Google Scholar] [CrossRef]
- El Jarroudi, M.; Kouadio, L.; Beyer, M.; Junk, J.; Hoffmann, L.; Tychon, B.; Maraite, H.; Bock, C.H.; Delfosse, P. Economics of a decision–support system for managing the main fungal diseases of winter wheat in the Grand-Duchy of Luxembourg. Field Crops Res. 2015, 172, 32–41. [Google Scholar] [CrossRef]
- Frahm, J.; Volk, T.; Johnen, A. Development of the PRO_PLANT decision-support system for plant protection in cereals, sugar-beet and rape. OEPP Bull./EPPO Bull. 1996, 26, 609–622. [Google Scholar] [CrossRef]
- Hagelskjær, L.; Jørgensen, L.N. A web-based decision support system for integrated management of cereal pests. Bull. OEPP/EPPO Bull. 2003, 33, 467–471. [Google Scholar] [CrossRef]
- Volk, T.; Johnen, A.; Newe, M.; Meier, H. ProPlant expert.com—The online consultation system on crop protection in cereals, rapeseed, potatoes and sugar beet: Experiences with cereal disease control in the region and possibilities for regional adaptations. In Proceedings of the Crop Protection Conference for the Baltic Sea Region, Poznan, Poland, 28–29 April 2003; Wolffhechel, H., Ed.; DIAS Report Plant Production No. 96. Danish Insitute of Agricultural Sciences: Tjele, Denmark, 2003; pp. 103–113. [Google Scholar]
- Burke, J.J.; Dunne, B. Field testing of six decision support systems for scheduling fungicide applications to control Mycosphaerella graminicola on winter wheat crops in Ireland. J. Agric. Sci. 2008, 146, 415–428. [Google Scholar] [CrossRef]
- Beyer, M.; El Jarroudi, M.; Junk, J.; Pogoda, F.; Dubos, T.; Görgen, K.; Hoffmann, L. Spring air temperature accounts for the bimodal temporal distribution of Septoria tritici epidemics in the winter wheat stands of Luxembourg. Crop Prot. 2012, 42, 250–255. [Google Scholar] [CrossRef]
- El Jarroudi, M.; Kouadio, L.; El Jarroudi, M.; Junk, J.; Bock, C.; Diouf, A.A.; Delfosse, P. Improving fungal disease forecasts in winter wheat: A critical role of intra-day variations of meteorological conditions in the development of Septoria leaf blotch. Field Crops Res. 2017, 213, 12–20. [Google Scholar] [CrossRef]
- Hansen, J.G.; Secher, B.J.N.; Jorgensen, L.N.; Welling, B. Thresholds for control of Septoria spp. in winter wheat based on precipitation and growth stage. Plant Pathol. 1994, 43, 183–189. [Google Scholar] [CrossRef]
- Hess, D.E.; Shaner, G. Effects of moisture and temperature on development of Septoria tritici in wheat. Phytopathology 1987, 77, 215–219. [Google Scholar] [CrossRef]
- Lovell, D.J.; Parker, S.R.; Hunter, T.; Royle, D.J.; Coker, R.R. Influence of crop growth and structure on the risk of epidemics by Mycosphaerella graminicola (Septoria tritici) in winter wheat. Plant Pathol. 1997, 46, 126–138. [Google Scholar] [CrossRef]
- Polley, R.W.; Thomas, M.R. Surveys of disease of wheat in England and Wales 1976–1988. Ann. Appl. Biol. 1991, 119, 1–20. [Google Scholar] [CrossRef]
- Shaner, G.; Finney, R.E. The effect of nitrogen fertilization on the expression of slow-mildewing resistance in knox wheat. Phytopathology 1977, 67, 1051–1056. [Google Scholar] [CrossRef]
- Tyldesley, J.B.; Thompson, N. Forecasting Septoria nodorum on winter wheat in England and Wales. Plant Pathol. 1980, 29, 9–20. [Google Scholar] [CrossRef]
- Wiik, L.; Ewaldz, T. Impact of temperature and precipitation on yield and plant diseases of winter wheat in southern Sweden 1983–2007. Crop Prot. 2009, 28, 952–962. [Google Scholar] [CrossRef]
- Eyal, Z. Integrated control of Septoria diseases of wheat. Plant Dis. 1981, 65, 763–768. [Google Scholar] [CrossRef]
- Shaw, M.W.; Royle, D.J. Factors determining the severity of epidemics of Mycosphaerella graminicola (Septoria tritici) on winter wheat in the UK. Plant Pathol. 1993, 42, 882–899. [Google Scholar] [CrossRef]
- Verreet, J.A.; Klink, H.; Hoffmann, G.M. Regional monitoring for disease prediction and optimization of plant protection measures: The IPM Wheat Model. Plant Dis. 2000, 84, 816–826. [Google Scholar] [CrossRef]
- Waggoner, P.E.; Aylor, D.E. Epidemiology: A science of patterns. Ann. Rev. Phytopathol. 2000, 38, 71–94. [Google Scholar] [CrossRef]
- Duvivier, M.; Dedeurwaerder, G.; De Proft, M.; Moreau, J.-M.; Legrève, A. Real-time PCR quantification and spatio-temporal distribution of airborne inoculum of Mycosphaerella graminicola in Belgium. Eur. J. Plant Pathol. 2013, 137, 325–341. [Google Scholar] [CrossRef]
- Shaner, G.; Buechley, G. Epidemiology of leaf blotch of soft red winter wheat caused by Septoria tritici and Stagonospora nodorum. Plant Dis. 1995, 79, 928–938. [Google Scholar] [CrossRef]
- Suffert, F.; Sache, I.; Lannou, C. Early stages of septoria tritici blotch epidemics of winter wheat: Build-up, overseasoning, and release of primary inoculum. Plant Pathol. 2011, 60, 166–177. [Google Scholar] [CrossRef]
- El Jarroudi, M.; Kouadio, L.; Junk, J.; Bock, C.H. Improved prediction of leaf emergence for efficacious crop protection: Assessing field variability in phyllotherms for upper leaves in winter wheat and winter barley. Agronomy 2020, 10, 1825. [Google Scholar] [CrossRef]
- MAVPC. Rapport D’activité 2021. Ministère de l’Agriculture, de la Viticulture et de la Protection des Consommateurs (MAVPC). (Verified February 2022). 2021. Available online: https://gouvernement.lu/dam-assets/fr/publications/rapport-activite/minist-agriculture-viticulture-protection-consommateurs/2021-rapport-activite-ma/2021-rapport-activite-ma.pdf (accessed on 19 October 2022).
- Larue, S. La localisation des Productions Agricoles en 1962 et en 2009: Une Comparaison des Cantons Luxembourgeois. STATEC, Institut National de la Statistique et des Études Économiques. 2012. Available online: https://statistiques.public.lu/en/publications/series/luxembourg/2012/16-12.html (accessed on 4 October 2022).
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- James, C.A. Manual of Assessment Keys for Plant Diseases; Publication No. 1458; Canada Department of Agriculture: Charlottetown, PE, Canada; APS: St. Paul, MN, USA, 1971. [Google Scholar]
- Tomerlin, J.R.; Howell, A. Distrain: A computer program for training people to estimate disease severity on cereal leaves. Plant Dis. 1988, 72, 455–459. [Google Scholar]
- El Jarroudi, M.; Kouadio, A.L.; Mackels, C.; Tychon, B.; Delfosse, P.; Bock, C.H. A comparison between visual estimates and image analysis measurements to determine Septoria leaf blotch severity in winter wheat. Plant Pathol. 2015, 64, 355–364. [Google Scholar] [CrossRef]
- Chiang, K.-S.; Bock, C.H.; Lee, I.H.; El Jarroudi, M.; Delfosse, P. Plant disease severity assessment—How rater bias, assessment method, and experimental design affect hypothesis testing and resource use efficiency. Phytopathology 2016, 106, 1451–1464. [Google Scholar] [CrossRef]
- Bock, C.H.; El Jarroudi, M.; Kouadio, L.A.; Mackels, C.; Chiang, K.-S.; Delfosse, P. Disease severity estimates—effects of rater accuracy and assessment methods for comparing treatments. Plant Dis. 2015, 99, 1104–1112. [Google Scholar] [CrossRef]
- BSA. BSA, Beschreibende Sortenliste, Getreide, Mais, Ölfüchte, Leguminosen, Hackfrüchte; Bundessortenamt: Hannover, Germany, 2008; pp. 78–127. [Google Scholar]
- Mahtour, A.; El Jarroudi, M.; Delobbe, L.; Hoffmann, L.; Maraite, H.; Tychon, B. Site-specific Septoria leaf blotch risk assessment in winter wheat using weather-radar rainfall estimates. Plant Dis. 2011, 95, 384–393. [Google Scholar] [CrossRef][Green Version]
- Mallows, C.L. Some Comments on C p. Technometrics 1973, 15, 661–675. [Google Scholar] [CrossRef]
- Snee, R.D. Validation of regression models: Methods and examples. Technometrics 1977, 19, 415–428. [Google Scholar] [CrossRef]
- Gladders, P.; Langton, S.D.; Barrie, I.A.; Hardwick, N.V.; Taylor, M.C.; Paveley, N.D. The importance of weather and agronomic factors for the overwinter survival of yellow rust (Puccinia striiformis) and subsequent disease risk in commercial wheat crops in England. Ann. Appl. Biol. 2007, 150, 371–382. [Google Scholar] [CrossRef]
- Gladders, P.; Paveley, N.D.; Barrie, I.A.; Hardwick, N.V.; Hims, M.J.; Langton, S.; Taylor, M.C. Agronomic and meteorological factors affecting the severity of leaf blotch caused by Mycosphaerella graminicola in commercial wheat crops in England. Ann. Appl. Biol. 2001, 138, 301–311. [Google Scholar]
- Parker, S.R.; Lovell, D.J.; Royle, D.J.; Paveley, N.D. Analysing epidemics of Septoria tritici for improved estimates of disease risk. In Chapter Six of "Septoria on Cereals: A Study of Pathosystems"; Bowyer, P., Anderson, H.M., Eds.; IACR—Long Ashton Research Station: Bristol, UK, 1999; p. 351. ISBN 0 85199269 2. [Google Scholar]
- Lovell, D.J.; Parker, S.R.; Hunter, T.; Welham, S.J.; Nichols, A.R. Position of inoculum in the canopy affects the risk of septoria tritici blotch epidemics in winter wheat. Plant Pathol. 2004, 53, 11–21. [Google Scholar]
- Andersson, B.; Djurle, A.; Ørum, J.E.; Jalli, M.; Ronis, A.; Ficke, A.; Jørgensen, L.N. Comparison of models for leaf blotch disease management in wheat based on historical yield and weather data in the Nordic-Baltic region. Agron. Sustain. Dev. 2022, 42, 42. [Google Scholar] [CrossRef]


| Site | Region | Year | Sowing Date | Cultivar | SLB Susceptibility a | Date of L3 Emergence | Date of GS 59 b | Date of GS 65 b | Date of GS 85 b | Harvest Date |
|---|---|---|---|---|---|---|---|---|---|---|
| Burmerange | Gutland | 2003 | 4 Oct. 2002 | Dekan | 4 | 14 Apr. | 28 May | 2 Jun. | 23 Jun. | 11 Jul. |
| (50°3′ N, 6°1′ E) | 2004 | 1 Oct. 2003 | Cubus | 6 | 18 Apr. | 1 Jun. | 12 Jun. | 5 Jul. | 2 Aug. | |
| 2005 | 13 Oct. 2004 | Cubus | 6 | 15 Apr. | 3 Jun | 6 Jun. | 4 Jul. | 4 Aug. | ||
| 2006 | 30 Sep. 2005 | Cubus | 6 | 15 Apr. | 30 May | 12 Jun. | 3 Jul. | 19 Jul. | ||
| 2007 | 11 Oct. 2006 | Cubus | 6 | 3 Apr. | 16 May | 23 May | 18 Jun. | 26 Jul. | ||
| 2008 | 6 Oct. 2007 | Cubus | 6 | 19 Apr. | 30 May | 4 Jun. | 23 Jun. | 5 Aug. | ||
| 2009 | 6 Oct. 2008 | Cubus | 6 | 17 Apr. | 30 May | 5 Jun. | 29 Jun. | 29 Jul. | ||
| Christnach | Gutland | 2003 | 2 Oct. 2002 | Flair | 4 | 22 Apr. | 5 Jun. | 10 Jun. | 7 Jul. | 23 Jul. |
| (49°45′ N, 6°14′ E) | 2004 | 13 Oct. 2003 | Flair | 4 | 23 Apr. | 10 Jun. | 22 Jun. | 12 Jul. | 12 Aug. | |
| 2005 | 27 Oct. 2004 | Rosario | 5 | 25 Apr. | 16 Jun. | 20 Jun. | 4 Jul. | 2 Aug. | ||
| 2006 | 12 Oct. 2005 | Flair | 4 | 25 Apr. | 12 Jun. | 15 Jun. | 3 Jul. | 25 Jul. | ||
| 2007 | 12 Oct. 2006 | Tommi | 4 | 11 Apr. | 21 May | 1 Jun. | 25 Jun. | 26 Jul. | ||
| 2008 | 23 Oct. 2007 | Flair | 4 | 30 Apr. | 4 Jun. | 9 Jun. | 7 Jul. | 5 Aug. | ||
| 2009 | 23 Oct. 2008 | Boomer | 5 | 27 Apr. | 2 Jun. | 11 Jun. | 6 Jul. | 7 Aug. | ||
| Everlange | Gutland | 2003 | 4 Oct. 2002 | Achat | 5 | 23 Apr. | 2 Jun. | 8 Jun. | 30 Jun. | 19 Jul. |
| (49°29′ N, 6°19′ E) | 2004 | 14 Oct. 2003 | Achat | 5 | 19 Apr. | 9 Jun. | 14 Jun. | 12 Jul. | 6 Aug. | |
| 2005 | 22 Oct. 2004 | Achat | 5 | 21 Apr. | 8 Jun. | 13 Jun. | 4 Jul. | 2 Aug. | ||
| 2006 | 10 Oct. 2005 | Achat | 5 | 23 Apr. | 8 Jun. | 12 Jun. | 3 Jul. | 7 Aug. | ||
| 2007 | 10 Oct. 2006 | Achat | 5 | 4 Apr. | 21 May | 2 Jun. | 2 Jul. | 26 Jul. | ||
| 2008 | 8 Oct. 2007 | Achat | 5 | 19 Apr. | 2 Jun. | 9 Jun. | 7 Jul. | 5 Aug. | ||
| 2009 | 13 Oct. 2008 | Achat | 5 | 20 Apr. | 4 Jun. | 8 Jun. | 6 Jul. | 6 Aug. | ||
| Reuler | Oesling | 2003 | 6 Nov. 2002 | Bussard | 6 | 30 Apr. | 12 Jun. | 16 Jun. | 7 Jul. | 5 Aug. |
| (50°11′ N, 5°15′ E) | 2004 | 16 Oct. 2003 | Bussard | 6 | 26 Apr. | 16 Jun. | 22 Jun. | 12 Jul. | 16 Aug. | |
| 2005 | 5 Oct. 2004 | Flair | 4 | 25 Apr. | 16 Jun. | 20 Jun. | 4 Jul. | 13 Aug. | ||
| 2006 | 13 Oct. 2005 | Dekan | 4 | 4 May | 14 Jun. | 19 Jun. | 3 Jul. | 8 Aug. | ||
| 2007 | 7 Oct. 2006 | Akteur | 6 | 15 Apr. | 29 May | 6 Jun. | 25 Jun. | 3 Aug. | ||
| 2008 | 10 Oct. 2007 | Schamane | 4 | 9 May | 8 Jun. | 12 Jun. | 7 Jul. | 14 Aug. | ||
| 2009 | 10 Oct. 2008 | Schamane | 4 | 30 Apr. | 15 Jun. | 21 Jun. | 6 Jul. | 18 Aug. |
| Burmerange | Christnach | Everlange | Reuler | |||||
|---|---|---|---|---|---|---|---|---|
| Cropping Season | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 2003 | 19.0 c | 21.4 | 60.6 b | 30.7 | 64.5 c | 23.5 | 22.5 c | 19.5 |
| 2004 | 43.7 b | 29.7 | 31.9 c | 29.4 | 41.2 d | 30.6 | 31.2 b | 29.3 |
| 2005 | 53.1 b | 30.7 | 14.1 d | 18.3 | 41.7 d | 25.1 | 23.8 bc | 25.6 |
| 2006 | 68.3 a | 25.1 | 17.8 d | 21.0 | 62.2 c | 24.1 | 3.2 d | 4.8 |
| 2007 | 73.5 a | 28.2 | 80.9 a | 16.2 | 85.1 a | 17.2 | 55.1 a | 27.7 |
| 2008 | 13.6 c | 22.7 | 17.2 d | 24.7 | 74.7 b | 24.6 | 10.1 d | 19.8 |
| 2009 | 44.2 b | 29.9 | 65.7 b | 31.3 | 68.2 bc | 32.9 | 8.0 d | 9.8 |
| Site | Variable a | R | Prob > |R| under H0: Rho = 0 b |
|---|---|---|---|
| Everlange | DSL5 | 0.82 | * |
| Tnw | −0.83 | * | |
| IDPROC(1) | 0.96 | *** | |
| P1 | 0.37 | ns | |
| P5 | 0.62 | ns | |
| ΣRain | 0.07 | ns | |
| ΣT | 0.75 | * | |
| IDPROC(2) | 0.42 | ns | |
| Burmerange | DSL5 | 0.80 | * |
| Tnw | 0.37 | ns | |
| IDPROC(1) | 0.84 | * | |
| P1 | 0.78 | * | |
| P5 | 0.78 | * | |
| ΣRain | 0.53 | ns | |
| ΣT | 0.91 | ** | |
| IDPROC(2) | 0.50 | ns | |
| Christnach | DSL5 | 0.84 | * |
| Tnw | −0.80 | * | |
| IDPROC(1) | 0.87 | ** | |
| P1 | −0.06 | ns | |
| P5 | 0.69 | ns | |
| ΣRain | 0.47 | ns | |
| ΣT | 0.76 | * | |
| IDPROC(2) | 0.66 | NS | |
| Reuler | DSL5 | 0.85 | * |
| Tnw | −0.92 | ** | |
| IDPROC(1) | −0.40 | ns | |
| P1 | −0.83 | ns | |
| P5 | −0.70 | ns | |
| ΣRain | −0.52 | ns | |
| ΣT | 0.72 | * | |
| IDPROC(2) | 0.83 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Jarroudi, M.; Kouadio, L.; Junk, J.; Maraite, H.; Tychon, B.; Delfosse, P. Assessing the Interplay between Weather and Septoria Leaf Blotch Severity on Lower Leaves on the Disease Risk on Upper Leaves in Winter Wheat. J. Fungi 2022, 8, 1119. https://doi.org/10.3390/jof8111119
El Jarroudi M, Kouadio L, Junk J, Maraite H, Tychon B, Delfosse P. Assessing the Interplay between Weather and Septoria Leaf Blotch Severity on Lower Leaves on the Disease Risk on Upper Leaves in Winter Wheat. Journal of Fungi. 2022; 8(11):1119. https://doi.org/10.3390/jof8111119
Chicago/Turabian StyleEl Jarroudi, Moussa, Louis Kouadio, Jürgen Junk, Henri Maraite, Bernard Tychon, and Philippe Delfosse. 2022. "Assessing the Interplay between Weather and Septoria Leaf Blotch Severity on Lower Leaves on the Disease Risk on Upper Leaves in Winter Wheat" Journal of Fungi 8, no. 11: 1119. https://doi.org/10.3390/jof8111119
APA StyleEl Jarroudi, M., Kouadio, L., Junk, J., Maraite, H., Tychon, B., & Delfosse, P. (2022). Assessing the Interplay between Weather and Septoria Leaf Blotch Severity on Lower Leaves on the Disease Risk on Upper Leaves in Winter Wheat. Journal of Fungi, 8(11), 1119. https://doi.org/10.3390/jof8111119






