Volatile Organic Compound (VOC) Profiles of Different Trichoderma Species and Their Potential Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal and Soil Sampling
2.2. VOC Analyses
2.2.1. Mass Spectrometer Analysis of the VOCs Produced by Trichoderma spp. Growing on PDA and in Soil
2.2.2. PTR-Qi-TOF-MS Data Analyses
2.3. Statistical Analyses
3. Results
VOC Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schulz-Bohm, K.; Martín-Sánchez, L.; Garbeva, P. Microbial volatiles: Small molecules with an important role in intra- and inter-kingdom interactions. Front. Microbiol. 2017, 8, 2484. [Google Scholar] [CrossRef]
- Kramer, R.; Abraham, W.R. Volatile sesquiterpenes from fungi: What are they good for? Phytochem. Rev. 2012, 11, 15–37. [Google Scholar] [CrossRef]
- Splivallo, R.; Novero, M.; Bertea, C.M.; Bossi, S.; Bonfante, P. Truffle volatiles inhibit growth and induce an oxidative burst in Arabidopsis thaliana. New Phytol. 2007, 175, 417–424. [Google Scholar] [CrossRef]
- Hung, R.; Lee, S.; Bennett, J.W. Fungal volatile organic compounds and their role in ecosystems. Appl. Microbiol. Biotechnol. 2015, 99, 3395–3405. [Google Scholar] [CrossRef]
- Mercier, J.; Jiménez, J.I. Demonstration of the biofumigation activity of Muscodor albus against Rhizoctonia solani in soil and potting mix. BioControl 2009, 54, 797–805. [Google Scholar] [CrossRef]
- Minerdi, D.; Bossi, S.; Gullino, M.L.; Garibaldi, A. Volatile Organic Compounds: A potential direct long-distance mechanism for antagonistic action of Fusarium oxysporum strain MSA 35. Environ. Microbiol. 2009, 11, 844–854. [Google Scholar] [CrossRef]
- Fialho, M.B.; Toffano, L.; Pedroso, M.P.; Augusto, F.; Pascholati, S.F. Volatile Organic Compounds produced by Saccharomyces cerevisiae inhibit the in vitro development of Guignardia citricarpa, the causal agent of citrus black spot. World J. Microbiol. Biotechnol. 2010, 26, 925–932. [Google Scholar] [CrossRef]
- Morath, S.U.; Hung, R.; Bennett, J.W. Fungal Volatile Organic Compounds: A review with emphasis on their biotechnological potential. Fungal Biol. Rev. 2012, 26, 73–83. [Google Scholar] [CrossRef]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Volatile 1-Octen-3-Ol induces a defensive response in Arabidopsis thaliana. J. Gen. Plant Pathol. 2007, 73, 35–37. [Google Scholar] [CrossRef]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Components of C6-aldehyde-induced resistance in Arabidopsis thaliana against a necrotrophic fungal pathogen, Botrytis cinerea. Plant Sci. 2006, 170, 715–723. [Google Scholar] [CrossRef]
- Van Hulten, M.; Pelser, M.; van Loon, L.C.; Pieterse, C.M.J.; Ton, J. Costs and benefits of priming for defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 5602–5607. [Google Scholar] [CrossRef]
- Siddiquee, S.; Cheong, B.E.; Taslima, K.; Kausar, H.; Hasan, M.M. Separation and identification of volatile compounds from liquid cultures of Trichoderma harzianum by GC-MS using three different capillary columns. J. Chromatogr. Sci. 2012, 50, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yap, M.; Behringer, G.; Hung, R.; Bennett, J.W. Volatile Organic Compounds emitted by Trichoderma species mediate plant growth. Fungal Biol. Biotechnol. 2016, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jud, W.; Ghirardo, A.; Antritter, F.; Benz, J.P.; Schnitzler, J.P.; Rosenkranz, M. Sniffing fungi—phenotyping of volatile chemical diversity in Trichoderma species. New Phytol. 2020, 227, 244–259. [Google Scholar] [CrossRef]
- Guo, Y.; Ghirardo, A.; Weber, B.; Schnitzler, J.P.; Philipp Benz, J.; Rosenkranz, M. Trichoderma species differ in their volatile profiles and in antagonism toward ectomycorrhiza Laccaria bicolor. Front. Microbiol. 2019, 10, 891. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Rai, N.; Limbu, K.; Koirala, G. Impact of Trichoderma sp. in agriculture: A mini-review. J. Biol. Today’s World 2020, 9, 227. [Google Scholar] [CrossRef]
- Bissett, J.; Gams, W.; Jaklitsch, W.; Samuels, G.J. Accepted Trichoderma names in the year 2015. IMA Fungus 2015, 6, 263–295. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Rajani, P.; Rajasekaran, C.; Vasanthakumari, M.M.; Olsson, S.B.; Ravikanth, G.; Uma Shaanker, R. Inhibition of plant pathogenic fungi by endophytic Trichoderma spp. through mycoparasitism and volatile organic compounds. Microbiol. Res. 2021, 242, 126595. [Google Scholar] [CrossRef]
- Phoka, N.; Suwannarach, N.; Lumyong, S.; Ito, S.-I.; Matsui, K.; Arikit, S.; Sunpapao, A. Role of volatiles from the endophytic fungus Trichoderma asperelloides-P1 in biocontrol potential and in promoting the plant growth of Arabidopsis thaliana. J. Fungi 2020, 6, 341. [Google Scholar] [CrossRef]
- Joo, J.H.; Hussein, K.A. Biological control and plant growth promotion properties of volatile compound-producing antagonistic Trichoderma spp. Front. Plant Sci. 2022, 13, 897668. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Pollastri, S.; Ruocco, M.; Monti, M.M.; Loreto, F. Volatile Organic Compounds in the interaction between plants and beneficial microorganisms. J. Plant Interact. 2022, 17, 840–852. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Herrera-Estrella, A.; López-Bucio, J. The 4-phosphopantetheinyl transferase of Trichoderma virens plays a role in plant protection against Botrytis cinerea through volatile organic compound emission. Plant Soil 2014, 379, 261–274. [Google Scholar] [CrossRef]
- Li, N.; Alfiky, A.; Wang, W.; Islam, M.; Nourollahi, K.; Liu, X.; Kang, S. Volatile compound-mediated recognition and inhibition between Trichoderma biocontrol agents and Fusarium oxysporum. Front. Microbiol. 2018, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Siddiquee, S. Recent advancements on the role and analysis of Volatile Compounds (VOCs) from Trichoderma. In Biotechnology and Biology of Trichoderma; Elsevier: Amsterdam, The Netherlands, 2014; pp. 139–175. [Google Scholar]
- Wheatley, R.; Hackett, C.; Bruce, A.; Kundzewiczd, A. Effect of substrate composition on production of volatile organic compounds from Trichoderma spp. inhibitory to wood decay fungi. Int. Biodeterior. Biodegrad. 1997, 39, 199–205. [Google Scholar] [CrossRef]
- Okull, D.O.; Beelman, R.B.; Gourama, H. Antifungal activity of 10-oxo-trans-8-decenoic acid and 1-octen-3-ol against Penicillium expansum in potato dextrose agar medium. J. Food Prot. 2003, 66, 1503–1505. [Google Scholar] [CrossRef]
- Reithner, B.; Brunner, K.; Schuhmacher, R.; Peissl, I.; Seidl, V.; Krska, R.; Zeilinger, S. The G protein α subunit Tga1 of Trichoderma atroviride is involved in chitinase formation and differential production of antifungal metabolites. Fungal Genet. Biol. 2005, 42, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Stoppacher, N.; Kluger, B.; Zeilinger, S.; Krska, R.; Schuhmacher, R. Identification and profiling of volatile metabolites of the biocontrol fungus Trichoderma atroviride by HS-SPME-GC-MS. J. Microbiol. Methods 2010, 81, 187–193. [Google Scholar] [CrossRef]
- Kottb, M.; Gigolashvili, T.; Großkinsky, D.K.; Piechulla, B. Trichoderma volatiles effecting Arabidopsis: From inhibition to protection against phytopathogenic fungi. Front. Microbiol. 2015, 6, 995. [Google Scholar] [CrossRef]
- Collins, R.P.; Halim, A.F. Characterization of the major aroma constituent of the fungus Trichoderma viride. J. Agric. Food Chem. 1972, 20, 437–438. [Google Scholar] [CrossRef]
- Claydon, N.; Allan, M.; Hanson, J.R.; Avent, A.G. Antifungal alkyl pyrones of Trichoderma harzianum. Trans. Br. Mycol. Soc. 1987, 88, 503–513. [Google Scholar] [CrossRef]
- Simon, A.; Dunlop, R.W.; Ghisalberti, E.L.; Sivasithamparam, K. Trichoderma koningii produces a pyrone compound with antibiotic properties. Soil Biol. Biochem. 1988, 20, 263–264. [Google Scholar] [CrossRef]
- Jeleń, H.; Błaszczyk, L.; Chełkowski, J.; Rogowicz, K.; Strakowska, J. Formation of 6-n-pentyl-2H-pyran-2-one (6-PAP) and other volatiles by different Trichoderma species. Mycol. Prog. 2014, 13, 589–600. [Google Scholar] [CrossRef]
- Garnica-Vergara, A.; Barrera-Ortiz, S.; Muñoz-Parra, E.; Raya-González, J.; Méndez-Bravo, A.; Macías-Rodríguez, L.; Ruiz-Herrera, L.F.; López-Bucio, J. The volatile 6-pentyl-2H-pyran-2-one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ETHYLENE INSENSITIVE 2 functioning. New Phytol. 2016, 209, 1496–1512. [Google Scholar] [CrossRef]
- Matysik, S.; Herbarth, O.; Mueller, A. Determination of Microbial Volatile Organic Compounds (MVOCs) by passive sampling onto charcoal sorbents. Chemosphere 2009, 76, 114–119. [Google Scholar] [CrossRef]
- Vita, F.; Taiti, C.; Pompeiano, A.; Bazihizina, N.; Lucarotti, V.; Mancuso, S.; Alpi, A. Volatile Organic Compounds in truffle (Tuber magnatum Pico): Comparison of samples from different regions of Italy and from different seasons. Sci. Rep. 2015, 5, 12629. [Google Scholar] [CrossRef]
- Schenkel, D.; Maciá-Vicente, J.G.; Bissell, A.; Splivallo, R. Fungi indirectly affect plant root architecture by modulating soil volatile organic compounds. Front. Microbiol. 2018, 9, 1847. [Google Scholar] [CrossRef]
- Schulz-Bohm, K.; Zweers, H.; de Boer, W.; Garbeva, P. A fragrant neighborhood: Volatile mediated bacterial interactions in soil. Front. Microbiol. 2015, 6, 1212. [Google Scholar] [CrossRef]
- Farneti, B.; Khomenko, I.; Cappellin, L.; Ting, V.; Romano, A.; Biasioli, F.; Costa, G.; Costa, F. Comprehensive VOC profiling of an apple germplasm collection by PTR-ToF-MS. Metabolomics 2015, 11, 838–850. [Google Scholar] [CrossRef]
- Li, T.; Holst, T.; Michelsen, A.; Rinnan, R. Amplification of plant volatile defence against insect herbivory in a warming Arctic tundra. Nat. Plants 2019, 5, 568–574. [Google Scholar] [CrossRef]
- Behrendt, T.; Williams, J.; Veres, P.R.; Klapthor, A.; Meixner, F.X.; Williams, J. Volatile organic compound emissions from soil: Using Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry (PTR-TOF-MS) for the real time observation of microbial processes. BiogeoSci. Discuss. 2014, 11, 12009–12038. [Google Scholar] [CrossRef]
- Mancuso, S.; Taiti, C.; Bazihizina, N.; Costa, C.; Menesatti, P.; Giagnoni, L.; Arenella, M.; Nannipieri, P.; Renella, G. Soil volatile analysis by Proton Transfer Reaction-Time of Flight Mass Spectrometry (PTR-TOF-MS). Appl. Soil Ecol. 2015, 86, 182–191. [Google Scholar] [CrossRef]
- Khomenko, I.; Stefanini, I.; Cappellin, L.; Cappelletti, V.; Franceschi, P.; Cavalieri, D.; Märk, T.D.; Biasioli, F. Non-invasive real time monitoring of yeast volatilome by PTR-ToF-MS. Metabolomics 2017, 13, 118. [Google Scholar] [CrossRef]
- Adams, R.I.; Lymperopoulou, D.S.; Misztal, P.K.; de Cassia Pessotti, R.; Behie, S.W.; Tian, Y.; Goldstein, A.H.; Lindow, S.E.; Nazaroff, W.W.; Taylor, J.W.; et al. Microbes and associated soluble and volatile chemicals on periodically wet household surfaces. Microbiome 2017, 5, 128. [Google Scholar] [CrossRef]
- Infantino, A.; Costa, C.; Aragona, M.; Reverberi, M.; Taiti, C.; Mancuso, S. Identification of different Fusarium spp. through MVOCS profiling by means of Proton-Transfer-Reaction Time-of-Flight (PTR-TOF-MS) analysis. J. Plant Pathol. 2017, 99, 663–669. [Google Scholar] [CrossRef]
- Misztal, P.K.; Lymperopoulou, D.S.; Adams, R.I.; Scott, R.A.; Lindow, S.E.; Bruns, T.; Taylor, J.W.; Uehling, J.; Bonito, G.; Vilgalys, R.; et al. Emission factors of microbial volatile organic compounds from environmental bacteria and fungi. Env. Sci. Technol. 2018, 52, 8272–8282. [Google Scholar] [CrossRef]
- Guo, Y.; Jud, W.; Weikl, F.; Ghirardo, A.; Junker, R.R.; Polle, A.; Benz, J.P.; Pritsch, K.; Schnitzler, J.P.; Rosenkranz, M. Volatile organic compound patterns predict fungal trophic mode and lifestyle. Commun. Biol. 2021, 4, 673. [Google Scholar] [CrossRef]
- Telagathoti, A.; Probst, M.; Khomenko, I.; Biasioli, F.; Peintner, U. High-throughput volatilome fingerprint using PTR–TOF–MS shows species-specific patterns in Mortierella and closely related genera. J. Fungi 2021, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Haidacher, S.; Hanel, G.; Hartungen, E.; Märk, L.; Seehauser, H.; Schottkowsky, R.; Sulzer, P.; Märk, T.D. A High resolution and high sensitivity Proton-Transfer-Reaction Time-of-Flight Mass Spectrometer (PTR-TOF-MS). Int. J. Mass. Spectrom. 2009, 286, 122–128. [Google Scholar] [CrossRef]
- Gutiérrez-Moreno, K.; Ruocco, M.; Monti, M.M.; de la Vega, O.M.; Heil, M. Context-dependent effects of Trichoderma seed inoculation on anthracnose disease and seed yield of bean (Phaseolus vulgaris): Ambient conditions override cultivar-specific differences. Plants 2021, 10, 1739. [Google Scholar] [CrossRef]
- Kullnig, C.M.; Krupica, T.; Woo, S.L.; Mach, R.L.; Rey, M.; Benitez, T.; Lorito, M.; Kubicek, C.P. Confusion abounds over identities of Trichoderma biocontrol isolates. Mycol. Res. 2001, 105, 770–772. [Google Scholar] [CrossRef]
- Chaverri, P.; Branco-Rocha, F.; Jaklitsch, W.; Gazis, R.; Degenkolb, T.; Samuels, G.J. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia 2015, 107, 558–590. [Google Scholar] [CrossRef]
- Ruocco, M.; Lanzuise, S.; Lombardi, N.; Woo, S.L.; Vinale, F.; Marra, R.; Varlese, R.; Manganiello, G.; Pascale, A.; Scala, V.; et al. Multiple roles and effects of a novel Trichoderma hydrophobin. Mol. Plant-Microbe. Interact. 2015, 28, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic. Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed]
- Ezra, D.; Jasper, J.; Rogers, T.; Knighton, B.; Grimsrud, E.; Strobel, G. Proton transfer reaction-mass spectrometry as a technique to measure volatile emissions of Muscodor albus. Plant Sci. 2004, 166, 1471–1477. [Google Scholar] [CrossRef]
- Speckbacher, V.; Ruzsanyi, V.; Wigger, M.; Zeilinger, S. The Trichoderma atroviride strains P1 and IMI 206040 differ in their light-response and VOC production. Molecules 2020, 25, 208. [Google Scholar] [CrossRef] [PubMed]
- Azzollini, A.; Boggia, L.; Boccard, J.; Sgorbini, B.; Lecoultre, N.; Allard, P.M.; Rubiolo, P.; Rudaz, S.; Gindro, K.; Bicchi, C.; et al. Dynamics of metabolite induction in fungal co-cultures by metabolomics at both volatile and non-volatile levels. Front. Microbiol. 2018, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Garbeva, P.; Hordijk, C.; Gerards, S.; de Boer, W. Volatile-mediated interactions between phylogenetically different soil bacteria. Front. Microbiol. 2014, 5, 289. [Google Scholar] [CrossRef]
- Piechulla, B.; Lemfack, M.C.; Kai, M. Effects of discrete bioactive microbial volatiles on plants and fungi. Plant Cell Env. 2017, 40, 2042–2067. [Google Scholar] [CrossRef]
- Crutcher, F.K.; Parich, A.; Schuhmacher, R.; Mukherjee, P.K.; Zeilinger, S.; Kenerley, C.M. A putative terpene cyclase, Vir4, is responsible for the biosynthesis of volatile terpene compounds in the biocontrol fungus Trichoderma virens. Fungal Genet. Biol. 2013, 56, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Asensio, D.; Peñuelas, J.; Filella, I.; Llusià, J. On-line screening of soil VOCs exchange responses to moisture, temperature and root presence. Plant Soil 2007, 291, 249–261. [Google Scholar] [CrossRef]
- Li, L.J.; You, M.Y.; Shi, H.A.; Ding, X.L.; Qiao, Y.F.; Han, X.Z. Soil CO2 emissions from a cultivated mollisol: Effects of organic amendments, soil temperature, and moisture. Eur. J. Soil Biol. 2013, 55, 83–90. [Google Scholar] [CrossRef]
- Schade, G.W.; Custer, T.G. OVOC emissions from agricultural soil in northern Germany during the 2003 european heat wave. Atmos Env. 2004, 38, 6105–6114. [Google Scholar] [CrossRef]
- Ruiz, J.; Bilbao, R.; Murillo, M.B. Adsorption of different VOC onto soil minerals from gas phase: Influence of mineral, type of VOC, and air humidity. Env. Sci. Technol. 1998, 32, 1079–1084. [Google Scholar] [CrossRef]
- Owen, S.M.; Clark, S.; Pompe, M.; Semple, K.T. Biogenic volatile organic compounds as potential carbon sources for microbial communities in soil from the rhizosphere of Populus tremula. FEMS Microbiol. Lett. 2007, 268, 34–39. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Lauber, C.L.; Fierer, N. Microbial consumption and production of volatile organic compounds at the soil-litter interface. Biogeochemistry 2010, 99, 97–107. [Google Scholar] [CrossRef]
- Pétriacq, P.; Williams, A.; Cotton, A.; McFarlane, A.E.; Rolfe, S.A.; Ton, J. Metabolite profiling of non-sterile rhizosphere soil. Plant J. 2017, 92, 147–162. [Google Scholar] [CrossRef]
- Zain, M.E.; El-Sheikh, H.H.; Soliman, H.G.; Khalil, A.M. Effect of certain chemical compounds on secondary metabolites of Penicillium janthinellum and P. duclauxii. J. Saudi Chem. Soc. 2011, 15, 239–246. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Steindorff, A.S.; Chenthamara, K.; Manganiello, G.; Henrissat, B.; Zhang, J.; Cai, F.; Kopchinskiy, A.G.; Kubicek, E.M.; Kuo, A.; et al. Evolution and comparative genomics of the most common Trichoderma species. BMC Genom. 2019, 20, 485. [Google Scholar] [CrossRef] [PubMed]
- Busman, M.; Roberts, E.; Proctor, R.H.; Maragos, C.M. Volatile organic compound profile fingerprints using DART–MS shows species-specific patterns in Fusarium mycotoxin producing fungi. J. Fungi 2022, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Lazazzara, V.; Vicelli, B.; Bueschl, C.; Parich, A.; Pertot, I.; Schuhmacher, R.; Perazzolli, M. Trichoderma spp. volatile organic compounds protect grapevine plants by activating defense-related processes against downy mildew. Physiol. Plant 2021, 172, 1950–1965. [Google Scholar] [CrossRef] [PubMed]
- Marra, R.; Lombardi, N.; D’Errico, G.; Troisi, J.; Scala, G.; Vinale, F.; Woo, S.L.; Bonanomi, G.; Lorito, M. Application of Trichoderma strains and metabolites enhances soybean productivity and nutrient content. J. Agric. Food Chem. 2019, 67, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Humphris, S.N.; Wheatley, R.E.; Bruce, A. The effects of specific volatile organic compounds produced by Trichoderma spp. on the growth of wood decay basidiomycetes. Holzforschung 2001, 55, 233–237. [Google Scholar] [CrossRef]
- Morawe, M.; Hoeke, H.; Wissenbach, D.K.; Lentendu, G.; Wubet, T.; Kröber, E.; Kolb, S. Acidotolerant bacteria and fungi as a sink of methanol-derived carbon in a deciduous forest soil. Front. Microbiol. 2017, 8, 1361. [Google Scholar] [CrossRef]
- Singh, J.; Singh, P.; Vaishnav, A.; Ray, S.; Rajput, R.S.; Singh, S.M.; Singh, H.B. Belowground fungal volatiles perception in okra (Abelmoschus esculentus) facilitates plant growth under biotic stress. Microbiol. Res. 2021, 246, 126721. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Zeng, L.; Jiang, H.; Mei, L.; Wang, Y. Trichoderma atroviride LZ42 releases volatile organic compounds promoting plant growth and suppressing Fusarium wilt disease in tomato seedlings. BMC Microbiol. 2022, 22, 88. [Google Scholar] [CrossRef] [PubMed]
- Scarselletti, R.; Faull, J.L. In vitro Activity of 6-pentyl-α-pyrone, a metabolite of Trichoderma harzianum, in the inhibition of Rhizoctonia solani and Fusarium oxysporum f. sp. lycopersici. Mycol. Res 1994, 98, 1207–1209. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Ruocco, M.; Woo, S.; Lorito, M. Trichoderma secondary metabolites that affect plant metabolism. Nat. Prod. Commun. 2012, 7, 1545–1550. [Google Scholar] [CrossRef]
- Li, Q.; Ning, P.; Zheng, L.; Huang, J.; Li, G.; Hsiang, T. Fumigant activity of volatiles of Streptomyces globisporus JK-1 against Penicillium italicum on Citrus microcarpa. Postharvest Biol. Technol. 2010, 58, 157–165. [Google Scholar] [CrossRef]


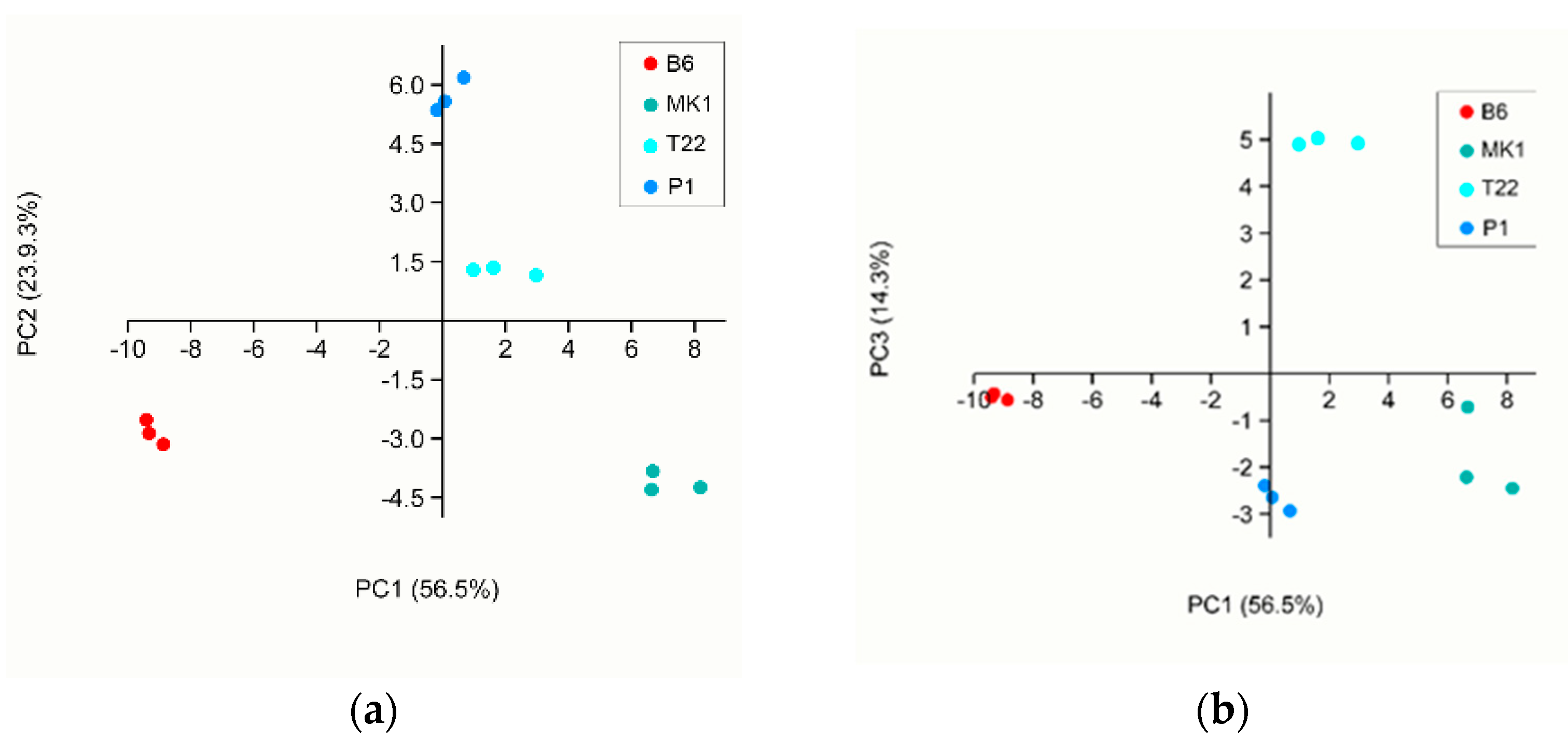
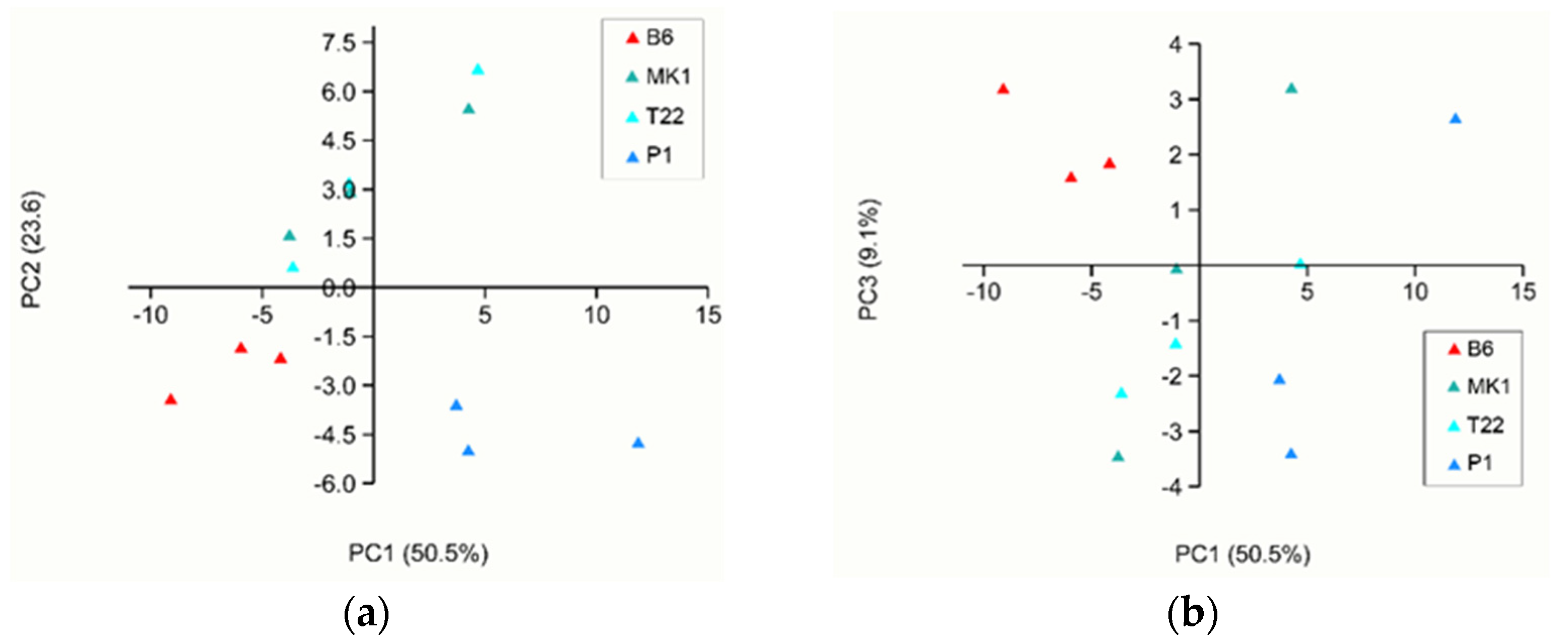
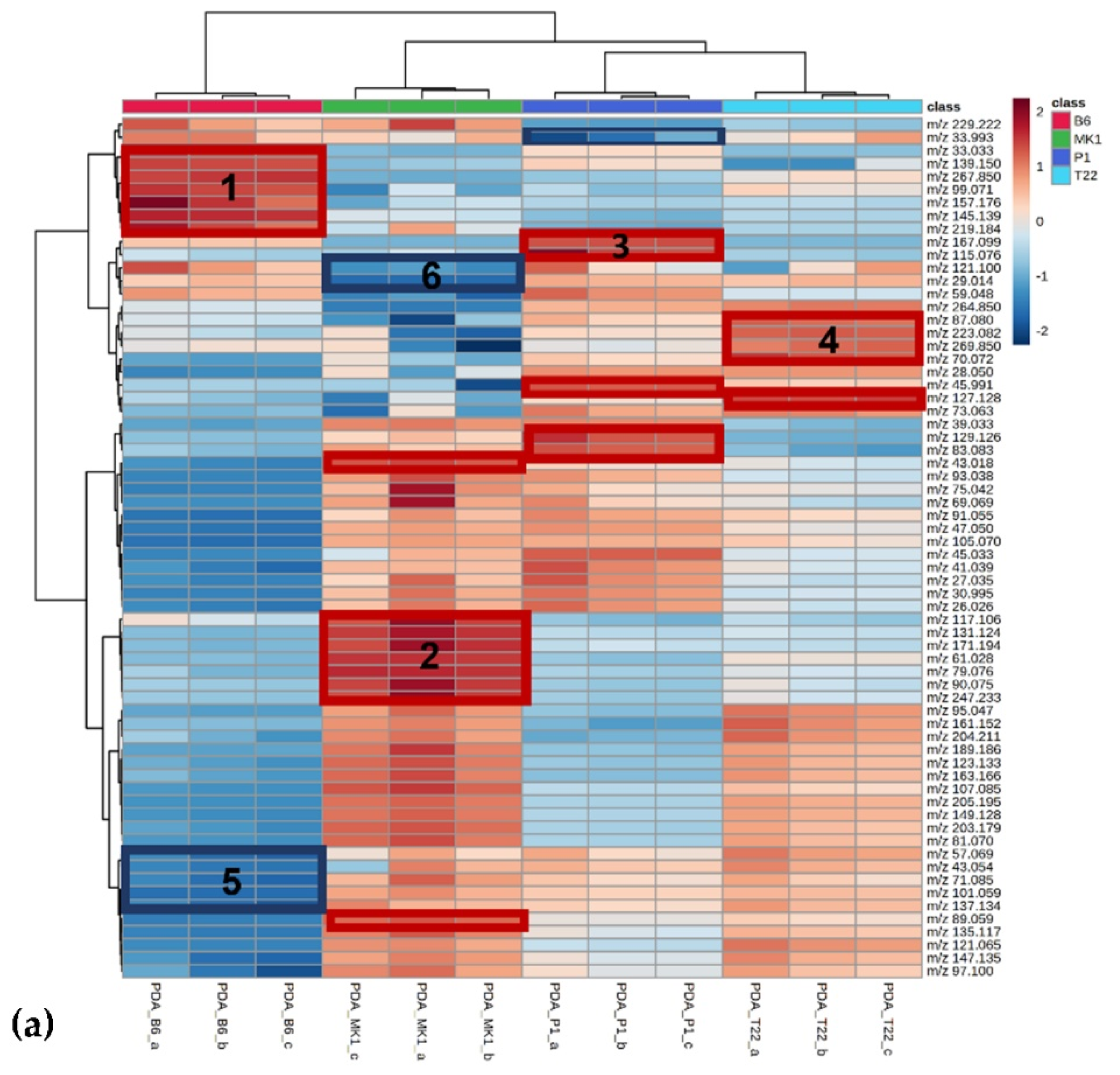
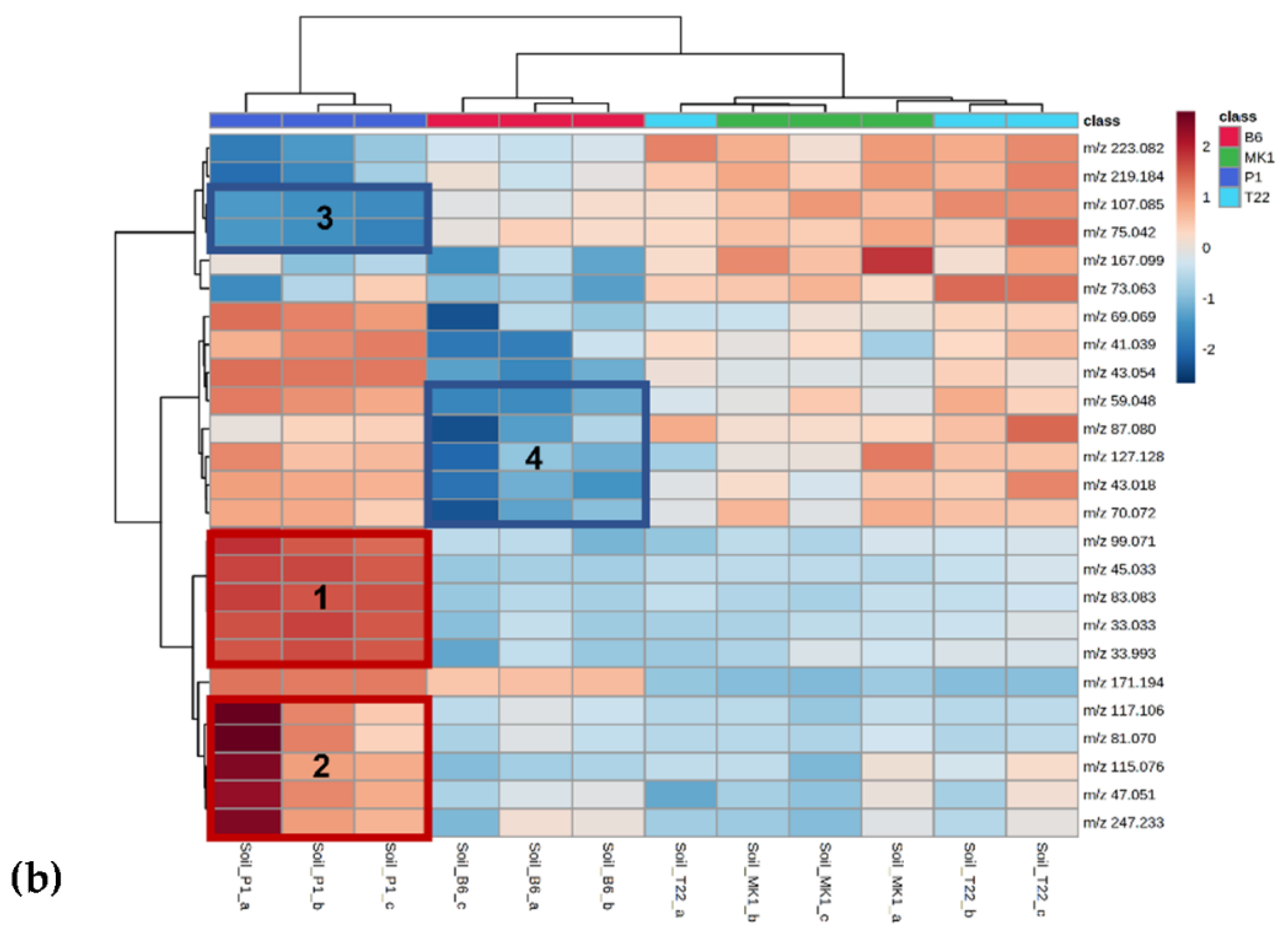
| B6 1 | %VOCs 2 | MK1 1 | %VOCs 2 | P1 1 | %VOCs 2 | T22 1 | %VOCs 2 |
|---|---|---|---|---|---|---|---|
| 29.014 | 2.23 | 43.018 | 2.13 | 47.051 | 2.21 | 29.014 | 2.04 |
| 59.048 | 2.07 | 47.051 | 2.10 | 45.033 | 2.15 | 47.051 | 2.01 |
| 33.033 | 2.02 | 61.028 | 2.06 | 29.014 | 2.05 | 205.195 | 1.93 |
| 41.039 | 1.87 | 89.059 | 2.01 | 41.039 | 1.98 | 57.069 | 1.91 |
| 43.018 | 1.82 | 205.195 | 2.00 | 43.018 | 1.97 | 43.018 | 1.90 |
| 33.993 | 1.81 | 93.038 | 1.88 | 59.048 | 1.95 | 41.039 | 1.85 |
| 57.069 | 1.79 | 41.039 | 1.86 | 93.038 | 1.85 | 43.054 | 1.77 |
| 145.139 | 1.78 | 45.033 | 1.83 | 57.069 | 1.85 | 61.028 | 1.75 |
| 47.051 | 1.77 | 29.014 | 1.81 | 131.124 | 1.77 | 45.033 | 1.75 |
| 139.150 | 1.73 | 57.069 | 1.79 | 26.026 | 1.76 | 89.059 | 1.72 |
| 27.035 | 1.68 | 149.128 | 1.75 | 27.035 | 1.75 | 71.085 | 1.71 |
| 219.184 | 1.67 | 71.085 | 1.73 | 167.099 | 1.74 | 59.048 | 1.70 |
| 61.028 | 1.66 | 131.124 | 1.71 | 105.070 | 1.73 | 73.063 | 1.69 |
| 111.046 | 1.64 | 135.117 | 1.69 | 45.991 | 1.73 | 95.047 | 1.68 |
| 26.026 | 1.64 | 105.070 | 1.69 | 43.054 | 1.73 | 149.128 | 1.68 |
| 73.063 | 1.63 | 90.075 | 1.68 | 39.033 | 1.70 | 121.065 | 1.67 |
| 69.069 | 1.62 | 26.026 | 1.67 | 71.085 | 1.68 | 33.993 | 1.64 |
| 39.033 | 1.60 | 27.035 | 1.66 | 73.063 | 1.67 | 27.035 | 1.63 |
| 157.176 | 1.58 | 39.033 | 1.65 | 89.059 | 1.62 | 105.070 | 1.63 |
| 45.991 | 1.57 | 81.070 | 1.64 | 33.993 | 1.61 | 26.026 | 1.63 |
| B6 1 | %VOCs 2 | MK1 1 | %VOCs 2 | P1 1 | %VOCs 2 | T22 1 | %VOCs 2 |
|---|---|---|---|---|---|---|---|
| 29.014 | 2.41 | 29.014 | 2.39 | 29.014 | 2.33 | 27.035 | 2.24 |
| 33.033 | 1.99 | 33.033 | 1.97 | 33.033 | 1.99 | 28.050 | 1.97 |
| 39.033 | 1.93 | 39.033 | 1.92 | 33.993 | 1.91 | 26.026 | 1.95 |
| 33.993 | 1.89 | 33.993 | 1.88 | 39.033 | 1.85 | 29.014 | 1.91 |
| 28.050 | 1.87 | 28.050 | 1.85 | 28.050 | 1.81 | 30.995 | 1.87 |
| 27.034 | 1.82 | 43.018 | 1.82 | 99.071 | 1.81 | 33.033 | 1.80 |
| 43.017 | 1.78 | 27.035 | 1.79 | 43.018 | 1.80 | 39.033 | 1.79 |
| 26.026 | 1.76 | 26.026 | 1.75 | 43.054 | 1.80 | 41.039 | 1.75 |
| 61.034 | 1.72 | 43.054 | 1.74 | 41.039 | 1.77 | 43.018 | 1.74 |
| 59.048 | 1.70 | 61.028 | 1.73 | 27.035 | 1.76 | 43.054 | 1.73 |
| 41.039 | 1.69 | 41.039 | 1.73 | 45.033 | 1.75 | 45.033 | 1.72 |
| 264.850 | 1.68 | 59.048 | 1.72 | 26.026 | 1.71 | 45.991 | 1.68 |
| 45.991 | 1.68 | 264.850 | 1.67 | 61.028 | 1.70 | 47.051 | 1.67 |
| 43.054 | 1.67 | 45.991 | 1.64 | 45.991 | 1.70 | 57.069 | 1.64 |
| 47.050 | 1.66 | 47.051 | 1.63 | 59.048 | 1.70 | 59.048 | 1.60 |
| 30.995 | 1.63 | 30.995 | 1.63 | 264.850 | 1.67 | 61.028 | 1.58 |
| 204.211 | 1.59 | 73.063 | 1.60 | 47.051 | 1.65 | 69.069 | 1.58 |
| 45.033 | 1.57 | 45.033 | 1.58 | 30.995 | 1.59 | 70.071 | 1.54 |
| 73.063 | 1.57 | 204.211 | 1.58 | 81.070 | 1.58 | 73.063 | 1.52 |
| 69.068 | 1.54 | 69.068 | 1.54 | 204.211 | 1.54 | 81.070 | 1.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gualtieri, L.; Monti, M.M.; Mele, F.; Russo, A.; Pedata, P.A.; Ruocco, M. Volatile Organic Compound (VOC) Profiles of Different Trichoderma Species and Their Potential Application. J. Fungi 2022, 8, 989. https://doi.org/10.3390/jof8100989
Gualtieri L, Monti MM, Mele F, Russo A, Pedata PA, Ruocco M. Volatile Organic Compound (VOC) Profiles of Different Trichoderma Species and Their Potential Application. Journal of Fungi. 2022; 8(10):989. https://doi.org/10.3390/jof8100989
Chicago/Turabian StyleGualtieri, Liberata, Maurilia Maria Monti, Francesca Mele, Assunta Russo, Paolo Alfonso Pedata, and Michelina Ruocco. 2022. "Volatile Organic Compound (VOC) Profiles of Different Trichoderma Species and Their Potential Application" Journal of Fungi 8, no. 10: 989. https://doi.org/10.3390/jof8100989
APA StyleGualtieri, L., Monti, M. M., Mele, F., Russo, A., Pedata, P. A., & Ruocco, M. (2022). Volatile Organic Compound (VOC) Profiles of Different Trichoderma Species and Their Potential Application. Journal of Fungi, 8(10), 989. https://doi.org/10.3390/jof8100989








