Peptaibol Production and Characterization from Trichoderma asperellum and Their Action as Biofungicide
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungi
2.2. Optimization of the Fermentation Media for Paib Production
2.2.1. Inoculum Preparation
2.2.2. Fermentation Media and Carbon Source Test
2.2.3. Elicitor Addition Test
2.2.4. Amino Acid Addition Test
2.3. Fermentation Process Modeling
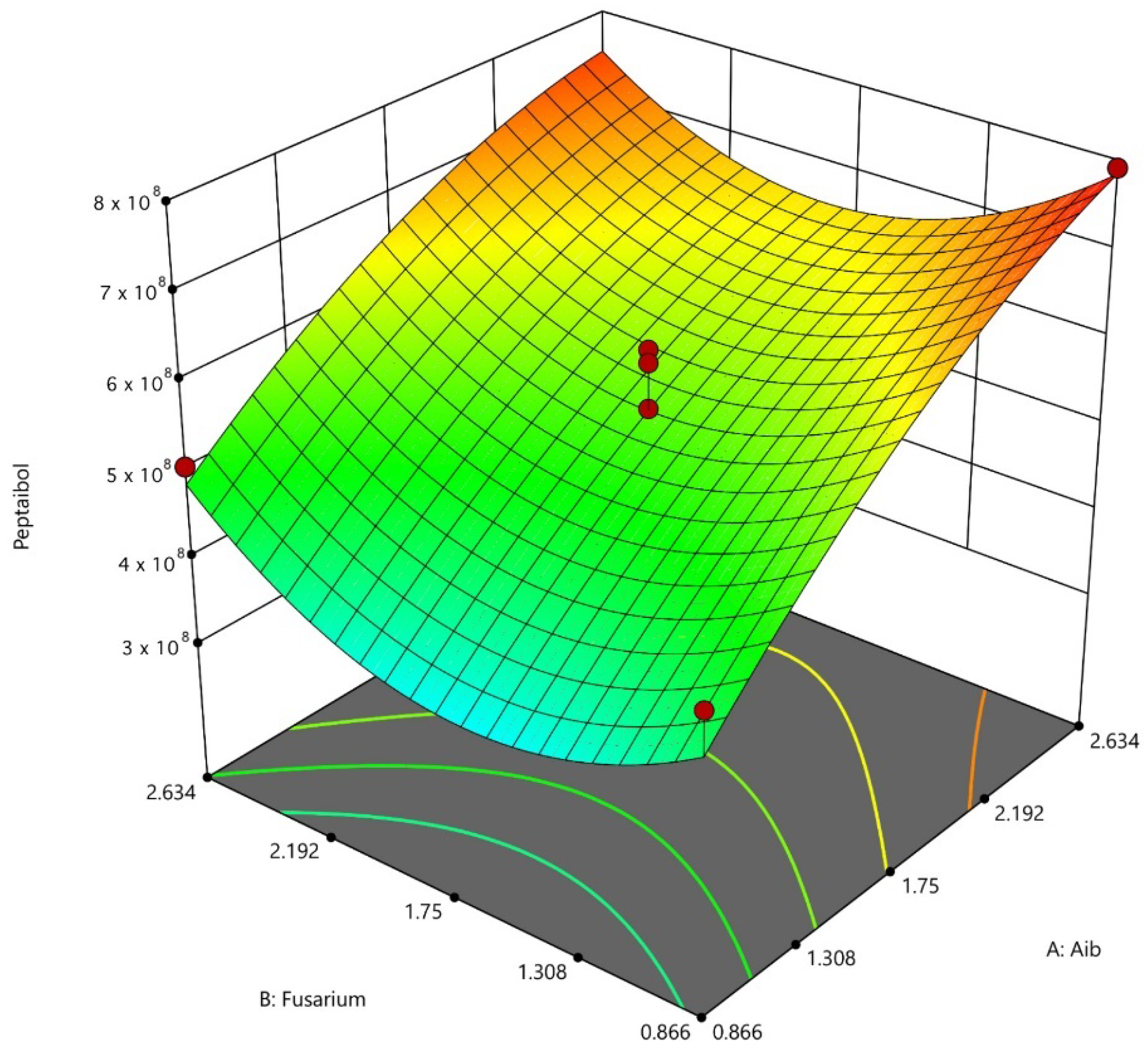
2.3.1. Model Approach
2.3.2. Model Validation
2.4. Mass Spectrometry
2.5. Paib Sequencing
2.6. Antifungal Activity of Paib from T. asperellum
2.6.1. Extract
2.6.2. Pathogenic Fungi In Vitro Growth Inhibition
2.6.3. A. alternata Growth Inhibition in Tomatoes
2.7. Effect of Paib on the Morphology of Phytopathogenic Fungi
2.7.1. Sample Preparation
2.7.2. Sample Fixation
3. Results and Discussion
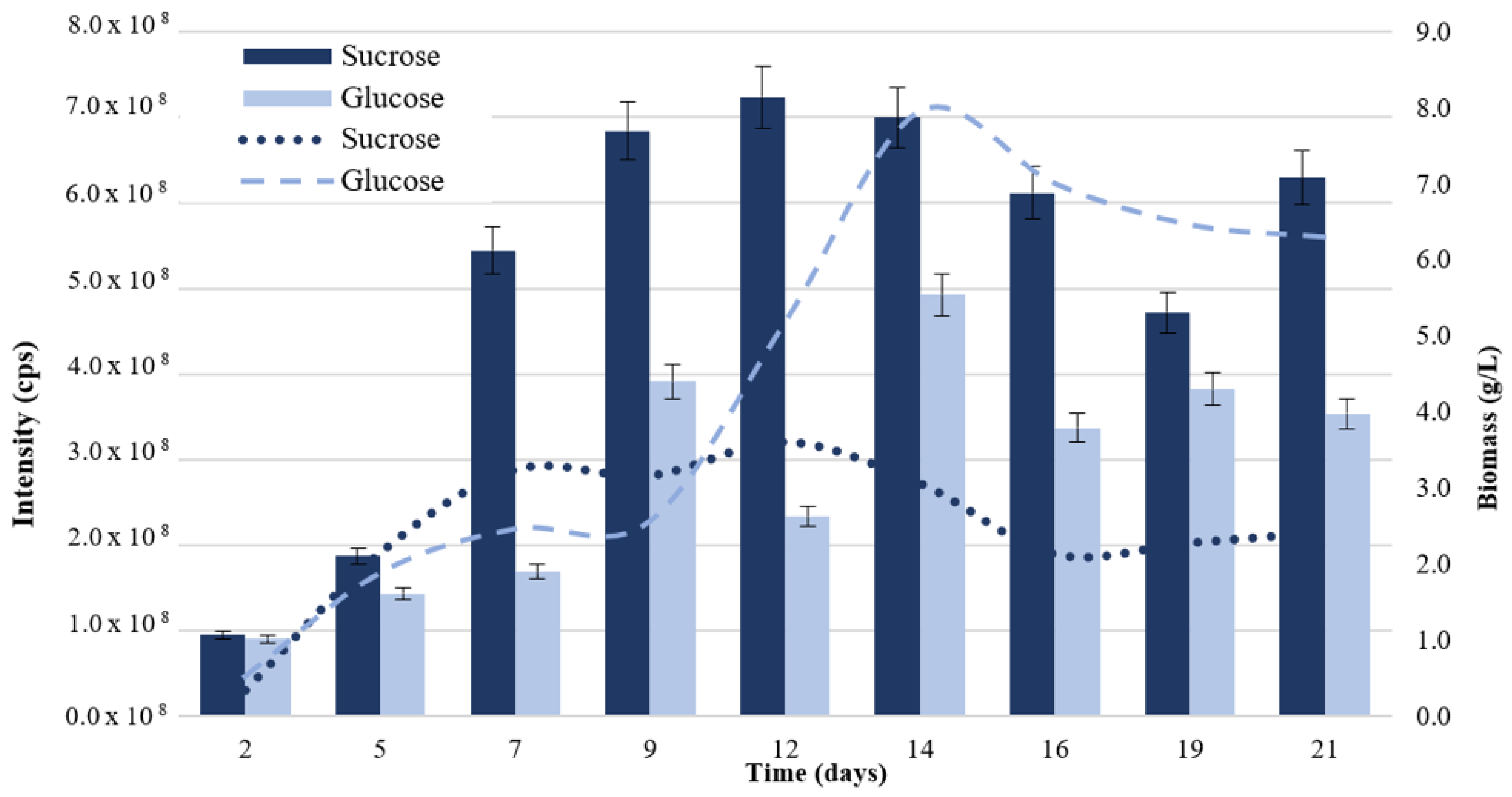
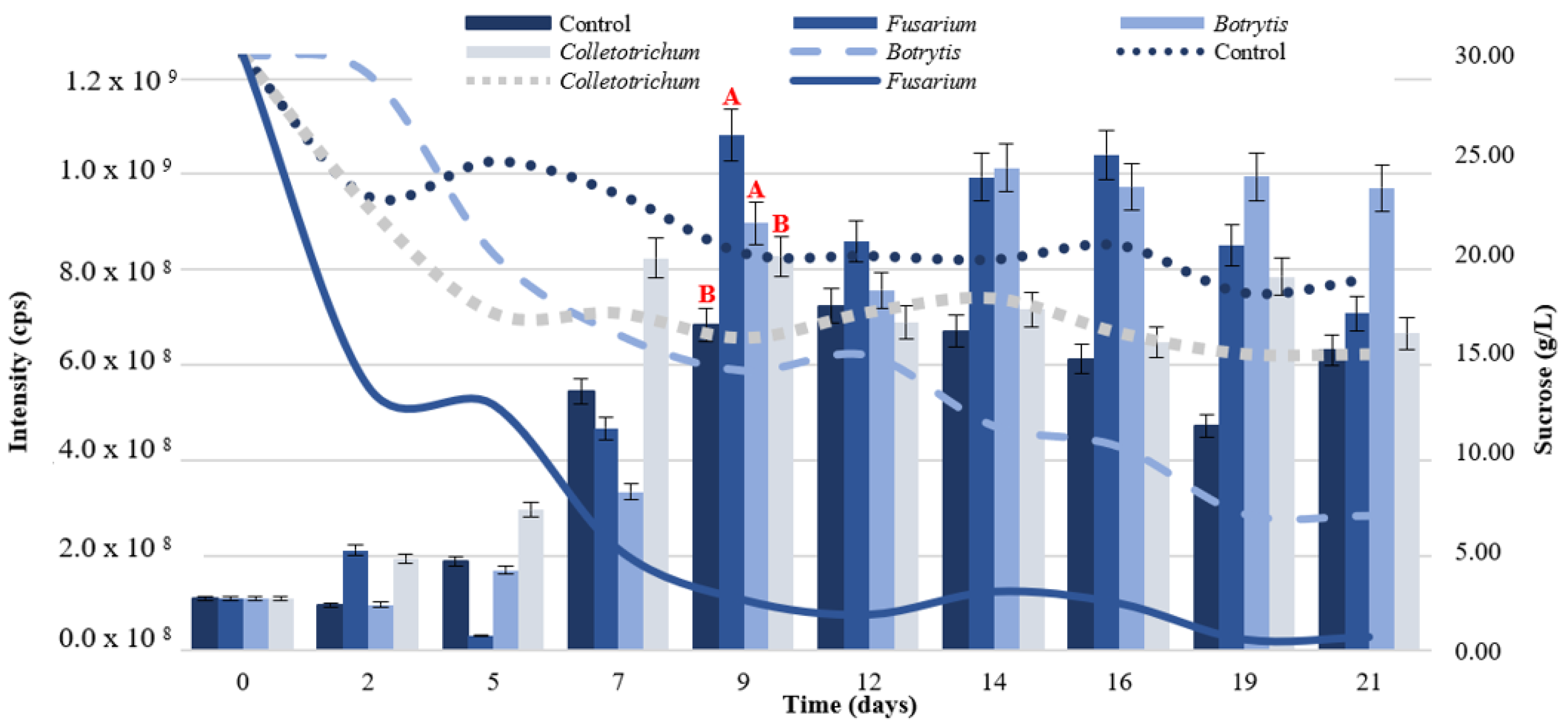
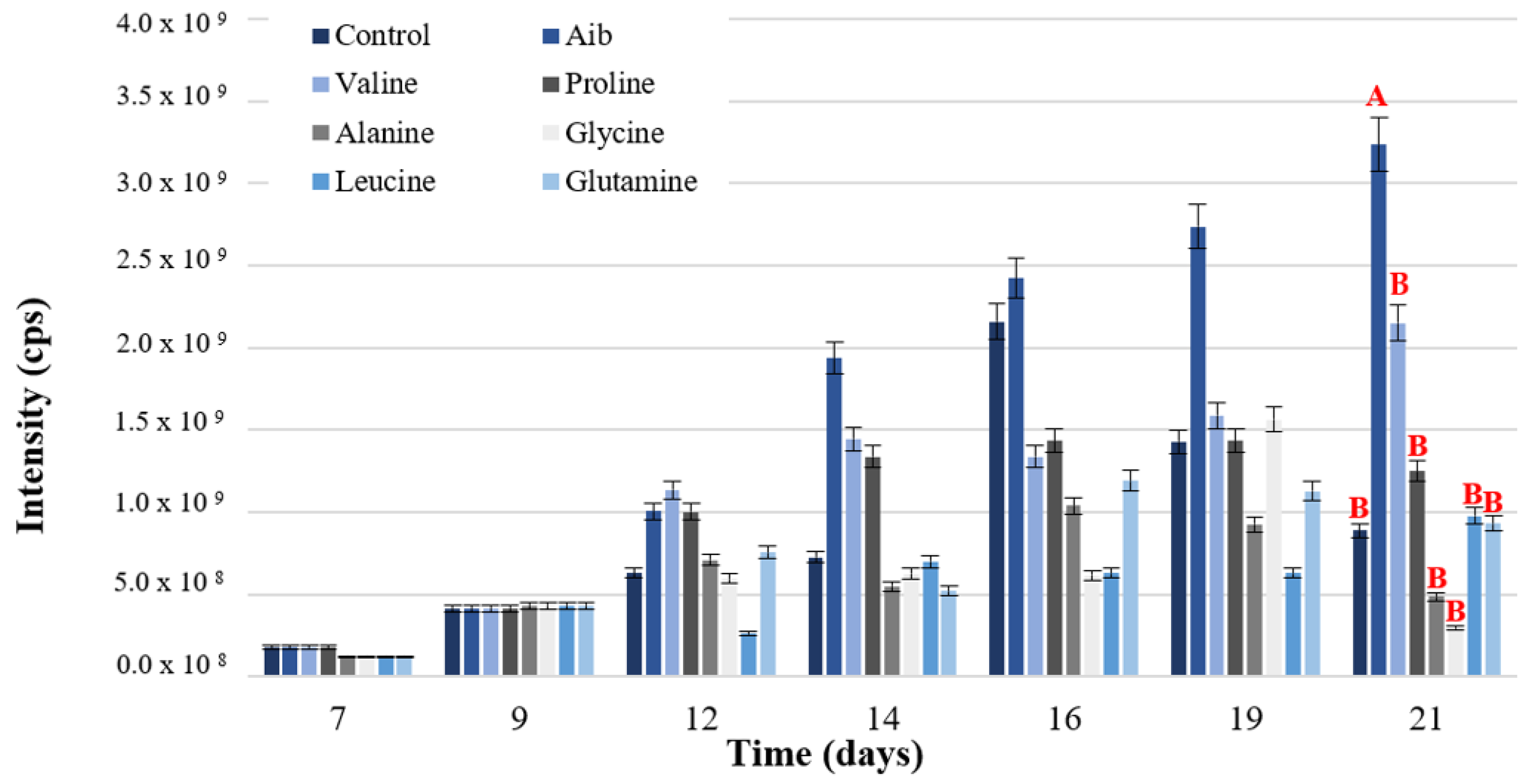
3.1. Optimization of Fermentation Media for Paib Production
3.1.1. Carbon Source Utilization Test
3.1.2. Elicitor Addition Test
3.1.3. Amino Acid Addition Test
3.2. Fermentation Process Modeling
3.3. Paib Sequence and Identification
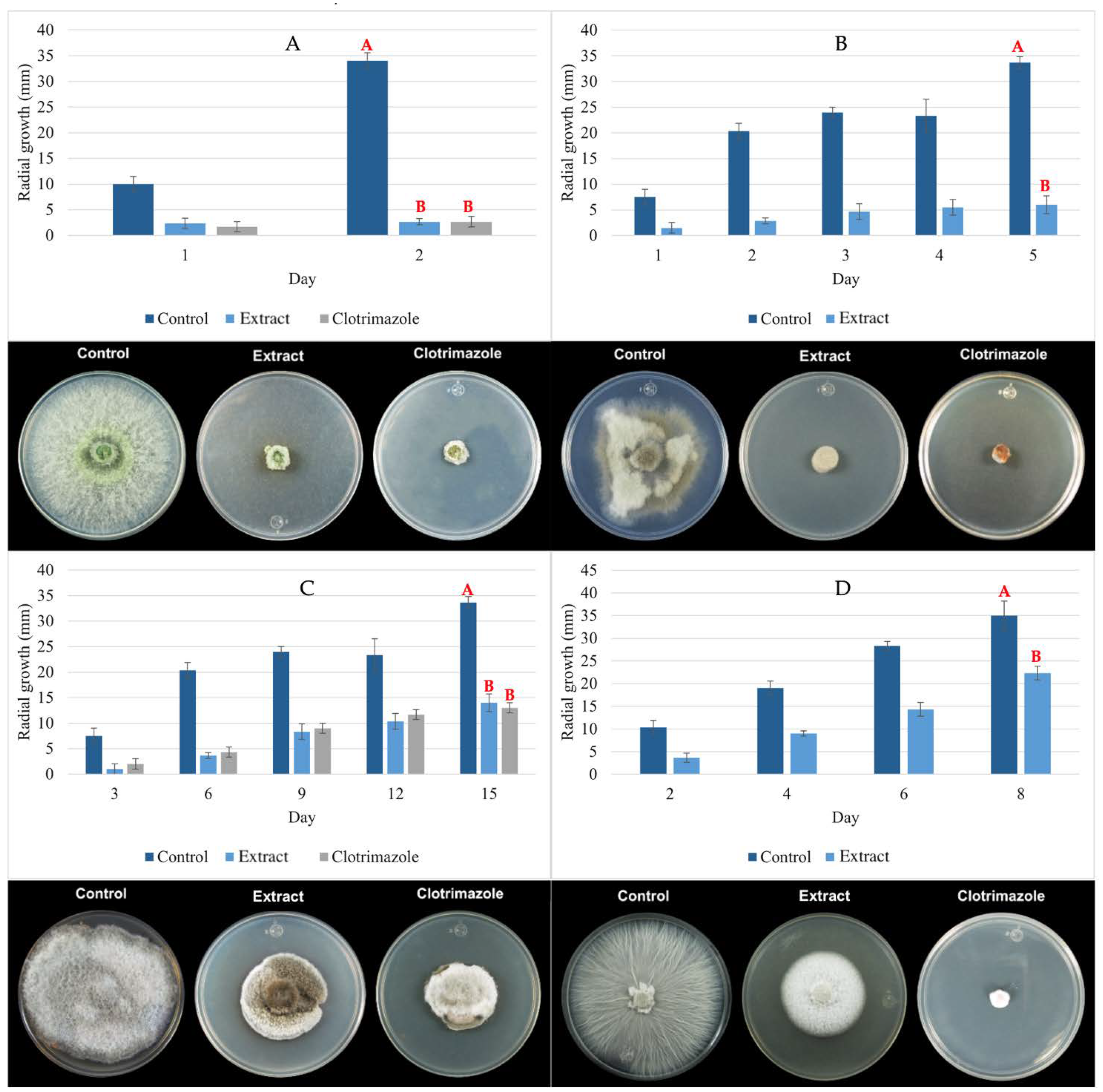
3.4. Antifungal Activity of Paib from T. asperellum
3.4.1. Pathogenic Fungi In Vitro Growth Inhibition
3.4.2. A. alternata Growth Inhibition in Tomatoes
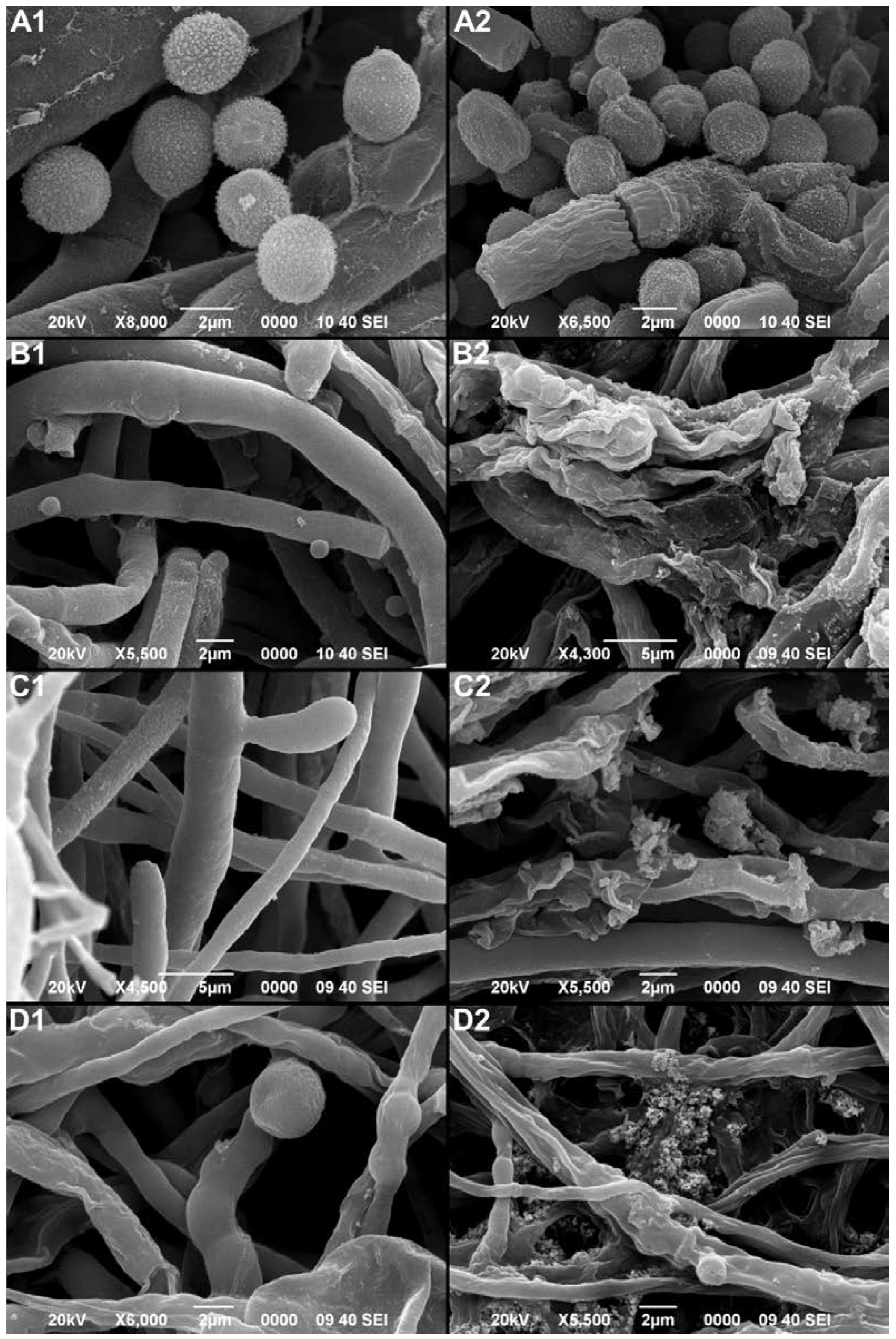
3.4.3. Effect of Paib on the Morphology of Phytopathogenic Fungi
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindsey, A.P.J.; Murugan, S.; Renitta, R.E. Microbial disease management in agriculture: Current status and future prospects. Biocatal. Agric. Biotechnol. 2020, 23, 101468. [Google Scholar] [CrossRef]
- Amaresh, Y.S.; Chennappa, G.; Avinash, S.; Naik, M.K.; Sreenivasa, M.Y. Trichoderma—A New Strategy in Combating Agriculture Problems; Elsevier B.V.: Amsterdam, The Netherlands, 2019; ISBN 978-0-12818-258-1. [Google Scholar]
- Krishnan, N.; Velramar, B.; Velu, R.K. Investigation of antifungal activity of surfactin against mycotoxigenic phytopathogenic fungus Fusarium moniliforme and its impact in seed germination and mycotoxicosis. Pestic. Biochem. Physiol. 2019, 155, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Solanum, L. El biocontrol de Alternaria alternata en tomate. Bioagro 2018, 30, 59–66. [Google Scholar]
- Carvalho, D.D.C.; Alves, E.; Barbosa Camargos, R.; Ferreira Oliveira, D.; Soares Scolforo, J.R.; de Carvalho, D.A.; Sâmia Batista, T.R. Plant extracts to control Alternaria alternata in murcott tangor fruits. Revista Iberoamericana de Micología 2011, 28, 173–178. [Google Scholar] [CrossRef]
- O’Brien, P.A. Biological control of plant diseases. Australas. Plant Pathol. 2017, 46, 293–304. [Google Scholar] [CrossRef]
- Monfil, V.O.; Casas-Flores, S. Molecular Mechanisms of Biocontrol in Trichoderma spp. and Their Applications in Agriculture; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-44459-576-8. [Google Scholar]
- Gupta, S.; Sharma, D.; Gupta, M. Climate change impact on Plant diseases: Opinion, trends and mitigation strategies. In Microbes for Climate Resilient Agriculture; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 41–56. [Google Scholar]
- Kumar, S.; Thaku, M.; Rani, A. Trichoderma: Mass production, formulation, quality control, delivery and its scope in commercialization in india for the management of plant diseases. Afr. J. Agric. Res. 2014, 9, 1461–1466. [Google Scholar] [CrossRef]
- You, J.; Yang, Z.; Stamps, B.W.; Stevenson, B.S.; Du, L.; Mitchell, C.A.; King, J.B.; Pan, N.; Bopassa, J.C.; Cichewicz, R.H.; et al. Unique Amalgamation of Primary and Secondary Structural Elements Transform Peptaibols into Potent Bioactive Cell-Penetrating Peptides. Proc. Natl. Acad. Sci. USA 2017, 114, E8957–E8966. [Google Scholar] [CrossRef]
- Das, S.; Ben Haj Salah, K.; Djibo, M.; Inguimbert, N. Peptaibols as a Model for the Insertions of Chemical Modifications. Arch. Biochem. Biophys. 2018, 658, 16–30. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Del-val, E.; Larsen, J. Interactions of Trichoderma with Plants, Insects, and Plant Pathogen Microorganisms: Chemical and Molecular Bases; Springer: Cham, Switzerland, 2019; pp. 1–28. [Google Scholar]
- Marik, T.; Urbán, P.; Tyagi, C.; Szekeres, A.; Leitgeb, B.; Vágvölgyi, M.; Manczinger, L.; Druzhinina, I.S.; Vágvölgyi, C.; Kredics, L. Diversity profile and dynamics of peptaibols produced by green mould Trichoderma species in interactions with their hosts agaricus bisporus and Pleurotus ostreatus. Chem. Biodivers. 2017, 14, e1700033. [Google Scholar] [CrossRef]
- Daniel, J.; Rodrigues, E. Peptaibols of Trichoderma. Nat. Prod. Rep. 2007, 24, 1128–1141. [Google Scholar] [CrossRef]
- Degenkolb, T.; Dçhren, H.; Nielsen, K.F.; Samuels, G.J.; Bruckner, H. Recent Advances and Future Prospects in Peptaibiotics, Hydrophobin and Mycotoxin Research and Their Importance for Chemotaxonomy of Trichoderma and Hypocrea. Chem. Biodivers. 2008, 5, 671–680. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced Systemic Resistance and Plant Responses to Fungal Biocontrol Agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef]
- Guha, S.; Ghimire, J.; Wu, E.; Wimley, W.C. Mechanistic Landscape of Membrane-Permeabilizing Peptides. Chem. Rev. 2019, 119, 6040–6085. [Google Scholar] [CrossRef]
- Keswani, C.; Singh, H.B.; Hermosa, R.; García-Estrada, C.; Caradus, J.; He, Y.W.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Vinale, F.; et al. Antimicrobial Secondary Metabolites from Agriculturally Important Fungi as next Biocontrol Agents. Appl. Microbiol. Biotechnol. 2019, 103, 9287–9303. [Google Scholar] [CrossRef]
- Ren, J.; Yang, Y.; Liu, D.; Chen, W.; Proksch, P.; Shao, B.; Lin, W. Sequential determination of new peptaibols asperelines G-Z12 produced by marine-derived fungus Trichoderma asperellum using ultrahigh pressure liquid chromatography combined with electrospray-ionization tandem mass spectrometry. J. Chromatogr. A 2013, 1309, 90–95. [Google Scholar] [CrossRef]
- Katoch, M.; Singh, D.; Kapoor, K.K.; Vishwakarma, R.A. Trichoderma lixii (IIIM-B4), an Endophyte of Bacopa monnieri L. producing peptaibols. BMC Microbiol. 2019, 19, 98. [Google Scholar] [CrossRef]
- Ito, A.; Kumagai, K.; Honda, S.; Shimatani, T.; Hosotani, N.; Saji, I. SPF-5506-A4, a New Peptaibol inhibitor of amyloid β-peptide formation produced by Trichoderma sp. J. Antibiot. 2009, 60, 184–190. [Google Scholar] [CrossRef]
- Xiao-Yan, S.; Qing-Tao, S.; Shu-Tao, X.; Xiu-Lan, C.; Cai-Yun, S.; Yu-Zhong, Z. Broad-spectrum antimicrobial activity and high stability of trichokonins from Trichoderma koningii SMF2 against plant pathogens. FEMS Microbiol. Lett. 2006, 260, 119–125. [Google Scholar] [CrossRef]
- Yang, P. The Gene Task1 Is Involved in Morphological Development, Mycoparasitism and Antibiosis of Trichoderma asperellum. Biocontrol Sci. Technol. 2017, 27, 620–635. [Google Scholar] [CrossRef]
- Stępień, Ł.; Popiel, D.; Gromadzka, K.; Basińska-Barczak, A.; Błaszczyk, L.; Ćwiek-Kupczyńska, H. Suppressive effect of Trichoderma spp. on toxigenic Fusarium species. Polish J. Microbiol. 2017, 66, 85–100. [Google Scholar] [CrossRef]
- Jadhav, R.N. Antagonistic and phosphate solubilization potential of Trichoderma sp. from rhizosphere of red gram cultivated in Marathwada Region. IJSRST 2018, 2, 101–105. [Google Scholar]
- Adnan, M.; Islam, W.; Shabbir, A.; Khan, K.A.; Ghramh, H.A.; Huang, Z.; Chen, H.Y.H.; Lu, G.-d. Plant defense against fungal pathogens by antagonistic fungi with Trichoderma in focus. Microb. Pathog. 2019, 129, 7–18. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gruber, S.; Bansal, R.; Mukherjee, P.K. Secondary Metabolism in Trichoderma—Chemistry Meets Genomics. Fungal Biol. Rev. 2016, 30, 74–90. [Google Scholar] [CrossRef]
- Aghcheh, R.K.; Braus, G.H. Importance of Stress Response Mechanisms in Filamentous Fungi for Agriculture and Industry. In Stress Response Mechanisms in Fungi; Springer International Publishing: Cham, Switzerland, 2018; pp. 189–222. [Google Scholar]
- Vinale, F.; Lorito, M.; Marra, R.; Woo, S.L.; Sivasithamparam, K.; Ghisalberti, E.L. Trichoderma–Plant–Pathogen Interactions. Soil Biol. Biochem. 2007, 40, 1–10. [Google Scholar] [CrossRef]
- Leitgeb, B.; Szekeres, A.; Manczinger, L.; Vágvölgyi, C.; Kredics, L. The History of Alamethicin: A Review of the Most Extensively Studied Peptaibol. Chem. Biodivers. 2007, 4, 1027–1051. [Google Scholar] [CrossRef]
- Li, F.F.; Brimble, M.A. Using Chemical Synthesis to Optimise Antimicrobial Peptides in the Fight against Antimicrobial Resistance. Proc. Pure Appl. Chem. 2019, 91, 181–198. [Google Scholar] [CrossRef]
- Marik, T.; Tyagi, C.; Balázs, D.; Urbán, P.; Szepesi, Á.; Bakacsy, L.; Endre, G.; Rakk, D.; Szekeres, A.; Andersson, M.A.; et al. Structural Diversity and Bioactivities of Peptaibol Compounds from the Longibrachiatum Clade of the Filamentous Fungal Genus Trichoderma. Front. Microbiol. 2019, 10, 1434. [Google Scholar] [CrossRef]
- Al-Ani, L.K.T. Bioactive secondary metabolites of Trichoderma spp. for efficient management of phytopathogens. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Springer: Singapore, 2019; pp. 125–143. [Google Scholar] [CrossRef]
- Harman, G.E.; Obregón, M.A.; Samuels, G.J.; Lorito, M. Changing models for commercialization and implementation of biocontrol in the developing and the developed world. Plant Dis. 2010, 94, 928–939. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Horwitz, B.A.; Herrera-Estrella, A.; Schmoll, M.; Kenerley, C.M. Trichoderma Research in the Genome Era. Annu. Rev. Phytopathol. 2013, 51, 105–129. [Google Scholar] [CrossRef]
- Ghazanfar, M.U.; Raza, M.; Raza, W.; Qamar, M.I. Trichoderma as Potential Biocontrol Agent, Its Exploitation in Agriculture: A Review. Plant Prot. 2018, 2, 109–135. [Google Scholar]
- Cai, F.; Druzhinina, I.S. In honor of John Bissett: Authoritative guidelines on molecular identification of Trichoderma. Fungal Divers. 2021, 107, 1–69. [Google Scholar] [CrossRef]
- Baranyi, J.; Pin, C.; Ross, T. Validating and Comparing Predictive Models. Int. J. Food Microbiol. 1999, 48, 159–166. [Google Scholar] [CrossRef]
- Degenkolb, T.; Gräfenhan, T.; Berg, A.; Nirenberg, H.I.; Gams, W.; Brückner, H. Peptaibiomics: Screening for Polypeptide Antibiotics (Peptaibiotics) from Plant-Protective Trichoderma Species. Chem. Biodivers. 2006, 3, 593–610. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Y.; Xie, S.T.; Chen, X.L.; Sun, C.Y.; Shi, M.; Zhang, Y.Z. Solid-State fermentation for trichokonins production from Trichoderma koningii SMF2 and preparative purification of trichokonin vi by a simple protocol. J. Biotechnol. 2007, 131, 209–215. [Google Scholar] [CrossRef]
- Touati, I.; Ruiz, N.; Thomas, O.; Druzhinina, I.S.; Atanasova, L.; Tabbene, O.; Elkahoui, S.; Benzekri, R.; Bouslama, L.; Pouchus, Y.F.; et al. Hyporientalin A, an anti-candida peptaibol from a marine Trichoderma orientale. World J. Microbiol. Biotechnol. 2018, 34, 98. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 8th ed.; Macmillan Learning: New York, NY, USA, 2021. [Google Scholar]
- Zeilinger, S.; Atanasova, L. Sensing and Regulation of Mycoparasitism-Relevant Processes in Trichoderma; Elsevier B.V.: Amsterdam, The Netherlands, 2020; ISBN 978-0-12819-453-9. [Google Scholar]
- Zhou, Y.R.; Song, X.Y.; Li, Y.; Shi, J.C.; Shi, W.L.; Chen, X.L.; Liu, W.F.; Liu, X.M.; Zhang, W.X.; Zhang, Y.Z. Enhancing peptaibols production in the biocontrol fungus Trichoderma longibrachiatum SMF2 by elimination of a putative glucose sensor. Biotechnol. Bioeng. 2019, 116, 3030–3040. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Horwitz, B.A.; Kenerley, C.M. Secondary Metabolism in Trichoderma—A Genomic Perspective. Microbiology 2012, 158, 35–45. [Google Scholar] [CrossRef]
- Bastos, A. Producción de Peptaiboles a Partir de Trichoderma asperellum y su Efecto Inhibitorio Contra Fitopatógenos de Papaya (Carica papaya); Instituto Tecnológico de Costa Rica: Cartago, Costa Rica, 2018. [Google Scholar]
- Carranza-Rodriguez, J. Desarrollo de un Protocolo Paraa Extracción y Purificación de Peptaiboles con Actividad Inhibitoria Obtenidos de Trichoderma asperellum para Controlar Hongos Fitopatógenos de los Géneros Fusarium y Rhizoctonia. Bachelor’s Thesis, Universidad de Costa Rica, Tacares, Costa Rica, 2019. [Google Scholar]
- Tamandegani, P.R.; Marik, T.; Zafari, D.; Balázs, D.; Vágvölgyi, C.; Szekeres, A.; Kredics, L. Changes in peptaibol production of Trichoderma species during in vitro antagonistic interactions with fungal plant pathogens. Biomolecules 2020, 10, 730. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Rawa, M.S.A.; Nogawa, T.; Okano, A.; Futamura, Y.; Nakamura, T.; Wahab, H.A.; Osada, H. A New Peptaibol, RK-026A, from the Soil Fungus Trichoderma sp. RK10-F026 by Culture Condition-Dependent Screening. Biosci. Biotechnol. Biochem. 2021, 85, 69–76. [Google Scholar] [CrossRef]
- Walker, G.M.; White, N.A. Introduction to Fungal Physiology. In Fungi: Biology and Applications; Kavanagh, K., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 1–35. [Google Scholar]
- Whitmore, L.; Wallace, B.A. The Peptaibol Database: A Database for Sequences and Structures of Naturally Occurring Peptaibols. Nucleic Acids Res. 2004, 32, D593–D594. [Google Scholar] [CrossRef]
- Chutrakul, C.; Alcocer, M.; Bailey, K.; Peberdy, J.F. The Production and Characterisation of Trichotoxin Peptaibols, by Trichoderma asperellum. Chem. Biodivers. 2008, 5, 1694–1706. [Google Scholar] [CrossRef]
- Stadnik, M.J.; Ei-Deeb, S.H.; Kreer, J.; Buchenauer, H. Effectiveness of α-Aminoisobutyric Acid as a Translocatable Fungistatic Agent against Blumeria Graminis ESP. Tritici in Wheat. J. Plant Pathol. 1999, 81, 103–111. [Google Scholar] [CrossRef]
- Ren, J.; Xue, C.; Tian, L.; Xu, M.; Chen, J.; Deng, Z.; Proksch, P.; Lin, W. Asperelines A–F, Peptaibols from the Marine-Derived Fungus Trichoderma asperellum. J. Nat. Prod. 2009, 72, 1036–1044. [Google Scholar] [CrossRef]
- Degenkolb, T.; Fognielsen, K.; Dieckmann, R.; Branco-Rocha, F.; Chaverri, P.; Samuels, G.J.; Thrane, U.; Vondçhren, H.; Vilcinskas, A.; Brückner, H. Peptaibol, Secondary-Metabolite, a Nd Hydrophobin P Attern of Commercial Biocontrol Agents Formulated with Species of the Trichoderma harzianum Complex. Chem. Biodivers. 2015, 12, 662–684. [Google Scholar] [CrossRef]
- Brito, J.P.C.; Ramada, M.H.S.; de Magalhães, M.T.Q.; Silva, L.P.; Ulhoa, C.J. Peptaibols from Trichoderma asperellum TR356 strain isolated from brazilian soil. J. Korean Phys. Soc. 2014, 3, 600. [Google Scholar] [CrossRef]
- Bruckner, H.; Konig, W.A.; Aydin, M.; Jung, G. Trichotoxin A40. Purification by Counter-Current Distribution and Sequencing of Isolated Fragments. Biochim. Biophys. Acta 1985, 827, 51–62. [Google Scholar] [CrossRef]
- Chugh, J.K.; Brückner, H.; Wallace, B.A. Model for a Helical Bundle Channel Based on the High-Resolution Crystal Structure of Trichotoxin_A50E. Biochemistry 2002, 41, 12934–12941. [Google Scholar] [CrossRef]
- Logrieco, A.; Bottalico, A.; Mulé, G.; Moretti, A.; Perrone, G. Epidemiology of Toxigenic Fungi and Their Associated Mycotoxins for Some Mediterranean Crops. Eur. J. Plant Pathol. 2003, 109, 645–667. [Google Scholar] [CrossRef]
- Notte, A.M.; Plaza, V.; Marambio-Alvarado, B.; Olivares-Urbina, L.; Poblete-Morales, M.; Silva-Moreno, E.; Castillo, L. Molecular Identification and Characterization of Botrytis cinerea Associated to the Endemic Flora of Semi-Desert Climate in Chile. Curr. Res. Microb. Sci. 2021, 2, 100049. [Google Scholar] [CrossRef]
- De Lucca, A.J. Hongos Patógenos Communes en la Agricultura y la Medicina. Rev. Iberoam. Micol. 2007, 24, 3–13. [Google Scholar] [CrossRef]
- Perrin, B.S.; Pastor, R.W. Simulations of Membrane-Disrupting Peptides I: Alamethicin Pore Stability and Spontaneous Insertion. Biophys. J. 2016, 111, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Dotson, B.R.; Soltan, D.; Schmidt, J.; Areskoug, M.; Rabe, K.; Swart, C.; Widell, S.; Rasmusson, A.G. The Antibiotic Peptaibol Alamethicin from Trichoderma Permeabilises Arabidopsis Root Apical Meristem and Epidermis but Is Antagonised by Cellulase-Induced Resistance to Alamethicin. BMC Plant Biol. 2018, 18, 165. [Google Scholar] [CrossRef]
- Haller, I. Mode of Action of Clotrimazole: Implications for Therapy. Am. J. Obstet. Gynecol. 1985, 152, 939–944. [Google Scholar] [CrossRef]
- Dalal, A.; Tushir, R.; Chauhan, A.; Bansal, R.; Kumar, P. Descriptive Review on Pharmacokinetics and Pharmacodynamics Profile of an Antifungal Agent: Clotrimazole. Certif. J. Tushir Eur. J. Pharm. Med. Res. 2022, 9, 204–216. [Google Scholar] [CrossRef]
- Fuente-Núñez, C.; de la Whitmore, L.; Wallace, B.A. Peptaibols. In Handbook of Biologically Active Peptides; Kastin Abba, J., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 150–156. ISBN 978-0-12385-095-9. [Google Scholar]
- Peltola, J.; Ritieni, A.; Mikkola, R.; Grigoriev, P.A.; Pócsfalvi, G.; Andersson, M.A.; Salkinoja-Salonen, M.S. Biological effects of Trichoderma harzianum peptaibols on mammalian cells. Appl. Environ. Microbiol. 2004, 70, 4996–5004. [Google Scholar] [CrossRef]
- Vestergaard, M.; Christensen, M.; Hansen, S.K.; Grønvall, D.; Kjølbye, L.R.; Vosegaard, T.; Schiøtt, B. How a Short Pore Forming Peptide Spans the Lipid Membrane. Biointerphases 2017, 12, 02D405. [Google Scholar] [CrossRef]
- Lorito, M.; Farkas, V.; Rebuffat, S.; Bodo, B. Cell Wall Synthesis Is a Major Target of Mycoparasitic Antagonism by Trichoderma harzianum. J. Bacteriol. 1996, 178, 6382–6385. [Google Scholar] [CrossRef]
- Szekeres, A.; Leitgeb, B.; Kredics, L.; Antal, Z.; Hatvani, L.; Manczinger, L.; Vágvölgyi, C. Peptaibols and Related Peptaibiotics of Trichoderma. Acta Microbiol. Immunol. Hung. 2005, 52, 137–168. [Google Scholar] [CrossRef]
- Duclohier, H.; Alder, G.M.; Bashford, C.L.; Brückner, H.; Chugh, J.K.; Wallace, B.A. Conductance Studies on Trichotoxin_A50E and Implications for Channel Structure. Biophys. J. 2004, 87, 1705–1710. [Google Scholar] [CrossRef][Green Version]
- Ageitos, J.M.; Sánchez-Pérez, A.; Calo-Mata, P.; Villa, T.G. Antimicrobial Peptides (AMPs): Ancient Compounds That Represent Novel Weapons in the Fight against Bacteria. Biochem. Pharmacol. 2017, 133, 117–138. [Google Scholar] [CrossRef]
- Salnikov, E.S.; De Zotti, M.; Bobone, S.; Mazzuca, C.; Raya, J.; Siano, A.S.; Peggion, C.; Toniolo, C.; Stella, L.; Bechinger, B. Trichogin GA IV Alignment and Oligomerization in Phospholipid Bilayers. ChemBioChem 2019, 20, 2141–2150. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B.A. Analysis of peptaibol sequence composition: Implications for in vivo synthesis and channel formation. Eur. Biophys. J. 2004, 33, 233–237. [Google Scholar] [CrossRef]
- Grigoletto, D.F.; Trivella, D.B.B.; Tempone, A.G.; Rodrigues, A.; Correia, A.M.L.; Lira, S.P. Antifungal compounds with anticancer potential from Trichoderma sp. P8BDA1F1, an endophytic fungus from Begonia venosa. Braz. J. Microbiol. 2020, 51, 989–997. [Google Scholar] [CrossRef]
- Akhtar, K.P.; Saleem, M.Y.; Asghar, M.; Haq, M.A. New Report of Alternaria alternaria causing leaf blight of tomato in Pakistan. Plant Pathol. 2004, 53, 816. [Google Scholar] [CrossRef]
- Iglesias, I.; Rodríguez-Rajo, F.J.; Méndez, J. Evaluation of the Different Alternaria Prediction Models on a Potato Crop in A Limia (NW of Spain). Aerobiologia 2007, 23, 27–34. [Google Scholar] [CrossRef]
- Santos Dória, M.; Silva Guedes, M.; de Andrade Silva, E.M.; Magalhães de Oliveira, T.; Pirovani, C.P.; Kupper, K.C.; Bastianel, M.; Micheli, F. Comparative proteomics of two citrus varieties in response to infection by the fungus Alternaria alternata. Int. J. Biol. Macromol. 2019, 136, 410–423. [Google Scholar] [CrossRef]
- Mejía, J.; Hernández, M. Evaluación de azoxystrobin en el control de la candelilla temprana (Alternaria solani) en el cultivo de tomate. Evaluation of Azoxystrobin on the Early Blight Control (Alternaria solani) in Tomatoes. Revista de la Facultad de Agronomía-Universidad del Zulia 2001, 18, 106–116. [Google Scholar]
- Soleimani, M.; Kirk, W. Enhance Resistance to Alternaria alternata causing potato brown leaf spot disease by using some plant defense inducers. J. Plant Prot. Res. 2012, 52, 83–90. [Google Scholar] [CrossRef]
- Yan, F.; Hu, H.; Lu, L.; Zheng, X. Rhamnolipids Induce Oxidative Stress Responses in Cherry Tomato Fruit to Alternaria alternata. Pest Manag. Sci. 2016, 72, 1500–1507. [Google Scholar] [CrossRef]
- Camiletti, B.X.; Lichtemberg, P.S.F.; Paredes, J.A.; Carraro, T.A.; Velascos, J.; Michailides, T.J. Characterization, Pathogenicity, and Fungicide Sensitivity of Alternaria Isolates Associated with Preharvest Fruit Drop in California Citrus. Fungal Biol. 2022, 126, 277–289. [Google Scholar] [CrossRef]
- De Linares, C.; Belmonte, J.; Canela, M.; de la Guardia, C.D.; Alba-Sanchez, F.; Sabariego, S.; Alonso-Pérez, S. Dispersal Patterns of Alternaria Conidia in Spain. Agric. For. Meteorol. 2010, 150, 1491–1500. [Google Scholar] [CrossRef]
- Schirmbock, M.; Lorito, M.; Wang, Y.L.; Hayes, C.K.; Arisan-Atac, I.; Scala, F.; Harman, G.E.; Kubicek, C.P. Parallel formation and synergism of hydrolytic enzymes and peptaibol antibiotics, molecular mechanisms involved in the antagonistic action of Trichoderma harzianum against phytopathogenic fungi. Appl. Environ. Microbiol. 1994, 60, 4364–4370. [Google Scholar] [CrossRef]
- Shakeri, J.; Foster, H.A. Proteolytic activity and antibiotic production by Trichoderma harzianum in relation to pathogenicity to insects. Enzym. Microb. Technol. 2007, 40, 961–968. [Google Scholar] [CrossRef]
- Engelberth, J.; Koch, T.; Kühnemann, F.; Boland, W. Channel-forming peptaibols are potent elicitors of plant secondary metabolism and tendril coiling. Angew. Chemie Int. Ed. 2000, 39, 1860–1862. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, D.D.; Dong, X.W.; Zhao, P.B.; Chen, L.L.; Song, X.Y.; Wang, X.J.; Chen, X.L.; Shi, M.; Zhang, Y.Z. Antimicrobial Peptaibols Induce Defense Responses and Systemic Resistance in Tobacco against Tobacco Mosaic Virus. FEMS Microbiol. Lett. 2010, 313, 120–126. [Google Scholar] [CrossRef][Green Version]
- Viterbo, A.; Wiest, A.; Brotman, Y.; Chet, I.; Kenerley, C. The 18mer peptaibols from Trichoderma virens elicit plant defence responses. Mol. Plant Pathol. 2007, 8, 737–746. [Google Scholar] [CrossRef]
- Shi, M.; Chen, L.; Wang, X.W.; Zhang, T.; Zhao, P.B.; Song, X.Y.; Sun, C.Y.; Chen, X.L.; Zhou, B.C.; Zhang, Y.Z. Antimicrobial peptaibols from Trichoderma pseudokoningii induce programmed cell death in plant fungal pathogens. Microbiology 2012, 158, 166–175. [Google Scholar] [CrossRef]
- Al-Askar, A.A.; Baka, Z.A.; Rashad, Y.M.; Ghoneem, K.M.; Abdulkhair, W.M.; Hafez, E.E.; Shabana, Y.M. Evaluation of Streptomyces griseorubens e44g for the biocontrol of Fusarium oxysporum f. sp. lycopersici: Ultrastructural and cytochemical investigations. Ann. Microbiol. 2015, 65, 1815–1824. [Google Scholar] [CrossRef]
- Pârvu, M.; Pârvu, A.E.; Crǎciun, C.; Barbu-Tudoran, L.; Tǎmaş, M. Antifungal activities of chelidonium majus extract on Botrytis cinerea in vitro and ultrastructural changes in its conidia. J. Phytopathol. 2008, 156, 550–552. [Google Scholar] [CrossRef]

| Variable | −α | −1 | 0 | 1 | α |
|---|---|---|---|---|---|
| Aib | 0.500 | 0.866 | 1.750 | 2.634 | 3.000 |
| Fusarium | 0.500 | 0.866 | 1.750 | 2.634 | 3.000 |
| Time (min) | A (%): Water/H+ | B (%): Methanol/H+ |
|---|---|---|
| 0 | 30 | 70 |
| 16 | 15 | 85 |
| 25 | 0 | 100 |
| 35 | 0 | 100 |
| Trial | Treatment (g/L) | Paib Production (cps) | ||
|---|---|---|---|---|
| Aib | F. oxysporum | Observed | Predicted | |
| 1 | 0.500 | 1.750 | 1.90 × 108 | 2.34 × 108 |
| 2 | 0.866 | 0.866 | 4.79 × 108 | 4.29 × 108 |
| 3 | 0.866 | 2.634 | 5.07 × 108 | 4.86 × 108 |
| 4 | 1.750 | 0.500 | 7.28 × 108 | 7.63 × 108 |
| 5 | 1.750 | 1.750 | 5.19 × 108 | 5.45 × 108 |
| 6 | 1.750 | 1.750 | 5.47 × 108 | 5.45 × 108 |
| 7 | 1.750 | 1.750 | 4.45 × 108 | 5.45 × 108 |
| 8 | 1.750 | 1.750 | 5.99 × 108 | 5.45 × 108 |
| 9 | 1.750 | 1.750 | 6.14 × 108 | 5.45 × 108 |
| 10 | 1.750 | 3.000 | 7.89 × 108 | 7.83 × 108 |
| 11 | 2.634 | 0.866 | 7.91 × 108 | 7.84 × 108 |
| 12 | 2.634 | 2.634 | 7.28 × 108 | 7.63 × 108 |
| 13 | 3.000 | 1.750 | 6.92 × 108 | 6.75 × 108 |
| Trial | Treatment (g/L) | Paib Production (cps) | ||
|---|---|---|---|---|
| Aib | F. oxysporum | Observed | Predicted | |
| 1 | 2.190 | 1.750 | 8.79 × 108 | 6.11 × 108 |
| 2 | 1.300 | 1.750 | 6.21 × 108 | 4.54 × 108 |
| 3 | 1.750 | 1.300 | 6.32 × 108 | 5.71 × 108 |
| 4 | 1.750 | 2.190 | 4.22 × 108 | 5.76 × 108 |
| 5 | 2.190 | 2.190 | 5.72 × 108 | 6.37 × 108 |
| 6 | 2.190 | 1.300 | 5.29 × 108 | 6.43 × 108 |
| 7 | 1.300 | 2.190 | 6.14 × 108 | 4.91 × 108 |
| 8 | 1.300 | 1.300 | 6.26 × 108 | 4.74 × 108 |
| 9 | 2.634 | 0.866 | 6.18 × 108 | 7.84 × 108 |
| 10 | 2.634 | 0.866 | 5.25 × 108 | 7.84 × 108 |
| Paib | m/z | N | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trichotoxin T5D2 1 | 1676 | Ac | Aib | Gly | Aib | Lxx | Aib | Gln | Aib | Aib | Ala | Ala | Ala | Aib | Pro | Lxx | Aib | Aib | Glu | Valol |
| Trichotoxin 1690 | 1691 | Ac | Aib | Gly | Aib | Lxx | Aib | Gln | Aib | Aib | Ala | Ala | Ala | Aib | Pro | Lxx | Aib | Vxx | Glu | Valol |
| Trichotoxin 1703A 3 | 1704 | Ac | Aib | Gly | Aib | Lxx | Aib | Gln | Aib | Aib | Aib | Ala | Ala | Aib | Pro | Lxx | Aib | Vxx | Gln | Valol |
| Trichotoxin A-40 2 | 1705 | Ac | Aib | Gly | Aib | Lxx | Aib | Gln | Aib | Aib | Aib | Ala | Aib | Aib | Pro | Lxx | Aib | Aib | Glu | Valol |
| Trichotoxin 1717A 3 | 1718 | Ac | Aib | Gly | Aib | Lxx | Aib | Gln | Aib | Aib | Aib | Ala | Aib | Aib | Pro | Lxx | Aib | Vxx | Gln | Valol |
| Trichotoxin A-50 G 1 | 1726 | Ac | Aib | Gly | Aib | Lxx | Aib | Gln | Aib | Aib | Aib | Ala | Ala | Aib | Pro | Lxx | Aib | Vxx | Gln | Valol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfaro-Vargas, P.; Bastos-Salas, A.; Muñoz-Arrieta, R.; Pereira-Reyes, R.; Redondo-Solano, M.; Fernández, J.; Mora-Villalobos, A.; López-Gómez, J.P. Peptaibol Production and Characterization from Trichoderma asperellum and Their Action as Biofungicide. J. Fungi 2022, 8, 1037. https://doi.org/10.3390/jof8101037
Alfaro-Vargas P, Bastos-Salas A, Muñoz-Arrieta R, Pereira-Reyes R, Redondo-Solano M, Fernández J, Mora-Villalobos A, López-Gómez JP. Peptaibol Production and Characterization from Trichoderma asperellum and Their Action as Biofungicide. Journal of Fungi. 2022; 8(10):1037. https://doi.org/10.3390/jof8101037
Chicago/Turabian StyleAlfaro-Vargas, Pamela, Alisson Bastos-Salas, Rodrigo Muñoz-Arrieta, Reinaldo Pereira-Reyes, Mauricio Redondo-Solano, Julián Fernández, Aníbal Mora-Villalobos, and José Pablo López-Gómez. 2022. "Peptaibol Production and Characterization from Trichoderma asperellum and Their Action as Biofungicide" Journal of Fungi 8, no. 10: 1037. https://doi.org/10.3390/jof8101037
APA StyleAlfaro-Vargas, P., Bastos-Salas, A., Muñoz-Arrieta, R., Pereira-Reyes, R., Redondo-Solano, M., Fernández, J., Mora-Villalobos, A., & López-Gómez, J. P. (2022). Peptaibol Production and Characterization from Trichoderma asperellum and Their Action as Biofungicide. Journal of Fungi, 8(10), 1037. https://doi.org/10.3390/jof8101037









