Exogenous Regulators Enhance the Yield and Stress Resistance of Chlamydospores of the Biocontrol Agent Trichoderma harzianum T4
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Media
2.2. Effects of Exogenous Regulators on the Production of Chlamydospores in T. harzianum T4
2.3. Survival Tests
2.4. Effects of Exogenous Regulators on Lipid Accumulation in the Chlamydospores of T. harzianum T4
2.5. Transmission Electron Microscopy (TEM)
2.6. Confocal Laser-Scanning Microscope
2.7. Effects of Exogenous Regulators on the Fatty Acid Types of the Chlamydospores of T. harzianum T4
2.8. qRT-PCR
2.9. Statistical Analysis
3. Results
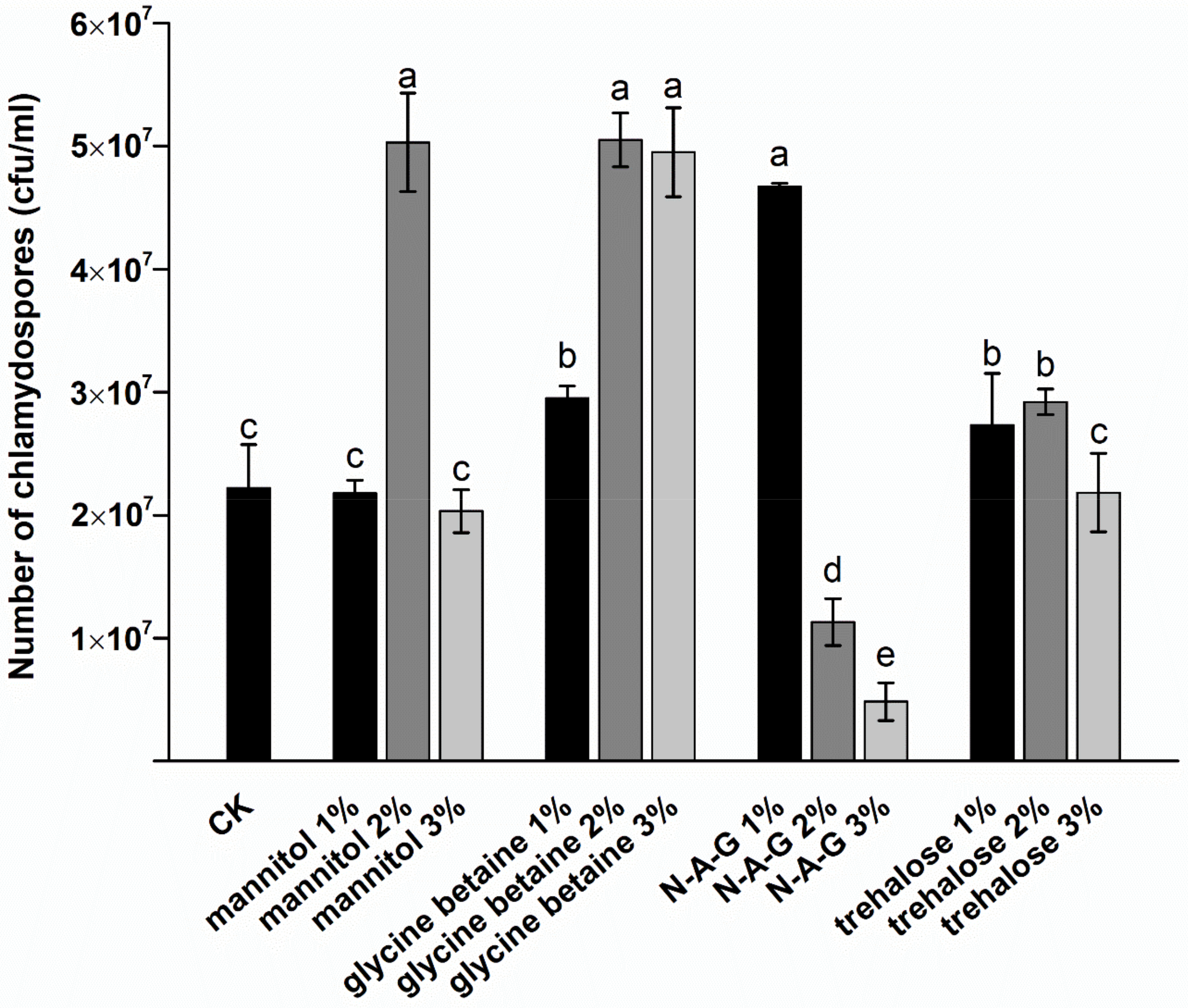
3.1. Induction of Chlamydospores by Exogenous Regulators
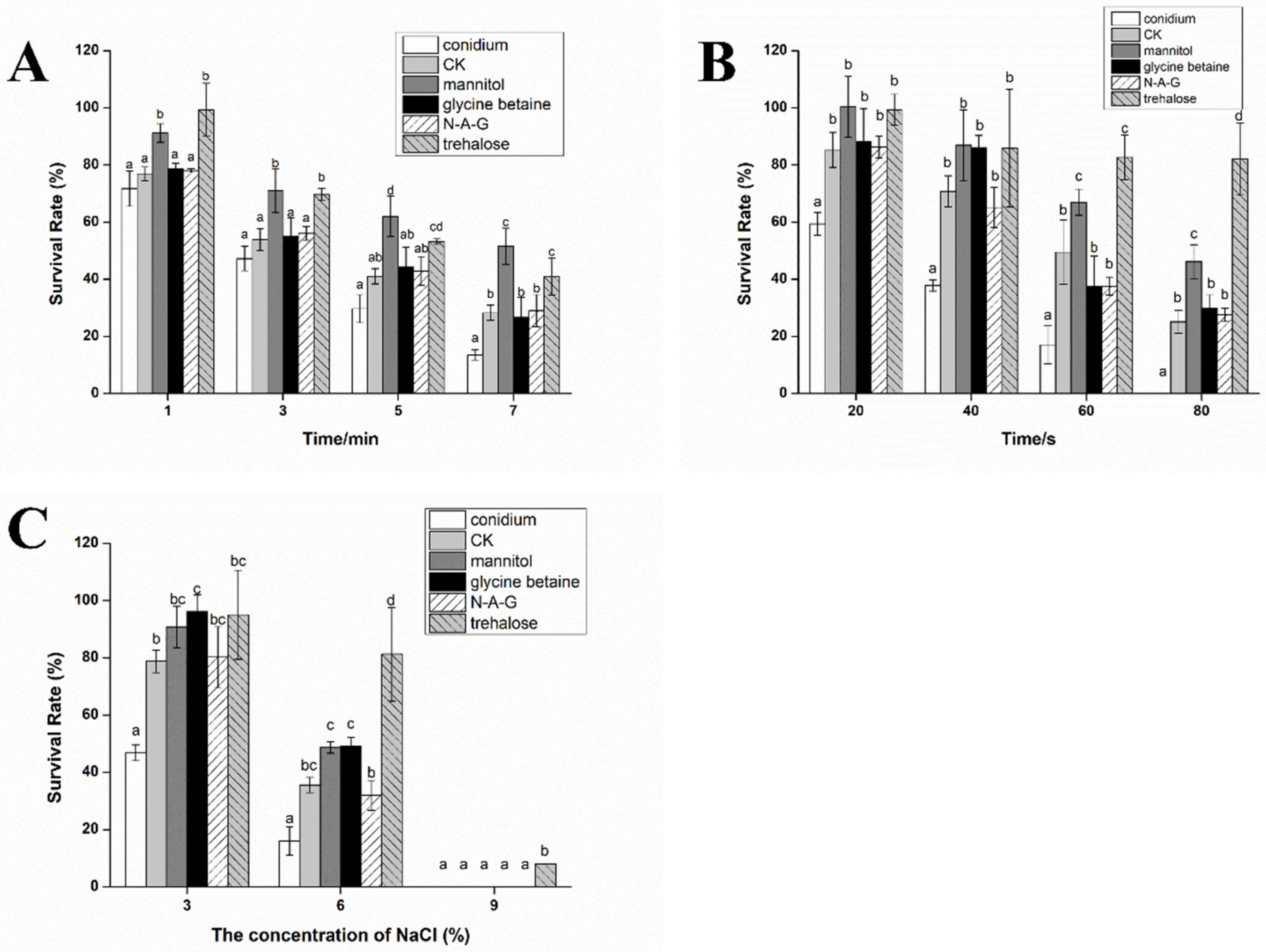
3.2. The Stress Resistance of Chlamydospores
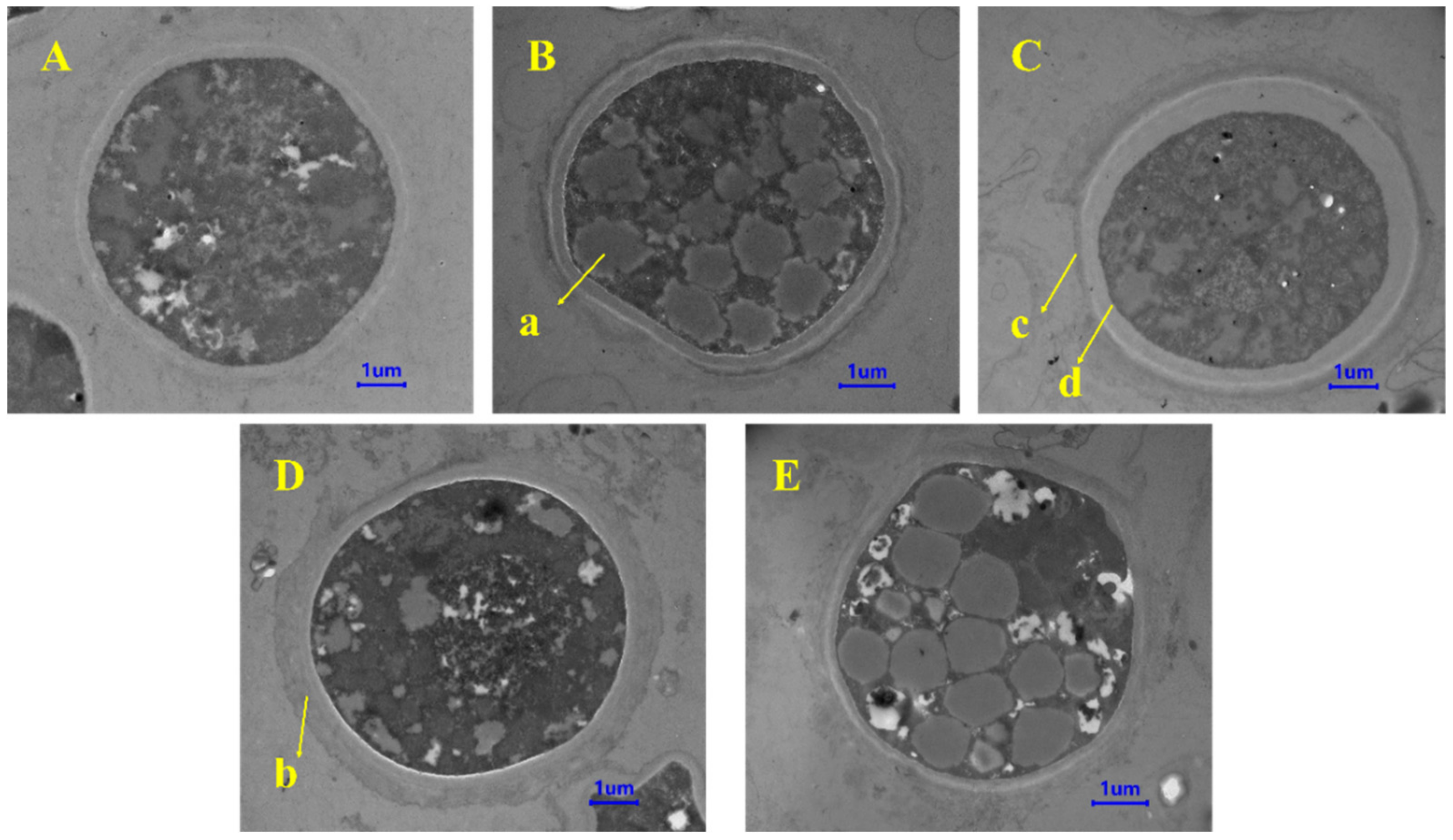
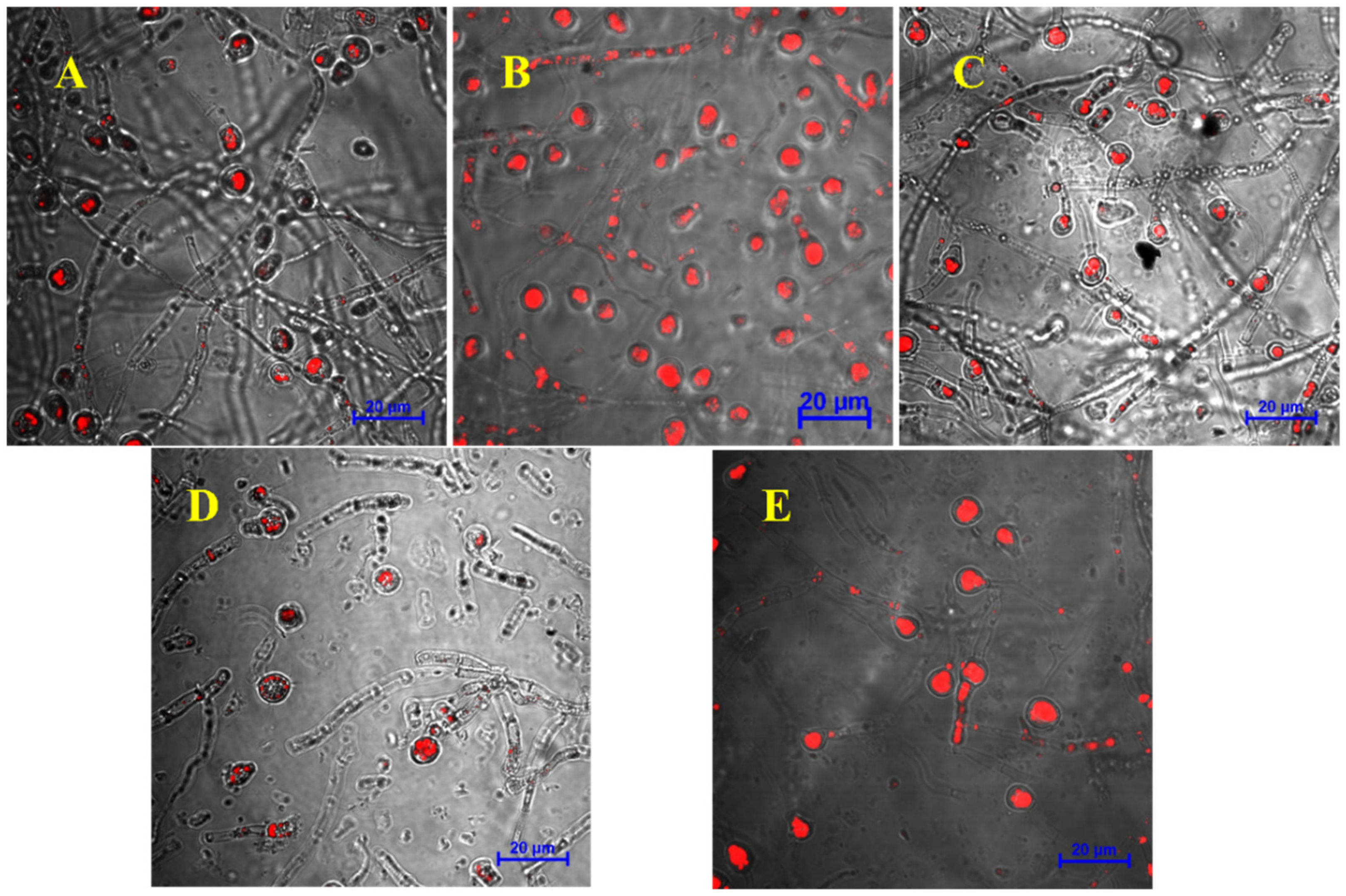
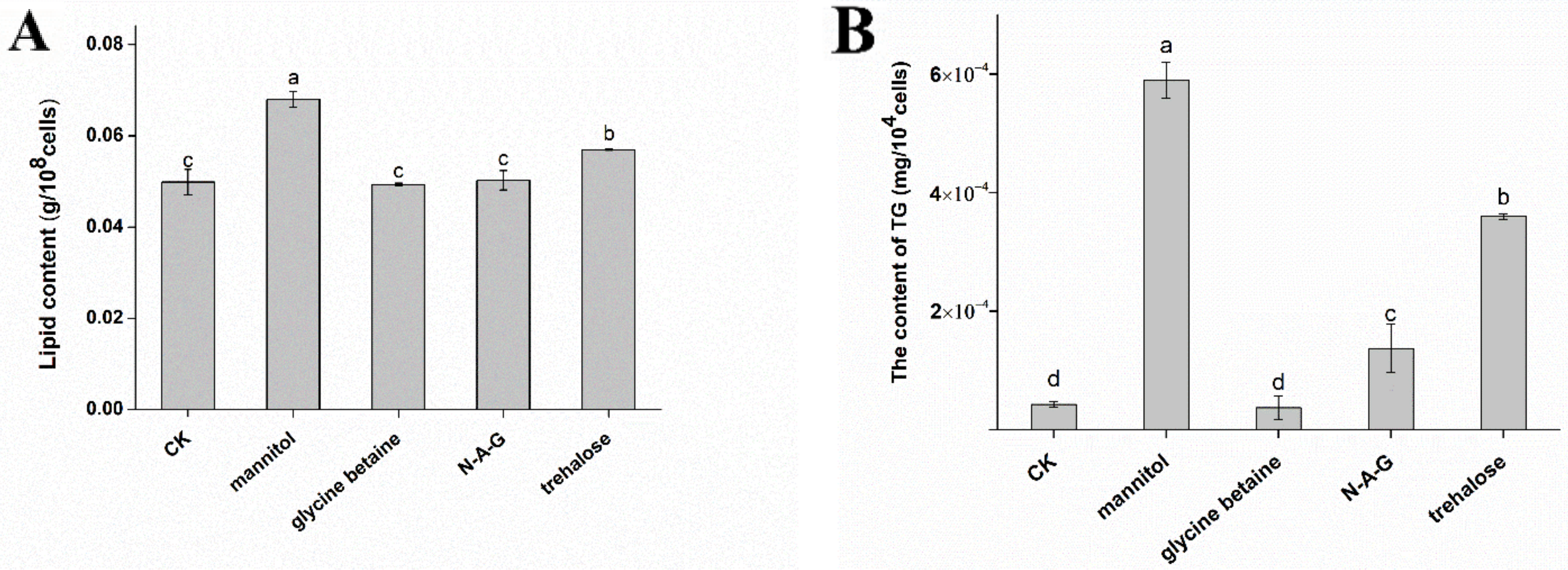
3.3. Effects of Exogenous Regulators on Lipid Accumulation in Chlamydospores of T. harzianum T4
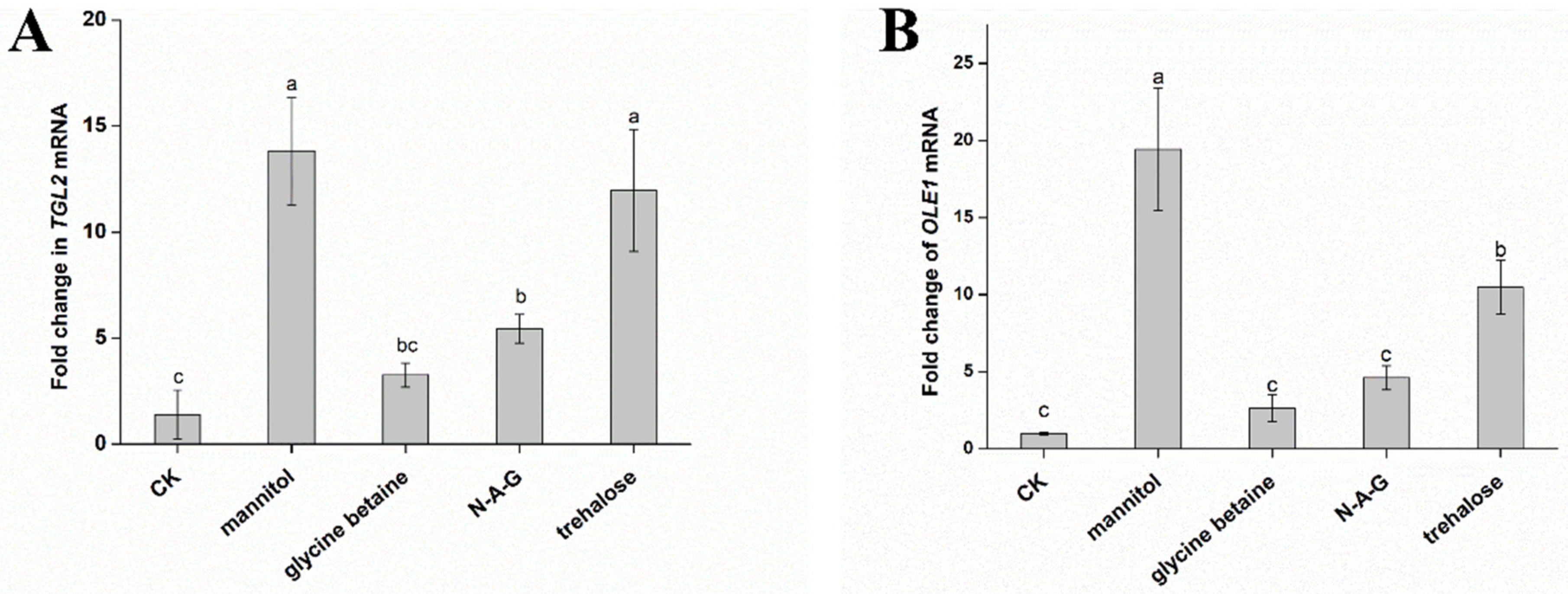
3.4. Effects of Exogenous Regulators on Fatty Acid Types of Chlamydospores of T. harzianum T4
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vinale, F.; Marra, R.; Scala, F.; Ghisalberti, E.L.; Sivasithamparam, K. Major secondary metabolites produced by two commercial Trichoderma strains active against different phytopathogens. Lett. Appl. Microbiol. 2006, 43, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Zeilinger, S.; Gruber, S.; Bansal, R.; Mukherjee, P.K. Secondary metabolism in Trichoderma—Chemistry meets genomics. Fungal Biol. Rev. 2016, 30, 74–90. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Rai, P.; Srivastava, A.K.; Kumar, S. Trichoderma for climate resilient agriculture. World J. Microbiol. Biotechnol. 2017, 33, 155. [Google Scholar] [CrossRef] [PubMed]
- Pei, W.L.; Wang, W. Preparation and Field Efficacy of Chlamydospore Water Dispersible Granule of Trichoderma harzianum. Chin. J. Biol. Control 2020, 36, 241–248. [Google Scholar]
- Fei, X.; Zhang, Y.; Xu, R.; Wang, W. Effect of Trichoderma harzianum T4 on Bacterial Community in Watermelon (Citrullus lanatus) Rhizosphere Soil. Chin. J. Biol. Control 2013, 29, 232–241. [Google Scholar]
- Yu, X.X.; Zhao, Y.T.; Cheng, J.; Wang, W. Biocontrol effect of Trichoderma harzianum T4 on brassica clubroot and analysis of rhizosphere microbial communities based on T-RFLP. Biocontrol Sci. Technol. 2015, 25, 1493–1505. [Google Scholar] [CrossRef]
- Dijksterhuis, J. Fungal spores: Highly variable and stress-resistant vehicles for distribution and spoilage. Food Microbiol. 2019, 81, 2–11. [Google Scholar] [CrossRef]
- Papavizas, G. Survival of Trichoderma harzianum in Soil and in Pea and Bean Rhizospheres. Phytopathology 1982, 72, 121–125. [Google Scholar] [CrossRef]
- Nyvall, R.F. Chlamydospores of Fusarium roseum ‘Graminearum’ as Survival Structures. Phytopathology 1970, 60, 1175. [Google Scholar] [CrossRef]
- Zamani, A.; Jeihanipour, A.; Edebo, L.; Niklasson, C.; Taherzadeh, M.J. Determination of Glucosamine and N-Acetyl Glucosamine in Fungal Cell Walls. J. Agric. Food Chem. 2008, 56, 8314–8318. [Google Scholar] [CrossRef]
- Liu, X.M.; Wu, X.L.; Gao, W.; Qu, J.B.; Chen, Q.; Huang, C.Y.; Zhang, J.X. Protective roles of trehalose in Pleurotus pulmonarius during heat stress response. J. Integr. Agric. 2019, 18, 428–437. [Google Scholar] [CrossRef]
- Magalhães, R.S.S.; Popova, B.; Braus, G.H.; Outeiro, T.F.; Eleutherio, E.C.A. The trehalose protective mechanism during thermal stress in Saccharomyces cerevisiae: The roles of Ath1 and Agt1. FEMS Yeast Res. 2018, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yu, C.; Zhao, Y.; Liu, S.; Wang, H.; Wang, C.; Guo, L.; Chen, M. Changes in Mannitol Content, Regulation of Genes Involved in Mannitol Metabolism, and the Protective Effect of Mannitol on Volvariella volvacea at Low Temperature. BioMed. Res. Int. 2019, 2019, 1493721. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Q.; Hao, G.; Liu, X.; Zhao, J.; Chen, Y.; Zhang, H.; Chen, W. The protective role of glycine betaine in Lactobacillus plantarum ST-III against salt stress. Food Control 2014, 44, 208–213. [Google Scholar] [CrossRef]
- Tang, D.; Wang, X.; Wang, J.; Wang, M.; Wang, Y.; Wang, W. Choline–betaine pathway contributes to hyperosmotic stress and subsequent lethal stress resistance in Pseudomonas protegens SN15-2. J. Biosci. 2020, 45, 85. [Google Scholar] [CrossRef]
- Jamialahmadi, K.; Soltani, F.; Nabavi Fard, M.; Behravan, J.; Mosaffa, F. Assessment of protective effects of glucosamine and N-acetyl glucosamine against DNA damage induced by hydrogen peroxide in human lymphocytes. Drug Chem. Toxicol. 2014, 37, 427–432. [Google Scholar] [CrossRef]
- Son, H.; Lee, J.; Lee, Y.W. Mannitol induces the conversion of conidia to chlamydospore-like structures that confer enhanced tolerance to heat, drought, and UV in Gibberella zeae. Microbiol. Res. 2012, 167, 608–615. [Google Scholar] [CrossRef]
- Silva-Udawatta, M.N.D.; Cannon, J.F. Roles of trehalose phosphate synthase in yeast glycogen metabolism and sporulation. Mol. Microbiol. 2001, 40, 1345–1356. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K. The cryoprotective effects of glycine betaine on bacteria. Trends Microbiol. 2002, 10, 311. [Google Scholar] [CrossRef]
- Wyatt, T.T.; Van Leeuwen, M.R.; Golovina, E.A.; Hoekstra, F.A.; Kuenstner, E.J.; Palumbo, E.A.; Snyder, N.L.; Cobus, V.; Alex, V.; Hallsworth, J.E. Functionality and prevalence of trehalose-based oligosaccharides as novel compatible solutes in ascospores of Neosartorya fischeri (Aspergillus fischeri) and other fungi. Environ. Microbiol. 2015, 17, 395–411. [Google Scholar] [CrossRef]
- Wang, R.Q.; Chen, G.; Chen, S.N.; Zhu, H.L.; Xiong, W.N.; Xu, M.; Jian, S.P. Metabolic changes of Neurospora crassa in the presence of oleic acid for promoting lycopene production. J. Biosci. Bioeng. 2021, 132, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wei, R.; Wang, L.; Lu, J.; Liu, H.; Zhang, W. Correlations of MMP-1, MMP-3, and MMP-12 with the degree of atherosclerosis, plaque stability and cardiovascular and cerebrovascular events. Exp. Ther. Med. 2017, 15, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- Adeyo, O.; Horn, P.J.; Lee, S.; Binns, D.D.; Chandrahas, A.; Chapman, K.D.; Goodman, J.M. The yeast lipin orthologue Pah1p is important for biogenesis of lipid droplets. J. Cell Biol. 2011, 192, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Li, M.; Yan, C.; Sun, J.; Peng, M.; Huang, Z.; Shi, P. Aspirin Causes Lipid Accumulation and Damage to Cell Membrane by Regulating DCI1/OLE1 in Saccharomyces cerevisiae. Microb. Drug Resist. 2020, 26, 857–868. [Google Scholar] [CrossRef]
- Choi, J.Y.; Stukey, J.; Hwang, S.Y.; Martin, C.E. Regulatory Elements That Control Transcription Activation and Unsaturated Fatty Acid-mediated Repression of the Saccharomyces cerevisiae OLE1 Gene. J. Biol. Chem. 1996, 271, 3581–3589. [Google Scholar] [CrossRef]
- Fang, Z.; Chen, Z.; Wang, S.; Shi, P.; Shen, Y.; Zhang, Y.; Xiao, J.; Huang, Z. Overexpression of OLE1 Enhances Cytoplasmic Membrane Stability and Confers Resistance to Cadmium in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2017, 83, e02319-16. [Google Scholar] [CrossRef]
- Patel, T.K.; Williamson, J.D. Mannitol in Plants, Fungi, and Plant–Fungal Interactions. Trends Plant Sci. 2016, 21, 486–497. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M.; Siddique, K.H.M. Role of Glycine Betaine in the Thermotolerance of Plants. Agronomy 2022, 12, 276. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Z.; Seleiman, M.F.; Rizwan, M.; YAVAŞ, İ.; Alhammad, B.A.; Shami, A.; Hasanuzzaman, M.; Kalderis, D. Glycine Betaine Accumulation, Significance and Interests for Heavy Metal Tolerance in Plants. Plants 2020, 9, 896. [Google Scholar] [CrossRef]
- Zhang, Y.; DeBosch, B.J. Microbial and metabolic impacts of trehalose and trehalose analogues. Gut Microbes 2020, 11, 1475–1482. [Google Scholar] [CrossRef]
- Caldas, T.; Demont-Caulet, N.; Ghazi, A.; Richarme, G. Thermoprotection by glycine betaine and choline. Microbiology 1999, 145, 2543–2548. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, G.L.; Chi, Z.; Hu, Z.; Chi, Z.M. Genetics of trehalose biosynthesis in desert-derived Aureobasidium melanogenum and role of trehalose in the adaptation of the yeast to extreme environments. Curr. Genet. 2018, 64, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Strippoli, V.; Simonetti, N. Specific induction of chlamydospore formation in Candida albicans by N-acetyl-d-glucosamine. Experientia 1975, 31, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, N.; Strippoli, V.; Cassone, A. Yeast-mycelial conversion induced by N-acetyl-D-glucosamine in Candida albicans. Nature 1974, 250, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Hrmová, M.; Drobnica, L. Induction of mycelial type of development in Candida albicans by the antibiotic monorden and N-acetyl-D-glucosamine. Mycopathologia 1982, 79, 55–64. [Google Scholar] [CrossRef]
- Torosantucci, A.; Angiolella, L.; Filesi, C.; Cassone, A. Protein Synthesis and Amino Acid Pool during Yeast-Mycelial Transition Induced by N-Acetyl-D-glucosamine in Candida albicans. Microbiology 1984, 130, 3285–3293. [Google Scholar] [CrossRef]
- Kimura, Y.; Kawasaki, S.; Yoshimoto, H.; Takegawa, K. Glycine Betaine Biosynthesized from Glycine Provides an Osmolyte for Cell Growth and Spore Germination during Osmotic Stress in Myxococcus xanthus. J. Bacteriol. 2010, 192, 1467–1470. [Google Scholar] [CrossRef]
- Huang, Q.C.; Xu, Z.R.; Han, X.Y.; Li, W.F. Changes in hormones, growth factor and lipid metabolism in finishing pigs fed betaine. Livest. Sci. 2006, 105, 78–85. [Google Scholar] [CrossRef]
- Boysen, A.K.; Durham, B.P.; Kumler, W.; Key, R.S.; Heal, K.R.; Carlson, L.T.; Groussman, R.D.; Armbrust, E.V.; Ingalls, A.E. Glycine betaine uptake and metabolism in marine microbial communities. Environ. Microbiol. 2022, 24, 2380–2403. [Google Scholar] [CrossRef]
- You, K.M.; Rosenfield, C.L.; Knipple, D.C. Ethanol Tolerance in the Yeast Saccharomyces cerevisiae Is Dependent on Cellular Oleic Acid Content. Appl. Environ. Microbiol. 2003, 69, 1499–1503. [Google Scholar] [CrossRef]
- Dulermo, T.; Lazar, Z.; Dulermo, R.; Rakicka, M.; Haddouche, R.; Nicaud, J.M. Analysis of ATP-citrate lyase and malic enzyme mutants of Yarrowia lipolytica points out the importance of mannitol metabolism in fatty acid synthesis. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Nakata, S.; Hio, M.; Takase, R.; Kawai, S.; Watanabe, D.; Hashimoto, W. Polyunsaturated fatty acids-enriched lipid from reduced sugar alcohol mannitol by marine yeast Rhodosporidiobolus fluvialis Y2. Biochem. Biophys. Res. Commun. 2020, 526, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Kempf, B.; Bremer, E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 1998, 170, 319–330. [Google Scholar] [CrossRef]
- Wang, X.; Tang, D.; Wang, W. Hyperosmotic Adaptation of Pseudomonas protegens SN15-2 Helps Cells to Survive at Lethal Temperatures. Biotechnol. Bioprocess Eng. 2020, 25, 403–413. [Google Scholar] [CrossRef]
- Crowe, L.M. Lessons from nature: The role of sugars in anhydrobiosis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 131, 505–513. [Google Scholar] [CrossRef]
- Crowe, J.H.; Leslie, S.B.; Crowe, L.M. Is Vitrification Sufficient to Preserve Liposomes during Freeze-Drying? Cryobiology 1994, 31, 355–366. [Google Scholar] [CrossRef]
- Li, S.; Yue, Q.; Zhou, S.; Yan, J.; Zhang, X.; Ma, F. Trehalose Contributes to Gamma-Linolenic Acid Accumulation in Cunninghamella echinulata Based on de Novo Transcriptomic and Lipidomic Analyses. Front. Microbiol. 2018, 9, 1296. [Google Scholar] [CrossRef]
- Ianutsevich, E.A.; Tereshina, V.M. Combinatorial impact of osmotic and heat shocks on the composition of membrane lipids and osmolytes in Aspergillus niger. Microbiology 2019, 165, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Nobusawa, T.; Yamakawa-Ayukawa, K.; Saito, F.; Nomura, S.; Takami, A.; Ohta, H. A homolog of Arabidopsis SDP1 lipase in Nannochloropsis is involved in degradation of de novo-synthesized triacylglycerols in the endoplasmic reticulum. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2019, 1864, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Ham, H.J.; Rho, H.J.; Shin, S.K.; Yoon, H.J. The TGL2 Gene of Saccharomyces cerevisiae Encodes an Active Acylglycerol Lipase Located in the Mitochondria *. J. Biol. Chem. 2010, 285, 3005–3013. [Google Scholar] [CrossRef]
- Fujimori, K.; Anamnart, S.; Nakagawa, Y.; Sugioka, S.; Ohta, D.; Oshima, Y.; Yamada, Y.; Harashima, S. Isolation and characterization of mutations affecting expression of the Δ9-fatty acid desaturase gene, OLE1, in Saccharomyces cerevisiae. FEBS Lett. 1997, 413, 226–230. [Google Scholar] [CrossRef]
- Li, P.; Fu, X.; Zhang, L.; Li, S. CRISPR/Cas-based screening of a gene activation library in Saccharomyces cerevisiae identifies a crucial role of OLE1 in thermotolerance. Microb. Biotechnol. 2019, 12, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wu, B.; Zhang, S.; Li, M.; Jiang, X. Transcriptome Dynamics Underlying Chlamydospore Formation in Trichoderma virens GV29-8. Front. Microbiol. 2021, 12, 1340. [Google Scholar] [CrossRef] [PubMed]
| Primer Name | Sequence (5′–3′) |
|---|---|
| TGL2 | CCGCCGTCAACATCATCG |
| ACCAGGTAATCCGCAAAGC | |
| OLE1 | ATGTCCGAACCCACAGCCTC |
| TTAAGCAGCGTCGGCGCTG | |
| 18SrRNA | GCAACGAGTAAAGCACCAGA |
| GGGGTTCATTCGTGTGTAGC |
| Fatty Acid Type | Number of Carbon Atoms | Control (%) | Mannitol (%) | Glycine Betaine (%) | N-A-G (%) | Trehalose (%) | |
|---|---|---|---|---|---|---|---|
| 1 | Palmitic acid | C16:0 | 39.049 ± 1.296 a | 19.577 ± 0.634 c | 33.497 ± 0.766 b | 33.039 ± 0.819 b | 21.498 ± 1.885 c |
| 2 | Linoleic acid | C18:2 | 9.171 ± 0.293 c | 35.419 ± 0.857 a | 14.187 ± 1.138 b | 10.037 ± 0.969 c | 34.771 ± 0.117 a |
| 3 | Oleic acid | C18:1 | 32.246 ± 1.949 c | 38.309 ± 1.165 ab | 36.717 ± 1.504 b | 40.081 ± 1.513 a | 36.561 ± 2.183 b |
| 4 | Stearic acid | C18:0 | 19.533 ± 0.959 a | 6.695 ± 0.25 c | 15.599 ± 0.198 b | 16.81 ± 0.252 b | 7.17 ± 0.397 c |
| 5 | Unsaturated index | 0.709 ± 0.066 d | 2.809 ± 0.124 a | 1.039 ± 0.084 c | 1.006 ± 0.03672 c | 2.502 ± 0.266 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Wang, Y.; Wang, X.; Wang, W. Exogenous Regulators Enhance the Yield and Stress Resistance of Chlamydospores of the Biocontrol Agent Trichoderma harzianum T4. J. Fungi 2022, 8, 1017. https://doi.org/10.3390/jof8101017
Zhu X, Wang Y, Wang X, Wang W. Exogenous Regulators Enhance the Yield and Stress Resistance of Chlamydospores of the Biocontrol Agent Trichoderma harzianum T4. Journal of Fungi. 2022; 8(10):1017. https://doi.org/10.3390/jof8101017
Chicago/Turabian StyleZhu, Xiaochong, Yaping Wang, Xiaobing Wang, and Wei Wang. 2022. "Exogenous Regulators Enhance the Yield and Stress Resistance of Chlamydospores of the Biocontrol Agent Trichoderma harzianum T4" Journal of Fungi 8, no. 10: 1017. https://doi.org/10.3390/jof8101017
APA StyleZhu, X., Wang, Y., Wang, X., & Wang, W. (2022). Exogenous Regulators Enhance the Yield and Stress Resistance of Chlamydospores of the Biocontrol Agent Trichoderma harzianum T4. Journal of Fungi, 8(10), 1017. https://doi.org/10.3390/jof8101017






