Functional Characterization of Melanin Decolorizing Extracellular Peroxidase of Bjerkandera adusta
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fungal Cell Cultivation
2.3. Crude Enzyme Preparation
2.4. Activity Assays
2.5. Effects of pH and Temperature
2.6. Stability Tests
3. Results
3.1. Preparation of the Peroxidase Solution from B. adusta Mycelial Cultures
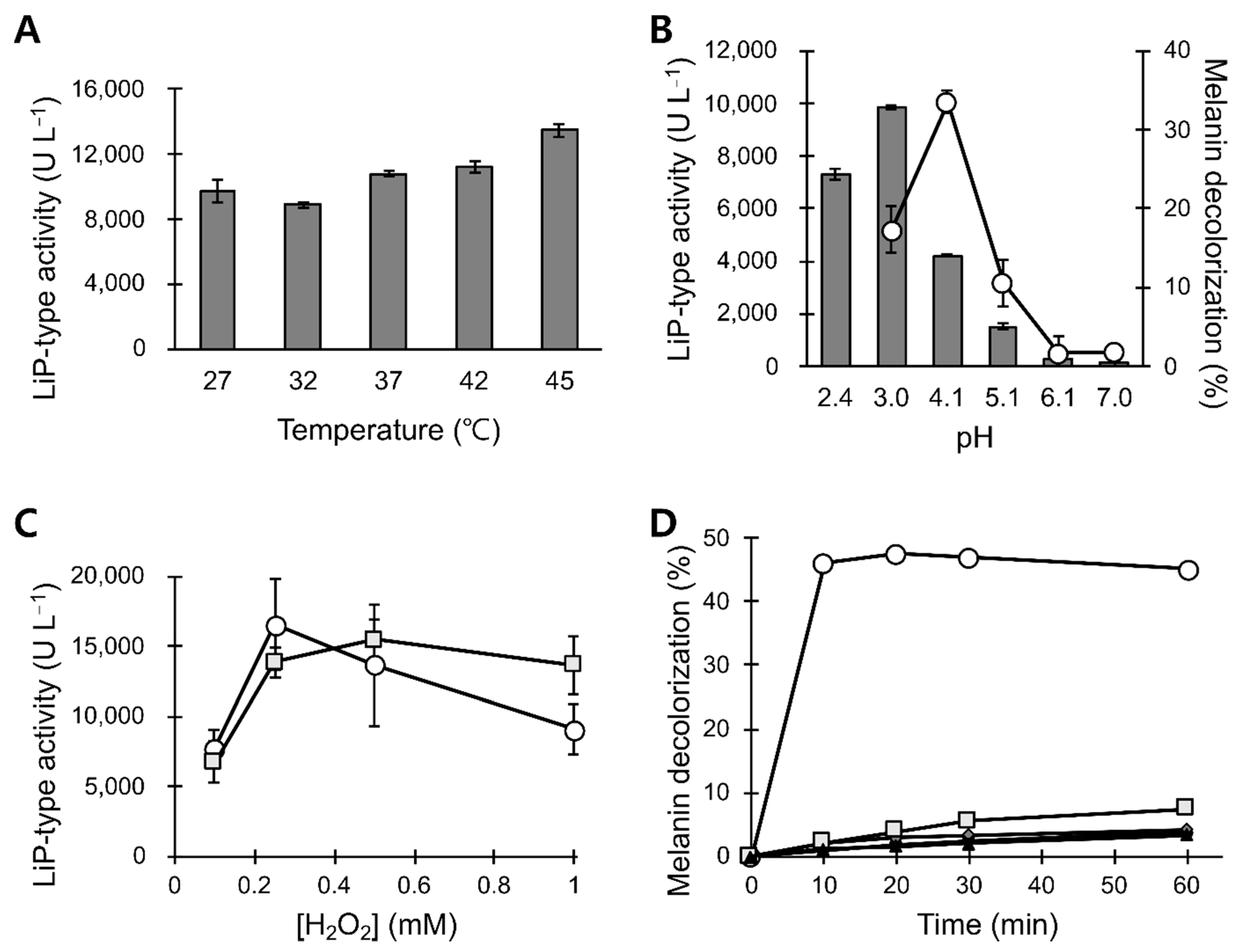
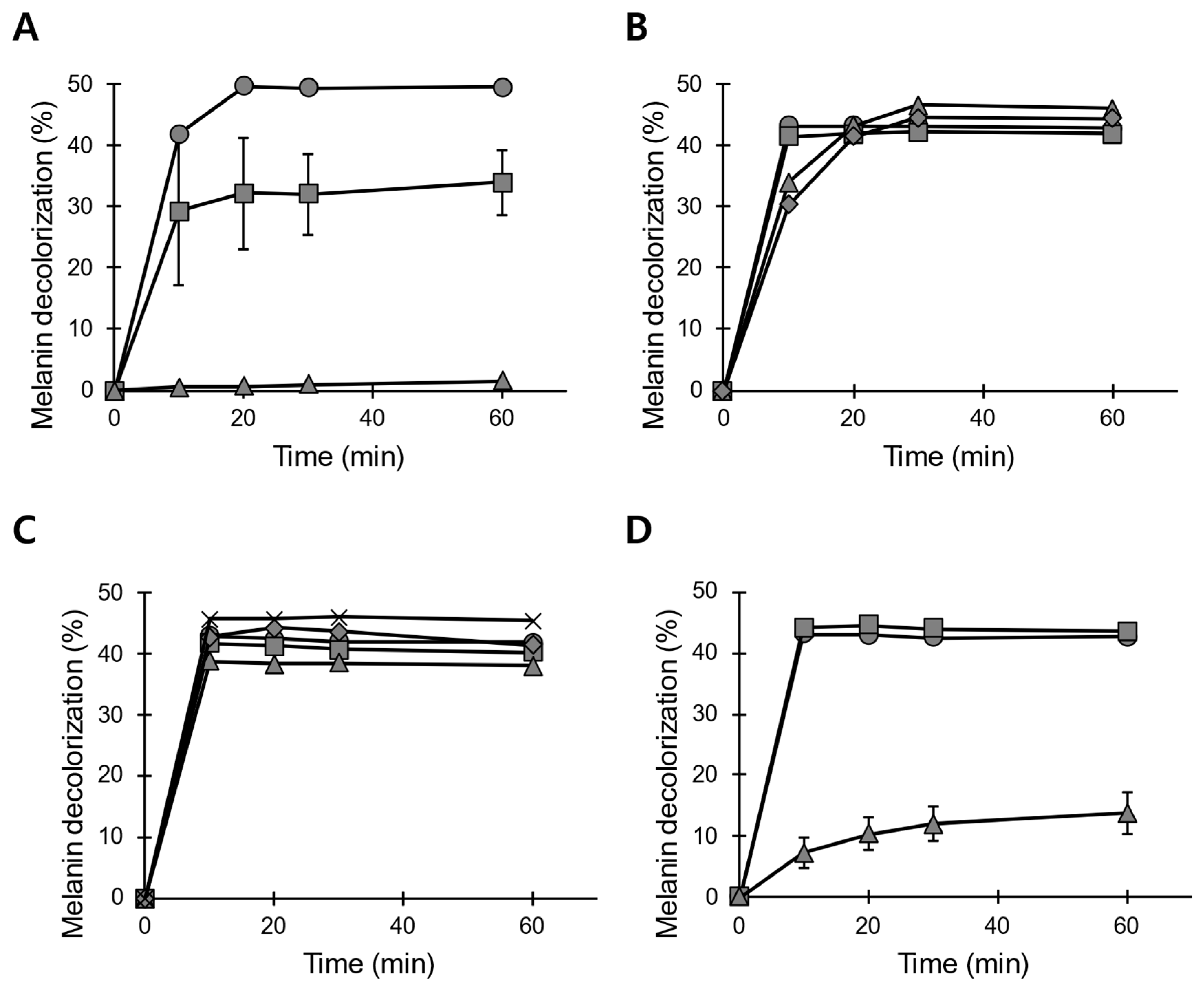
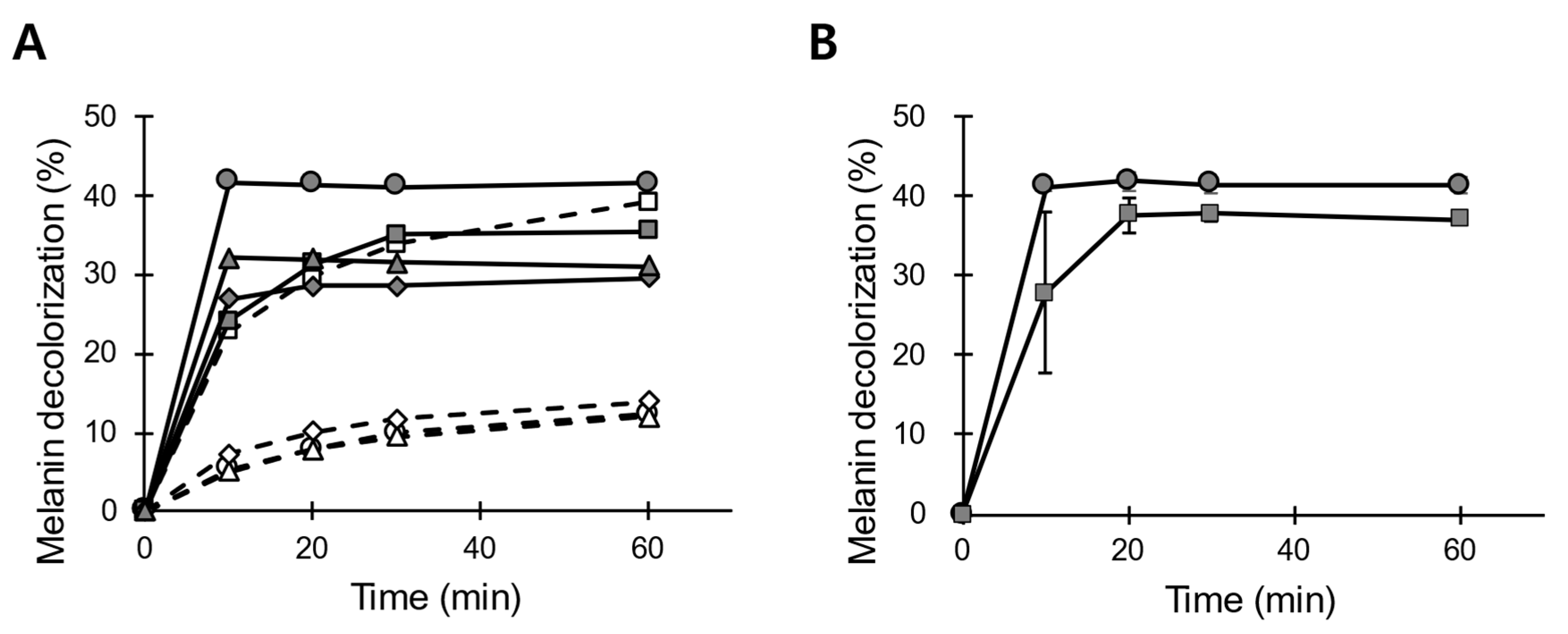
3.2. LiP-Type Activity in POX_Ba Is Likely from VP
3.3. pH and Temperature Stability of POX_Ba
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vashi, N.; Kundu, R. Facial hyperpigmentation: Causes and treatment. Br. J. Dermatol. 2013, 169, 41–56. [Google Scholar] [CrossRef]
- Singh, B.K.; Kim, E.-K. P-Protein: A Novel Target for Skin-whitening Agent. Biotechnol. Bioprocess Eng. 2019, 24, 76–84. [Google Scholar] [CrossRef]
- Joly-Tonetti, N.; Wibawa, J.I.D.; Bell, M.; Tobin, D. Melanin fate in the human epidermis: A reassessment of how best to detect and analyse histologically. Exp. Dermatol. 2016, 25, 501–504. [Google Scholar] [CrossRef] [Green Version]
- Saade, D.; Maymone, M.; De La Garza, H.; Secemsky, E.; Kennedy, K.; Vashi, N. Trends in Use of Prescription Skin Lightening Creams. Int. J. Environ. Res. Public Health 2021, 18, 5650. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, J.; Chazarra, S.; Garcia-Carmona, F. Kojic Acid, a Cosmetic Skin Whitening Agent, is a Slow-binding Inhibitor of Catecholase Activity of Tyrosinase. J. Pharm. Pharmacol. 2011, 46, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Dooley, T. Topical skin depigmentation agents. J. Dermatol. Treat. 1997, 8, 275–283. [Google Scholar] [CrossRef]
- Sugimoto, K.; Nishimura, T.; Nomura, K.; Sugimoto, K.; Kuriki, T. Inhibitory Effects of α-Arbutin on Melanin Synthesis in Cultured Human Melanoma Cells and a Three-Dimensional Human Skin Model. Biol. Pharm. Bull. 2004, 27, 510–514. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Park, H.Y.; Jung, K.H.; Kim, D.H.; Rho, H.S.; Choi, K. Anti-melanogenic Effects of Kojic Acid and Hydroxycinnamic Acid Derivatives. Biotechnol. Bioprocess Eng. 2020, 25, 190–196. [Google Scholar] [CrossRef]
- Seiberg, M.; Paine, C.; Sharlow, E.; Eisinger, M.; Shapiro, S.S.; Andrade-Gordon, P.; Costanzo, M. Inhibition of Melanosome Transfer Results in Skin Lightening1. J. Investig. Dermatol. 2000, 115, 162–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.I.; Lee, H.Y.; Lee, J.E.; Myung, C.H.; Hwang, J.S. Inhibitory effect of 2-methyl-naphtho[1,2,3-de]quinolin-8-one on melanosome transport and skin pigmentation. Sci. Rep. 2016, 6, 29189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimbow, K.; Obata, H.; Pathak, M.A.; Fitzpatrick, T.B. Mechanism of Depigmentation by Hydroquinone. J. Investig. Dermatol. 1974, 62, 436–449. [Google Scholar] [CrossRef] [Green Version]
- Mohorčič, M.; Friedrich, J.; Renimel, I.; André, P.; Mandin, D.; Chaumont, J.-P. Production of melanin bleaching enzyme of fungal origin and its application in cosmetics. Biotechnol. Bioprocess Eng. 2007, 12, 200–206. [Google Scholar] [CrossRef]
- Nagasaki, K.; Kumazawa, M.; Murakami, S.; Takenaka, S.; Koike, K.; Aoki, K. Purification, Characterization, and Gene Cloning of Ceriporiopsis sp. Strain MD-1 Peroxidases That Decolorize Human Hair Melanin. Appl. Environ. Microbiol. 2008, 74, 5106–5112. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.; Kim, Y.-H.; Ahn, J.-Y.; Lee, H.-C.; Oh, S.-J.; Chung, B.-W.; Min, J. Melanin reduction by peroxidase activity in lysosome-related organelle extracts from hen egg whites, HeLa cells, and Saccharomyces cerevisiae. Mol. Cell. Toxicol. 2015, 11, 441–447. [Google Scholar] [CrossRef]
- Kim, B.S.; Blaghen, M.; Hong, H.; Lee, K. Purification and characterization of a melanin biodegradation enzyme from Geotrichum sp. Int. J. Cosmet. Sci. 2016, 38, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.J.; Khan, M.F.; Kim, Y.H. Recombinant lignin peroxidase-catalyzed decolorization of melanin using in-situ generated H2O2 for application in whitening cosmetics. Int. J. Biol. Macromol. 2019, 136, 20–26. [Google Scholar] [CrossRef]
- Sadaqat, B.; Khatoon, N.; Malik, A.Y.; Jamal, A.; Farooq, U.; Ali, M.I.; He, H.; Liu, F.-J.; Guo, H.; Urynowicz, M.; et al. Enzymatic decolorization of melanin by lignin peroxidase from Phanerochaete chrysosporium. Sci. Rep. 2020, 10, 20240. [Google Scholar] [CrossRef] [PubMed]
- Jeon, G.; Kim, C.; Cho, U.M.; Hwang, E.T.; Hwang, H.S.; Min, J. Melanin-Decolorizing Activity of Antioxidant Enzymes, Glutathione Peroxidase, Thiol Peroxidase, and Catalase. Mol. Biotechnol. 2021, 63, 150–155. [Google Scholar] [CrossRef]
- Mauricio, T.; Karmon, Y.; Khaiat, A. A randomized and placebo-controlled study to compare the skin-lightening efficacy and safety of lignin peroxidase cream vs. 2% hydroquinone cream. J. Cosmet. Dermatol. 2011, 10, 253–259. [Google Scholar] [CrossRef]
- Zhong, S.-M.; Sun, N.; Liu, H.-X.; Niu, Y.; Wu, Y. Reduction of facial pigmentation of melasma by topical lignin peroxidase: A novel fast-acting skin-lightening agent. Exp. Ther. Med. 2015, 9, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.H.; Cho, J.S.; Lee, B.S.; Kim, E.K. Decolorization of melanin by lignin peroxidase from Phanerochaete chrysosporium. Biotechnol. Bioprocess Eng. 2004, 9, 256–260. [Google Scholar] [CrossRef]
- Rothschild, N.; Levkowitz, A.; Hadar, Y.; Dosoretz, C.G. Manganese Deficiency Can Replace High Oxygen Levels Needed for Lignin Peroxidase Formation by Phanerochaete chrysosporium. Appl. Environ. Microbiol. 1999, 65, 483–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Sungusia, M.G.; Sawada, T.; Kuwahara, M. Lignin-degrading enzyme production by Bjerkandera adusta immobilized on polyurethane foam. J. Biosci. Bioeng. 1999, 88, 41–47. [Google Scholar] [CrossRef]
- Heinfling, A.; Martínez, M.J.; Martínez, A.T.; Bergbauer, M.; Szewzyk, U. Purification and characterization of peroxidases from the dye-decolorizing fungus Bjerkandera adusta. FEMS Microbiol. Lett. 1998, 165, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Linke, D.; Leonhardt, R.; Eisele, N.; Petersen, L.M.; Riemer, S.; Nimtz, M.; Berger, R.G. Carotene-degrading activities from Bjerkandera adusta possess an application in detergent industries. Bioprocess Biosyst. Eng. 2015, 38, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Bouacem, K.; Rekik, H.; Jaouadi, N.Z.; Zenati, B.; Kourdali, S.; El Hattab, M.; Badis, A.; Annane, R.; Bejar, S.; Hacene, H.; et al. Purification and characterization of two novel peroxidases from the dye-decolorizing fungus Bjerkandera adusta strain CX-9. Int. J. Biol. Macromol. 2018, 106, 636–646. [Google Scholar] [CrossRef]
- Mester, T.; Field, J.A. Characterization of a Novel Manganese Peroxidase-Lignin Peroxidase Hybrid Isozyme Produced by Bjerkandera Species Strain BOS55 in the Absence of Manganese. J. Biol. Chem. 1998, 273, 15412–15417. [Google Scholar] [CrossRef] [Green Version]
- Ertan, H.; Siddiqui, K.S.; Muenchhoff, J.; Charlton, T.; Cavicchioli, R. Kinetic and thermodynamic characterization of the functional properties of a hybrid versatile peroxidase using isothermal titration calorimetry: Insight into manganese peroxidase activation and lignin peroxidase inhibition. Biochimie 2012, 94, 1221–1231. [Google Scholar] [CrossRef]
- Tuisel, H.; Sinclair, R.; Bumpus, J.; Ashbaugh, W.; Brock, B.J.; Aust, S.D. Lignin peroxidase H2 from Phanerochaete chrysosporium: Purification, characterization and stability to temperature and pH. Arch. Biochem. Biophys. 1990, 279, 158–166. [Google Scholar] [CrossRef]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef]
- Nie, G.; Aust, S.D. Effect of Calcium on the Reversible Thermal Inactivation of Lignin Peroxidase. Arch. Biochem. Biophys. 1997, 337, 225–231. [Google Scholar] [CrossRef]
- Verdín, J.; Pogni, R.; Baeza, A.; Baratto, M.C.; Basosi, R.; Vázquez-Duhalt, R. Mechanism of versatile peroxidase inactivation by Ca2+ depletion. Biophys. Chem. 2006, 121, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.J.; Field, J.A.; Teunissen, P.; Dobson, A.D. Stabilization of lignin peroxidases in white rot fungi by tryptophan. Appl. Environ. Microbiol. 1997, 63, 2543–2548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnarme, P.; Jeffries, T.W. Mn(II) Regulation of Lignin Peroxidases and Manganese-Dependent Peroxidases from Lignin-Degrading White Rot Fungi. Appl. Environ. Microbiol. 1990, 56, 210–217. [Google Scholar] [CrossRef] [Green Version]
- A Brown, J.; Alic, M.; Gold, M.H. Manganese peroxidase gene transcription in Phanerochaete chrysosporium: Activation by manganese. J. Bacteriol. 1991, 173, 4101–4106. [Google Scholar] [CrossRef] [Green Version]
- Périé, F.H.; Gold, M.H. Manganese regulation of manganese peroxidase expression and lignin degradation by the white rot fungus Dichomitus squalens. Appl. Environ. Microbiol. 1991, 57, 2240–2245. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baik, J.; Purkayastha, A.; Park, K.H.; Kang, T.J. Functional Characterization of Melanin Decolorizing Extracellular Peroxidase of Bjerkandera adusta. J. Fungi 2021, 7, 762. https://doi.org/10.3390/jof7090762
Baik J, Purkayastha A, Park KH, Kang TJ. Functional Characterization of Melanin Decolorizing Extracellular Peroxidase of Bjerkandera adusta. Journal of Fungi. 2021; 7(9):762. https://doi.org/10.3390/jof7090762
Chicago/Turabian StyleBaik, Jina, Anwesha Purkayastha, Kyung Hye Park, and Taek Jin Kang. 2021. "Functional Characterization of Melanin Decolorizing Extracellular Peroxidase of Bjerkandera adusta" Journal of Fungi 7, no. 9: 762. https://doi.org/10.3390/jof7090762
APA StyleBaik, J., Purkayastha, A., Park, K. H., & Kang, T. J. (2021). Functional Characterization of Melanin Decolorizing Extracellular Peroxidase of Bjerkandera adusta. Journal of Fungi, 7(9), 762. https://doi.org/10.3390/jof7090762




