Abstract
Patients with severe COVID-19, such as individuals in intensive care units (ICU), are exceptionally susceptible to bacterial and fungal infections. The most prevalent fungal infections are aspergillosis and candidemia. Nonetheless, other fungal species (for instance, Histoplasma spp., Rhizopus spp., Mucor spp., Cryptococcus spp.) have recently been increasingly linked to opportunistic fungal diseases in COVID-19 patients. These fungal co-infections are described with rising incidence, severe illness, and death that is associated with host immune response. Awareness of the high risks of the occurrence of fungal co-infections is crucial to downgrade any arrear in diagnosis and treatment to support the prevention of severe illness and death directly related to these infections. This review analyses the fungal infections, treatments, outcome, and immune response, considering the possible role of the microbiome in these patients. The search was performed in Medline (PubMed), using the words “fungal infections COVID-19”, between 2020–2021.
Keywords:
fungal infection; COVID-19; SARS-CoV-2; immune response; Candida; Aspergillus; Mucor; immune response; microbiome 1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiologic agent of coronavirus disease 2019 (COVID-19), has infected millions of patients worldwide, and placed an unprecedented stress on healthcare systems [1,2,3,4]. This disease has predisposed a relatively high number of patients to acute respiratory distress syndrome, and co-infections are a frequent complication [5,6], especially with prolonged hospital stays [7]. Changes in humans’ microbiota have been recently observed in COVID-19 patients [1], with patients often being colonized or infected by microorganisms responsible for secondary infections (co-infections or superinfections), often caused by bacteria and fungal pathogens [5,7,8,9]. Indeed, several opportunistic infections following severe respiratory viral infections have been recognized in COVID-19 patients [2]—particularly, a higher incidence of fungal co-infections (Figure 1) [10,11,12]. For example, in Spain, the incidence of candidemia cases was higher in the first and second waves and lower during the third wave, also with a prevalence of invasive pulmonary aspergillosis (IPA) cases [11]. Moreover, the coronavirus-associated pulmonary aspergillosis (CAPA) showed to affect up to 30% of ventilated patients with COVID-19 admitted in intensive care units (ICU) [13], and, in a hospital in Pisa (Italy), 21.9% of 315 hospitalized patients with COVID-19 had a superinfection [14].
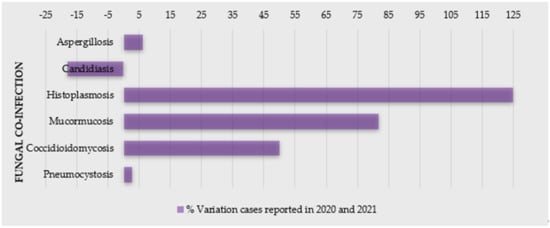
Figure 1.
Percentage of variation of cases of COVID-19 patients with fungal co-infections reported in 2020 and 2021 (source: PubMed).
The main pathogens related to co-infections are reported to be Enterobacterales (44.9%), non-fermenting Gram-negative bacilli (15.6%), Gram-positive bacteria (15.6%), and fungi (5.5%) [14]. In COVID-19 patients, the most fungi related to co-infections are Aspergillus spp., Candida albicans, Candida glabrata, Candida dubliniensis, Candida parapsilosis sensu stricto, Candida tropicalis, and Candida krusei (Pichia kudriavzevii) [8]. Moreover, these cases have been indicated as mainly primary and catheter-related infections [15].
There is still lack of information regarding the long-term impact of secondary infections on the outcome of hospitalized COVID-19 patients [9,16]. Patients with co-infection undergoing invasive mechanical ventilation showed to be 3.8 times more likely to die than those without positive cultures [9]. In order to perform an efficient treatment and reduce mortality, it is important to make an accurate early identification [12]; however, these co-infections raise difficulties on diagnosis, treatment (including broad-spectrum antimicrobial drugs, mechanical ventilation, extracorporeal membrane oxygenation), prognosis, and even increase the disease the symptoms and mortality of COVID-19 [8,12,15,17,18,19].
The repercussions of SARS-CoV-2 infections on future global antimicrobial resistance must be explored profoundly [3,16]. In Valencia (Spain), the antifungal consumption increased in 2020 compared to previous year, especially echinocandins, voriconazole, and isavuconazole [11]. Considering that the antimicrobials drugs for COVID-19 patients, both on and during admission, are almost all prescribed uncertainly in clinical settings, there is expected an increase in drug-resistant infections [3].
Lastly, considering the immune response, there has been represented a host dysregulation triggered by SARS-CoV-2 infection, which has been hypothesized as a causal pathway for the increasingly reported mainly fungal (oral) manifestations associated with COVID-19 [20,21]. Additionally, the alteration in human microbiota (due to SARS-CoV-2 infection), which can also indicate the progression of COVID-19, may contribute to bacterial, fungal, or viral infections and affect the immune system [1]. In these patients, this is normally described as an increase in pro-inflammatory markers, such as IL-1, IL-6, and tumor necrosis alpha (TNF-α), less CD4 interferon-gamma expression, and a decreased number of CD4 and CD8 cells, which increase susceptibility to bacterial and fungal infections [12].
The present review aims to analyze the prevalence of fungal infections, immune response, and the role of the microbiome in COVID-19 patients.
2. Fungal Infections as a Co-Morbidity of COVID-19
Fungal co-infections are frequent in the COVID-19 patients; therefore, its awareness is important for proper diagnosis and, subsequently, efficient treatment of the fungal co-infections for reducing morbidity and mortality. Due to a general neglected approach towards fungal tropical diseases, morbidity and mortality is expected to worsen in the context of the COVID-19 pandemic [22]. SARS related to COVID-19 disease is known to increase the risk of invasive fungal infections (IFI) [23,24]. In addition, patients suffering from endemic mycoses and COVID-19 co-infection seem to be an at-risk population and have a poor prognosis. A significant number of cases of COVID-19-associated candidiasis, aspergillosis, mucormycosis, and histoplasmosis have been reported so far from the different region of the world [22,25,26,27]. Some reports even state that COVID-19 increases the mortality rate in the patients having fungal infections, but the case reports suggest that individuals with COVID-19 are more susceptible to a fungal infection mostly because of impaired immune responses, which further increases the awareness of clinicians for more effective diagnosis and treatment [28,29].
2.1. Candidiasis
One of the major complications of severe COVID-19 cases are yeast infections. They are mainly caused primarily by Candida spp., which are associated with a high mortality rate, due to a longer ICU stay, catheterization, and broad-spectrum antibiotic use [6] (Table 1). Nucci et al. observed stable incidence of candidemia in their hospital during an 18-year period (1.3 episodes per 1000 admissions), but since March 2020, an increase in cases diagnosed with candidemia was noticed [30]. Compared with non-COVID-19 patients, COVID-19 patients with candidemia were more likely to be under mechanical ventilation [30]. Katz et al. evaluated the association between COVID-19 and oral and systemic candidiasis [25]. Generally, candidiasis was significantly associated with increased risk for COVID-19, whereas oral candidiasis showed an insignificant trend [25].

Table 1.
Clinical characteristics of COVID-19 patients reported with candidiasis.
Both fungi and virus display highly distinctive patterns of sudden emergence, and are based on simple infection-driven, human-to-human transmission [31]. In times of SARS-CoV-2, the vigilance of multidrug-resistant Candida spp. (e.g., Candida auris, C. glabrata, and Candida duobushaemulonii [17,32,33]) is extremely important. Data regarding multidrug-resistant Candida spp. in COVID-19 patients are scarce [32]. C. auris, an emerging pathogen known for a reduced susceptibility to antifungals, is spread across all continents [5], and it is easily transmitted between healthcare professionals. Both C. auris and SARS-CoV-2 have been found on hospital surfaces including on bedrails, intravenous (IV) poles, beds, air conditioner ducts, windows, and hospital floors [5]. Hospital-acquired C. auris infections in coronavirus disease patients may lead to adverse outcomes and additional strain on healthcare resources [34]. Moreover, the standard COVID-19 critical care of using mechanical ventilation and protracted ventilator-assisted management makes these patients potentially susceptible to colonization and infections by C. auris [5]. For example, during April–July 2020 in New Delhi (India), C. auris accounted for two-thirds of cases, and the case-fatality rate was very high (60%) [4]. In a phylogenetic molecular clock study (Genoa, Italy), Di Pilato and colleagues showed that all C. auris isolates were resistant to amphotericin B, voriconazole, and fluconazole at a high level, owing to mutations in ERG11 (K143R) and TACB1 (A640V) genes. Critically, C. auris could be easily spread because of the COVID-19 pandemic [35]. After the first C. auris-colonized case was diagnosed in a COVID-19 patient in ICU at a hospital in Salvador, Brazil, a multidisciplinary team conducted a local C. auris prevalence investigation [36]. Remarkably, findings revealed that among body swabs collected from 47 patients, eight samples from the axillae were positive for C. auris. Contaminated axillary monitoring thermometer helped to C. auris dissemination. Re-use of these devices must imply a careful disinfection or they should be replaced by other temperature monitoring methods [36]. Moreover, in 2020, the Florida Department of Health was alerted to three C. auris bloodstream infections and one urinary tract infection (UTI) in four patients with COVID-19 who had received care in the same COVID-19 ICU ward [37]. A report from in a tertiary academic center (United States, May 2014 to October 2020) showed that in an entire sample (non-COVID-19 and COVID-19 groups), C. albicans accounted for a minority of isolates collected [38]. Compared to non-COVID-19 patients with candidemia, COVID-19 patients had lower ICU admission sequential organ failure assessment score, but longer ICU stays and central venous catheter dwell times at candidemia detection [38].
Surveillance data assessed differences in candidemia patients with and without a prior COVID-19 diagnosis [28]. COVID-19 patients with candidemia lacked established underlying conditions associated with candidemia but had two times the mortality rate versus candidemia patients without COVID-19 [28]. Over a two-year period, patients followed in the ICU of Ankara City Hospital, Turkey, were divided into pre-pandemic and pandemic periods [29]. In multivariate logistic regression analysis, corticosteroid use, presence of sepsis, and age older than 65 years were independent risk factors for mortality in candidemia patients [29]. Indeed, candidemia with high mortality is reported as a more serious problem for COVID-19 patients due to its increased and earlier incidence, and a higher rate of mortality [28,29].
2.2. Aspergillosis
Aspergillosis is one of the most common opportunistic fungal co-infections caused by some Aspergillus spp., which particularly affects immunocompromised persons, such as COVID-19 patients. It critically affects the respiratory system, leading to a mild/serious lung infection, known as pulmonary aspergillosis, a serious form of aspergillosis, which becomes worse over time and does not have an effective treatment [26,41]. Clinical characteristics of the COVID-19 patients co-infected with aspergillosis can be analyzed in Table 2. Based on the available literature, it is suggested to keep a low threshold to investigate for COVID-19 associated pulmonary aspergillosis (CAPA), since an early detection and respective treatment may significantly improve outcomes. Moreover, prolonged courses of steroids should not be given unless further conclusive evidence is available [42], because steroids suppress the immune system, making the patient more susceptible to secondary infections. A rapid and aggressive treatment approach with judicious use of steroids while treating co-infections turns out to be the best possible outcome and solution.

Table 2.
Clinical characteristics of COVID-19 patients reported with aspergillosis.
2.3. Histoplasmosis
Histoplasmosis is a systemic mycosis, highly endemic in certain regions of America and Asia, including Brazil and India. It is caused by a dimorphic fungus, Histoplasma capsulatum, which predominately occurs in soil containing large amounts of bird or bat droppings. The infection occurs through the inhalation of fungal microconidia after perturbation of these environmental sources [50]. Similarly to aspergillosis, the disease is usually associated with immunosuppressive conditions, clinically presenting severe acute disseminated forms. Underlying lung disorders can predispose individuals to chronic pulmonary histoplasmosis, whereas acute and subacute pulmonary forms mainly occur in healthy individuals after a large fungal inoculum inhalation [50,51]. These clinical forms are less known, often misdiagnosed as bacterial pneumonia and pulmonary tuberculosis (Table 3). In the case of this particular fungal disease, it was indicated that most patients who received steroids for COVID-19 treatment developed histoplasmosis (Table 3). Histoplasmosis is mainly associated with COVID-19 patients with AIDS, and there are very few studies on the co-infection of H. capsulatum and COVID-19 [27,52]. Actually, the important findings were all patients of COVID-19 having co-infection of H. capsulatum survived after antifungal treatment with amphotericin B and itraconazole (Table 3) [27,52,53,54,55].

Table 3.
Clinical characteristics of COVID-19 patients reported with histoplasmosis.
2.4. Mucormycosis
The presence of hyphal infiltration of sinus tissue and a temporal course of less than four weeks defines mucormycosis [56,57]. The most common species related to mucormycosis are Rhizopus spp. and Mucor spp., but recently, a new Cunninghamella species, Cunninghamella bigelovii, was described [58]. Clinically, rhino-cerebral mucormycosis (RCM) can have atypical symptoms and signs that are similar to complicated sinusitis, such as crusting, nasal blockage, facial pain, proptosis and chemosis, edema, ptosis, and even ophthalmoplegia, as well as fever and headache and symptoms of intracranial extension [59,60]. A black eschar can be found on the hard palate or in the nasal cavity, but it is not typical [61,62]. Mycotic infiltration of blood vessels, thrombosis with vasculitis, acute neutrophilic infiltrate, bleeding, and tissue infarction are all histological characteristics [63].
Without early treatment and identification, this illness may advance quickly, with reported death rates of 50–80%, due to intra-orbital and cerebral complications. Even with timely treatment of underlying illnesses, diagnosis, and surgical intervention, therapy is frequently ineffective, resulting in infection spread and eventually death [64].
Recently, there has been a shift in the occurrence of sinus mucormycosis infection, and patients have been identified more often. A dramatic increase in cases of invasive fungal sinusitis, especially mucormycosis, has occurred in the past months, with many patients needing drastic surgical operations to treat this illness [65,66]. The use of steroids to control COVID-19 may be directly related to the suppression in immunity; thus, it also allows the colonization of opportunistic fungi, leading to mucormycosis, during any stages of the disease (Table 4) [23].

Table 4.
Clinical characteristics of COVID-19 patients reported with mucormycosis.
2.5. Cryptococcus
Cryptococcus neoformans is also related to a very serious opportunistic infection in immunocompromised patients. It has been reported that C. neoformans can infect COVID-19 patients. Mohamad Y et al. described the importance of early suspicion of C. neoformans infections in patients with immunocompromised state, considering that Cryptococci patients have a high risk of mortality [98]. In the current perspective, the use of immunosuppressive drugs should be justified and to be alert for infections such as C. neoformans, which can cause sepsis and mortality [98]. Studies have shown that almost all patients with COVID-19 having co-infection of C. neoformans did not survive, even after treatment with fluconazole and amphotericin B (Table 5).

Table 5.
Clinical characteristics of COVID-19 patients reported with cryptococcosis and other fungal infections.
2.6. Other Fungal Infections
Some other types of fungal infections have also been reported along with COVID-19. This is the case of Coccidioides immitis and Pneumocystis jirovecii (Table 5). Although co-infection with P. jirovecii is considered life-threatening, according to recent publications, patients improved clinically when treated with common drugs, such as trimethoprim–sulfamethoxazole [99,100]. Similarly to the other cases, during these co-infections, steroids had a negative impact on COVID-19-associated fungal co-infections conditions [100,101].
3. Role of Immune Response against the Most Clinically Relevant Fungal Infections in COVID-19 Patients: Two Sides of a Coin
The profound role of the host immune system to fight against fungal pathogens has been extensively described. Generally, two mainly types of immune cells, related to innate and adaptive immunity, dynamically contribute to effective immunity to eliminate the fungal pathogens [114,115].
Since COVID-19 patients are immunosuppressed, the adaptive form of immunity (lymphocytopenia in lymphocytes T CD4+ and CD8+) is remarkably declined, thus having a defective immune response [12]. As such, it is quite reasonable speculate that these factors establish a favorable environment for the acquisition of persistent fungal co-infections [116,117,118]. Moreover, collateral effects of host recognition pathways, which are desirable for the activation of antiviral immunity, may unexpectedly contribute to a highly permissive inflammatory environment. This, of course, favors fungal pathogenesis and predisposes patients to opportunistic fungal infections, with an exceptional chance of inducing the pathogenicity in high-risk patients [119]. In the beginning of the COVID-19 pandemic, the most predominant fungal infections were pulmonary aspergillosis [116] and candidiasis [6]. Recently, mucormycosis and cryptococcosis [12] are also among the main opportunistic fungal infections in vulnerable groups.
It is known that invasive yeast infections (IYF), especially candidiasis, are dramatically rising in COVID-19 patients, causing complications mainly related to oral infections [120] and candidemia [121]. Some reports have indicated that, in spite of impaired immune response of COVID-19, immune cells accounting for immunity in candidiasis are still present. Thus, it is probable that relevant clinical risk factors play critical role in developing IYF on COVID-19 cases [6]. This is the case of wide-spectrum antibiotic/steroid use, prolonged ICU stays and central venous catheters, transplant patients, chemotherapy/radiotherapy, patients under invasive or noninvasive ventilation, and diabetic individuals [6,119].
Changes in immune phenotype and cytokine release by whole blood stimulation assays against A. fumigatus and C. albicans were evaluated in order to mimic secondary infections in critically ill COVID-19-infected patients. In comparison to healthy controls, these patients had an immune phenotype considered increased in HLA-DR+CD38+ and PD-1+ CD4+ and CD8+ T cells, with high CD8+CD244+ lymphocytes. Some monocyte activation markers—IL-6, IL-8, TNF, IL-10, and sIL2Rα—were increased; however, IL-1β levels were low. Moreover, A. fumigatus antigen stimulation triggered an immune response, with no difference between COVID-19 patients and healthy controls, but a reduced monocyte CD80 upregulation. Regarding C. albicans responses, there was a lower release of IL-6, TNF, IL-1α, and IL-1β, and it was concluded that COVID-19 cases are more susceptible to Candida spp. infections [10]. Despite the marked immune dysregulation in COVID-19, no prominent defects have been reported in immune cells that are critically required for immunity to Candida spp. [6].
Among secondary infections in COVID-19 patients, APA showed to be related to a high rate of mortality and morbidity in patients with severe pneumonia. However, many features of the disease indicate that there are several diagnostic and therapeutic challenges that still need to be uncovered, since some cases with CAPA are undiagnosed through the lack of clinical awareness and global emergence of triazole resistance [122]. Moreover, a proper host immune response not only can protect against coronavirus, but may also restore immune hemostasis to reduce the risk of CAPA in COVID-19 patients [123]. The inflammatory cytokine cascade impairs the lung epithelial cells by producing large amounts of cytokine IL1α, which results in the production of IL1β from activated neutrophils and monocytes. Furthermore, the innate system also produces an extra level of nucleotide-binding leucine-rich repeat-containing proteins, or NOD-like receptors (NLRs), especially NLRP3 inflammasome, subsequently enhancing the level of IL6 and triggering detrimental responses associated with cytokine cascade [124]. In some cases of patients with aspergillosis, an increased level of IL6 is noticed (in epithelial cells), suggesting that a co-infection of COVID-19 may contribute to the severity of this clinical feature, owing to the augmented level of cytokines [125]. In this regard, in a large population of COVID-19 patients, the use of l IL6 receptor antagonist was stated to stimulate and sustain the immune response related to clinical development [126]. In contrast, trials in animal models with IL6 deficiency showed an association of this deficiency and a predisposition to CAPA [127], and thus, both IL6 receptor antagonist and antifungal are considered as prophylaxis in severe COVID-19 patients.
While there is much to be learned, our current understanding regarding co-infections in COVID-19 patients has provided some alternative immunotherapeutic strategies. This includes endogenous pathways of immunomodulation, which are recognized as a way to re-equilibrate the immune system, to overwhelm its complexity in COVID-19, and to prevent secondary infections, particularly aspergillosis [128]. For instance, thymosin α1, an endogenous thymic peptide with a wide range of immunomodulatory activities, could have beneficial effects on the activation of the immune system, and on balancing impaired immune responses, also inducing the indoleamine 2,3-dioxygenase 1 pathway [129,130,131]. Surprisingly, thymosin α1 effectively induced the antifungal activity, through the promotion of IFN and Th1 responses. Accordingly, it stimulates such responses in cases with active COVID-19 infection, but has no protective effects when used in prophylaxis [132,133,134]. Moreover, thymosin α1 could enhance immunomodulatory responses to vaccine and, subsequently, reduce COVID-19-associated secondary infections, specifically in elderly people [133,134]. Collectively, the normalization of immune responses might be an effective way of fighting aspergillosis. In controversy, it is arguable that Anakinra, a recombinant version of IL-1 receptor antagonist [135], could also restore immune responses for protection against aspergillosis in COVID-19 patients [136]. This drug has a favorable safety profile, and its efficacy against aspergillosis has been established as a result of unbalanced inflammasome activation in cystic fibrosis patients [137] and chronic granulomatous disease CGD, which leads to susceptibility to aspergillosis [138]. Likewise, there is a controversial idea on the protective role of aryl hydrocarbon receptor (AhR), a xenobiotic receptor, in COVID-19 patients susceptible to aspergillosis. Still, its beneficial therapeutic effects were linked to a reduction in the mucosal damage and re-establishment of the protection against gut infection, by stimulation production of IL-12. Hence, more studies will be required to assess the therapeutic purpose of AhR [139,140,141]. Recently, a study indicated that intravenous immunoglobulin (IVIg), collected from recovered patients (especially at the same geographic area), decreases inflammation of intestinal epithelial cells in newly infected subjects, and eradicates overgrowth of C. albicans in murine gut [142].
The application of effective natural compounds enhancing the capacity of the immune system are also drawing attention. Indeed, there are new insights into promising agents that can reduce the risk of infectious disease, specifically fungal pathogens, in susceptible individuals with COVID-19. Among them, honey and its ingredients showed a potential benefit towards inflammation disease and microbial pathogens such as fungal agents; however, further studies are needed on the application of honey [143]. In addition, β-glucan, a natural immunomodulatory component derived from Saccharomyces cerevisiae, was suggested to bolster innate immune responses in COVID-19 patients prior to infection, and any microbial infection as prophylaxis [144]. However, clinical trials are still needed to confirm its efficacy and to further study the distinctive effects of β-glucans from different sources.
4. Antifungal Resistance and Therapeutic Approaches in COVID-19 Patients
In recent years, we have been witnessing an incredible number of emerging resistant species related to a higher morbidity and mortality rates [145]. It has been estimated that, in 2050, antimicrobial resistance (AMR) could be responsible for 10 million deaths and treatment costs as high as USD 100 trillion [145]. This is also relevant in fungal infections. Indeed, the antifungal resistance phenomenon is especially critical in emerging resistant species, such as C. auris [17].
As seen during hospitalization, patients with COVID-19 are more predisposed to co-infections with bacterial and/or fungal pathogens (e.g., C. albicans and A. flavus [146,147]), which is likely to influence mortality rates [148,149,150]. Zhou et al. reported that almost 50% of mortalities accrued in patients had secondary bacterial and fungal infections [151]. This is the reason why antibiotics have been prescribed for hospitalized patients, for example, as a prophylactic measure against secondary infections, regardless of the susceptibility of the microorganism, promoting the emergence of multiple drug-resistant microbial species [3].
Since the onset of the COVID-19 pandemic, there are still few data on the prevalence of co-infections in patients with COVID-19 pneumonia. Yet, some studies already mention the problem of co-infections and drug resistance, which is the case of Candida spp. and COVID-19-associated superinfection mycosis, and its high potential for antifungal resistance [152]. Indeed, around 21% of patients who were under treatment with antifungals (voriconazole, isavuconazole, and caspofungin) showed no survival benefit [153]. Arastehfar et al. described COVID-19-associated candidemia (CAC) among seven Iranian patients. Half of patients with C. albicans were refractory to both azoles and echinocandins. Despite antifungal therapy, the high mortality of patients with CAC unveiled the severity of the disease in these patients. This, of course, also draws attention of the underestimation of the importance of an early diagnosis and timely initiation of antifungal therapy [121]. Another case reported a patient with COVID-19 CAPA caused by a triazole-resistant A. fumigatus, which highlights the need for surveillance triazole-resistant fungal species, particularly in CAPA cases [154]. Furthermore, early screening for IA and the necessity to identify isolates for pan-azole resistance should be considered in respiratory specimen in COVID-19 CAPA in ICU hospitalized patients [155].
Regarding antifungal susceptibility pattern of oropharyngeal candidiasis (OPC), a study carried out in Iranian COVID-19 patients showed that the majority of the Candida isolates were susceptible to all three classes of antifungal drugs (azoles, polyenes, and echinocandins). The only exception was one isolate of Pichia kudriavzevii and C. dubliniensis, which were caspofungin-resistant [156]. Long-term use of azoles may result in the selection of less sensitive species, such as P. kudriavzevii, C. dubliniensis, and C. glabrata, and in the development of drug resistance, even in previously susceptible Candida spp. [157]. Further studies should be carried out to design appropriate prophylaxis strategies in OPC.
Likewise, C. glabrata was recently linked to a possible fatal blood stream infection in a type-2 diabetes patient diagnosed with COVID-19. After 13 days of caspofungin treatment, C. glabrata with FKS-associated pan-echinocandin resistance was isolated from the patient [39]. Similarly, C. auris has been recovered from two-thirds of 15 cases of candidemia in India, with a high rate of fatality (60%). All C. auris isolates were resistant to fluconazole, and 40% were resistant to amphotericin B. This resistance to both classes of drug is highly concerning, because the use of other antifungals (such as echinocandins) are limited in developing countries [17]. In resource-limited countries, C. auris diagnostics are still a challenge, alerting the global medical community about the potential of C. auris as a critical factor in COVID-19 patients [158]. This also emphasizes the importance of early diagnosis and screening for antimicrobial drug-resistant co-infections, to reduce unfavorable outcomes in COVID-19 patients.
Commercial antibiotics and antifungals used for treatment of infectious diseases are almost all cytotoxic in high doses, which limits the use of these synthetic drugs. In this context, novel antimicrobial agents are among the most popular therapeutic strategies currently being applied, with minimal side effects to reduce AMR. New drug delivery systems including nano-carriers, liposomes, nano-mesopores, and nano carbon tubes plus the natural or bioactive compounds are promising therapeutic agents that have recently interested researchers [159,160]. In addition, many essentials oils, plant extracts, and essences have also been investigated and the results showed that these compounds have potential antibacterial, antifungal, and antiviral specification [161,162,163]. Still, further investigations are required to prove their activity in the future.
5. Role of the Microbiome and Probiotics to Fight COVID-19
At the beginning of the COVID-19 pandemic, several studies confirmed the prominent role of the immune system to defeat pathogens in COVID-19 cases [164]. Numerous clinical and scientific studies instill a promising window, considering that the gastrointestinal (GI) tract has a fundamental role to enhance the host immunity of COVID-19 [149,165]. In this regard, (normal) microbiota are described as the population of microorganisms (e.g., fungi, virus, bacteria) which particularly exist in the gut, with beneficial activity for the host (e.g., production of vitamins, facilitation of digestion, and stimulation of immune response against pathogens) [1,166,167,168]. Therefore, physiological changes in the intestinal tract easily lead to infection and inflammation disorders.
SARS-CoV-2 induces infections through binding angiotensin-converting enzyme 2 (ACE2) receptor, which is expressed on the cell surface of esophagus, lung, liver, and intestinal epithelium [169,170,171]. The microbiota that colonize the epithelial membrane of skin, oral cavity, and gut play an essential role to boost immunity in targeted tissues to fight and inhibit the adhesion of several pathogens [166]. In addition, during the fermentation process, the metabolites produced by microbiota can inhibit ACE2 receptors and suppress the implantation of viruses. As a result, blocking ACE2 receptor or blocking viral proteins could avoid any development of this viral infection [172,173,174].
Previous reports demonstrated that, in healthy cases, the primary community of microbiota in the oral cavity includes Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Spirochaetes, and Fusobacteria, while the major fungal species includes Candida spp., followed by Cladosporium spp., Aureobasidium spp., and Saccharomycetales [175,176]. The diversity of microbiota changes with age, since the population of microbiota in infants is less than adults, and in elders, is less than young people, which supports the evidence that the elderly are more susceptible to COVID-19 infection [177]. Besides, the gut microbiota also regulate the intestine mucosal site through the production of metabolites such as short chain fatty acids (SCFs), which can restore the secretion of immunoglobulins, effector cells, and anti-inflammatory factors (e.g., NF-kβ and TNF-α) in healthy individuals via affected pattern recognition receptors (PRR) [171]. On the other hand, microbiota metabolites binding with toll-like receptors (TLR) consequently regulate the immune responses and increased expression of T regulatory lymphocytes, cytokines, and chemokine to inhibit viral infection. It has been indicated that a direct relationship exists between microbiota and COVID-19 infection. Bacillus subtilis reduced the infectivity of COVID-19 [174]. Moreover, in COVID-19 patients’ lungs, the microbiota compounds were altered. The changes were thought to have an essential role in the COVID-19 immunity, severity of clinical presentation, and outcome [178].
As microbiota can affect antiviral immunity, probiotics are indicated as having anti-viral, anti-inflammatory, and anti-allergic activities, and to decrease the time duration and rate of respiratory viral infections [167,168,179,180]. Commensal fungi are well-known mycobiota, which directly and indirectly impact virus pathogenesis in the lungs [173]. The main fungal population in healthy cases include Candida spp., followed by Cladosporium spp., Saccharomysetales, and Aureobasidium spp. [1]. Based on previous studies, the mycobiome is significantly altered in COVID-19 patients when compared with healthy subjects [181,182]. Indeed, probiotic microorganisms, including Saccharomyces spp., Lactobacillus spp. and Bifidobacterium spp., are broadly used in the food industry, having important functions in innate immune response and modulating of immune cells such as B and T lymphocytes, macrophages, and dendritic cells [183]. The possible mechanism of probiotic immune modulation includes the activation of TLRs [184], regulation of gene expression and signaling pathways in the host cells [159,185]. It has also been disclosed that bacteria metabolites regulate the mucosal immunity via interacting with TLRs, cytokines, chemokines, and expression of NF-kB. In fact, it is known that several signals received from the lower GI tract can be transmitted to other mucosal surfaces, such as the respiratory tract, thereby enhancing protection against infection [171]. Although lungs possess their own microbiota, the inhibition of viral replication by lung–gut microbiota interaction indirectly influences the immune response of the respiratory tract [186,187]. This interaction occurs as host–microbe or microbe–microbe and affects the course of the respiratory infection. Importantly, any imbalance in communities of lung–gut microbiomes (dysbiosis) has been related to severe respiratory infections [187,188]. Intestinal dysbiosis was indicated to cause inflammation and weaker response to pathogens [189]. Even though our knowledge about fungal microbiota and probiotics is restricted, it is, for example, supported that supplementation with the combination of Streptococcus thermophilus and Bifidobacterium bifidum promote the reduction of viral infections [190]. The oral administration of Lactobacillus acidophilus in mice indicated a decrease in inflammation and damage on lung tissues after 24 h of a pulmonary infection. The diversity of the microbiome in a population creates a different range of severity of infections in each individual. The use of probiotics might open a new insight into the management of fungal pathogens, but there is much to uncover about the probable side effectiveness of clinical application of those in COVID-19 cases [191,192,193].
6. Final Remarks
Opportunistic fungal infections are of concern in COVID-19 patients. Categorically, these patients can develop fungal infections throughout any stages of this disease [23]. At the beginning, COVID-19 was highly associated with pulmonary aspergillosis and candidemia (invasive candidiasis), which were increasingly recognized as the main fungal diseases. Conversely, in recent months, a pointedly growing shift to other fungal infections has been ongoing. This is the case of infections related to Mucor and Rhizopus genera, Cryptococcus spp. and other less common species.
In general, data show that COVID-19 patients in ICU seem are more susceptible to fungal infections, when compared with patients without ICU admission, due to their immunosuppression status (the same case of HIV patients). Moreover, probably the incidence of aspergillosis is higher in COVID-19 patients, as the virus particularly affects the respiratory system. Correspondingly, in COVID-19 patients, the mortality rate is high in the case of co-infections (both bacterial and fungal species).
A highly complex interplay of predisposing factors, such as previous respiratory pathology, diabetes, nosocomial infection sources, and immunosuppressive therapy, is linked to co-infections. Furthermore, the neglected attitude towards fungal (tropical) diseases over the years, and the financial support for their diagnosis, treatment, and research, which is much lower than those available for other infectious diseases, leads to a similar mortality percentage [194]. Moreover, as COVID-19 patients are under immunosuppressive conditions, particularly T CD4+ and CD8+ lymphocytopenia, this provides an encouraging background for the occurrence of persistent fungal co-infections [116,117,118]. It is quite evident that any systemic immune alterations or the use of steroids to control COVID-19 may be directly related to the suppression in immunity, which also allows the colonization of opportunistic fungi. Hence, there is an urgent need to use steroids judiciously, prepare more comprehensive guidelines, and improve the steroid characterization for their efficacy, types, dose, duration of therapy, route of administration, and interaction with other drugs in order to improve COVID-19 treatments and prevent the increased probability and risk of developing a fungal infection secondary to the disease.
Investing more in precise guidelines related to the correct administration of antifungal agents and promoting more effective doses to increase the success of antifungal treatments is also imperative. Based on WHO guidelines for the control of resistant species, antimicrobial treatment/prophylaxis must be restricted, except when undertaking clinical indication [195]. This, obviously, also draws attention to the underestimation of the importance of an early diagnosis and timely initiation of antifungal therapy [121]. Of course, the adoption of certain precautions is also essential, such as hand washing and disinfecting surfaces with antiseptic agents.
Lastly, it also needs to be highlighted that several factors, such as lack of appropriate equipment to early screen and identify fungal infections, can result in many cases remaining undiagnosed. Subsequently, when the efficient treatment is not achieved on time and the multidrug-resistant phenomenon persists, this results in clinical failure outcomes in COVID-19 patients [196].
Author Contributions
Conceptualization, M.R. and C.F.R.; methodology, M.R. and C.F.R.; validation: C.F.R.; investigation, all authors; writing—original draft preparation, all authors; writing—review and editing, all authors; supervision, C.F.R. All authors have read and agreed to the published version of the manuscript.
Funding
C.F.R. would like to that the support by Base Funding—UIDB/00511/2020 of the Laboratory for Process Engineering, Environment, Biotechnology and Energy—LEPABE—funded by national funds through the FCT/MCTES (PIDDAC). S.K. and A.K. thank the Shri Ramswaroop Memorial University, Barabanki (UP), India, and the National Institute of Technology, Raipur (CG), India, for continuous support and assistance during the work and scientific writing. L.Č. thanks the Slovak Research and Development Agency under contract No. APVV-15-0347 and the grant of VEGA 1/0537/19 from the Ministry of Education, Science, Research, and Sport of the Slovak Republic.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Soltani, S.; Zakeri, A.; Zandi, M.; Kesheh, M.M.; Tabibzadeh, A.; Dastranj, M.; Faramarzi, S.; Didehdar, M.; Hafezi, H.; Hosseini, P.; et al. The Role of Bacterial and Fungal Human Respiratory Microbiota in COVID-19 Patients. BioMed Res. Int. 2021, 2021, 6670798. [Google Scholar] [CrossRef] [PubMed]
- Talento, A.F.; Hoenigl, M. Fungal Infections Complicating COVID-19: With the Rain Comes the Spores. J. Fungi 2020, 6, 279. [Google Scholar] [CrossRef]
- Rawson, T.M.; Wilson, R.C.; Holmes, A. Understanding the role of bacterial and fungal infection in COVID-19. Clin. Microbiol. Infect. 2021, 27, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Tarai, B.; Singh, A.; Sharma, A. Multidrug-resistant Candida auris infections in critically Ill Coronavirus disease patients, India, April–July 2020. Emerg. Infect. Dis. 2020, 26, 2694–2696. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Sharma, A. The lurking scourge of multidrug resistant Candida auris in times of COVID-19 pandemic. J. Glob. Antimicrob. Resist. 2020, 22, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Carvalho, A.; Nguyen, M.H.; Hedayati, M.T.; Netea, M.G.; Perlin, D.S.; Hoenigl, M. COVID-19-associated candidiasis (CAC): An underestimated complication in the absence of immunological predispositions? J. Fungi 2020, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Kubin, C.J.; McConville, T.H.; Dietz, D.; Zucker, J.; May, M.; Nelson, B.; Istorico, E.; Bartram, L.; Small-Saunders, J.; Sobieszczyk, M.E.; et al. Characterization of Bacterial and Fungal Infections in Hospitalized Patients with Coronavirus Disease 2019 and Factors Associated with Health Care-Associated Infections. Open Forum Infect. Dis. 2021, 8, ofab201. [Google Scholar] [CrossRef]
- Chen, X.; Liao, B.; Cheng, L.; Peng, X.; Xu, X.; Li, Y.; Hu, T.; Li, J.; Zhou, X.; Ren, B. The microbial coinfection in COVID-19. Appl. Microbiol. Biotechnol. 2020, 104, 7777–7785. [Google Scholar] [CrossRef]
- Silva, D.L.; Lima, C.M.; Magalhães, V.C.R.; Baltazar, L.M.; Peres, N.T.A.; Caligiorne, R.B.; Moura, A.S.; Fereguetti, T.; Martins, J.C.; Rabelo, L.F.; et al. Fungal and bacterial coinfections increase mortality of severely ill COVID-19 patients. J. Hosp. Infect. 2021, 113, 145–154. [Google Scholar] [CrossRef]
- Moser, D.; Biere, K.; Han, B.; Hoerl, M.; Schelling, G.; Woehrle, T.; Chouke, A. COVID-19 Impairs Immune Response to Candida albicans. Front. Immunol. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Mulet Bayona, J.V.; Tormo Palop, N.; Salvador García, C.; Fuster Escrivá, B.; Chanzá Aviñó, M.; Ortega García, P.; Gimeno Cardona, C. Impact of the SARS-CoV-2 Pandemic in Candidaemia, Invasive Aspergillosis and Antifungal Consumption in a Tertiary Hospital. J. Fungi 2021, 7, 440. [Google Scholar] [CrossRef]
- Bhatt, K.; Agolli, A.; Patel, M.H.; Garimella, R.; Devi, M.; Garcia, E.; Amin, H.; Domingue, C.; Del Castillo, R.G.; Sanchez-Gonzalez, M. High mortality co-infections of COVID-19 patients: Mucormycosis and other fungal infections. Discoveries 2021, 9, e126. [Google Scholar] [CrossRef]
- Bienvenu, A.L.; Bleyzac, N.; Richard, J.C.; Leboucher, G. No time for pending confirmation of invasive fungal disease in critically ill COVID-19 patients-think empirical treatment. Crit. Care 2020, 24, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Tiseo, G.; Giordano, C.; Leonildi, A.; Menichini, M.; Vecchione, A.; Pistello, M.; Guarracino, F.; Ghiadoni, L.; Forfori, F.; et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: A prospective observational study. J. Antimicrob. Chemother. 2020, 76, 1078–1084. [Google Scholar] [CrossRef]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Azzam Lopez, A.; Diez-Remesal, Y.; Martinez Castro, N.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Hays, J.P.; Kemp, A.; Okechukwu, R.; Murugaiyan, J.; Ekwanzala, M.D.; Ruiz Alvarez, M.J.; Paul-Satyaseela, M.; Iwu, C.D.; Balleste-Delpierre, C.; et al. The potential impact of the COVID-19 pandemic on global antimicrobial and biocide resistance: An AMR Insights global perspective. JAC-Antimicrobial Resist. 2021, 3, dlab038. [Google Scholar] [CrossRef] [PubMed]
- Černáková, L.; Roudbary, M.; Brás, S.; Tafaj, S.; Rodrigues, C.F. Candida auris: A Quick Review on Identification, Current Treatments, and Challenges. Int. J. Mol. Sci. 2021, 22, 4470. [Google Scholar] [CrossRef]
- Salmanton-Garcia, J.; Sprute, R.; Stemler, J.; Bartoletti, M.; Dupont, D.; Valerio, M.; Garcia-Vidal, C.; Falces-Romero, I.; Machado, M.; de la Villa, S.; et al. COVID-19-Associated Pulmonary Aspergillosis, March–August 2020. Emerg. Infect. Dis. 2021, 27, 1077–1086. [Google Scholar] [CrossRef]
- Danion, F.; Letscher-Bru, V.; Guitard, J.; Sitbon, K.; Dellière, S.; Angoulvant, A.; Desoubeaux, G.; Botterel, F.; Bellanger, A.-P.; Gargala, G.; et al. High mortality of COVID-19 associated mucormycosis in France: A nationwide retrospective study. medRxiv 2021. [Google Scholar] [CrossRef]
- Riad, A.; Gomaa, E.; Hockova, B.; Klugar, M. Oral candidiasis of COVID-19 patients: Case report and review of evidence. J. Cosmet. Dermatol. 2021, 20, 1580–1584. [Google Scholar] [CrossRef] [PubMed]
- Rajendra Santosh, A.B.; Muddana, K.; Bakki, S.R. Fungal Infections of Oral Cavity: Diagnosis, Management, and Association with COVID-19. SN Compr. Clin. Med. 2021, 3, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Nargesi, S.; Bongomin, F.; Hedayati, M.T. The impact of COVID-19 pandemic on AIDS-related mycoses and fungal neglected tropical diseases: Why should we worry? PLoS Negl. Trop. Dis. 2021, 15, e0009092. [Google Scholar] [CrossRef] [PubMed]
- Gangneux, J.-P.; Bougnoux, M.-E.; Dannaoui, E.; Cornet, M.; Zahar, J.R. Invasive fungal diseases during COVID-19: We should be prepared. J. Mycol. Med. 2020, 30, 100971. [Google Scholar] [CrossRef]
- Verweij, P.E.; Alanio, A. Fungal infections should be part of the core outcome set for COVID-19. Lancet Infect. Dis. 2021, 21, e145. [Google Scholar] [CrossRef]
- Katz, J. Prevalence of candidiasis and oral candidiasis in COVID-19 patients: A cross-sectional pilot study from the patients’ registry in a large health center. Quintessence Int. 2021, 52, 714–718. [Google Scholar] [PubMed]
- Prattes, J.; Valentin, T.; Hoenigl, M.; Talakic, E.; Reisinger, A.C.; Eller, P. Invasive pulmonary aspergillosis complicating COVID-19 in the ICU—A case report. Med. Mycol. Case Rep. 2021, 31, 2–5. [Google Scholar] [CrossRef]
- Messina, F.A.; Marin, E.; Caceres, D.H.; Romero, M.; Depardo, R.; Priarone, M.M.; Rey, L.; Vázquez, M.; Verweij, P.E.; Chiller, T.M.; et al. Coronavirus Disease 2019 (COVID-19) in a Patient with Disseminated Histoplasmosis and HIV—A Case Report from Argentina and Literature Review. J. Fungi 2020, 6, 275. [Google Scholar] [CrossRef]
- Seagle, E.E.; Jackson, B.R.; Lockhart, S.R.; Georgacopoulos, O.; Nunnally, N.S.; Roland, J.; Barter, D.M.; Johnston, H.L.; Czaja, C.A.; Kayalioglu, H.; et al. The landscape of candidemia during the COVID-19 pandemic. Clin. Infect. Dis. 2021, ciab562. [Google Scholar] [CrossRef]
- Kayaaslan, B.; Eser, F.; Kaya Kalem, A.; Bilgic, Z.; Asilturk, D.; Hasanoglu, I.; Ayhan, M.; Tezer Tekce, Y.; Erdem, D.; Turan, S.; et al. Characteristics of candidemia in COVID-19 patients; increased incidence, earlier occurrence and higher mortality rates compared to non-COVID-19 patients. Mycoses 2021, 64, 1083–1091. [Google Scholar] [CrossRef]
- Nucci, M.; Barreiros, G.; Guimarães, L.F.; Deriquehem, V.A.S.; Castiñeiras, A.C.; Nouér, S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses 2021, 64, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Steele, E.J.; Gorczynski, R.M.; Lindley, R.A.; Tokoro, G.; Temple, R.; Wickramasinghe, N.C. Origin of new emergent Coronavirus and Candida fungal diseases—Terrestrial or cosmic? Cosm. Genet. Evol. 2020, 106, 75–100. [Google Scholar]
- Awada, B.; Alam, W.; Chalfoun, M.; Araj, G.; Bizri, A.R. COVID-19 and Candida duobushaemulonii superinfection: A case report. J. Med. Mycol. 2021, 31, 101168. [Google Scholar] [CrossRef]
- Rodrigues, C.; Rodrigues, M.; Silva, S.; Henriques, M. Candida glabrata Biofilms: How Far Have We Come? J. Fungi 2017, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Chowdhary, A.; Sharma, C.; Meis, J.F. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLOS Pathog. 2017, 13, e1006290. [Google Scholar] [CrossRef] [PubMed]
- Di Pilato, V.; Codda, G.; Ball, L.; Giacobbe, D.R.; Willison, E.; Mikulska, M.; Magnasco, L.; Crea, F.; Vena, A.; Pelosi, P.; et al. Molecular Epidemiological Investigation of a Nosocomial Cluster of C. auris: Evidence of Recent Emergence in Italy and Ease of Transmission during the COVID-19 Pandemic. J. Fungi 2021, 7, 140. [Google Scholar] [CrossRef]
- De Almeida, J.N.; Brandão, I.B.; Francisco, E.C.; Almeida, S.L.R.; Oliveira Dias, P.; Pereira, F.M.; Santos Ferreira, F.; Andrade, T.S.; Miranda Costa, M.M.; Souza Jordão, R.T.; et al. Axillary Digital Thermometers uplifted a multidrug-susceptible Candida auris outbreak among COVID-19 patients in Brazil. Mycoses 2021, 64, 1062–1072. [Google Scholar] [CrossRef]
- Prestel, C.; Anderson, E.; Forsberg, K.; Lyman, M.; de Perio, M.A.; Kuhar, D.; Edwards, K.; Rivera, M.; Shugart, A.; Walters, M.; et al. Candida auris Outbreak in a COVID-19 Specialty Care Unit—Florida, July–August 2020. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 56–57. [Google Scholar] [CrossRef]
- Macauley, P.; Epelbaum, O. Epidemiology and Mycology of Candidaemia in non-oncological medical intensive care unit patients in a tertiary center in the United States: Overall analysis and comparison between non-COVID-19 and COVID-19 cases. Mycoses 2021, 64, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Posteraro, B.; Torelli, R.; Vella, A.; Leone, P.M.; De Angelis, G.; De Carolis, E.; Ventura, G.; Sanguinetti, M.; Fantoni, M. Pan-Echinocandin-Resistant Candida glabrata Bloodstream Infection Complicating COVID-19: A Fatal Case Report. J. Fungi 2020, 6, 163. [Google Scholar] [CrossRef]
- Hanson, B.M.; Dinh, A.Q.; Tran, T.T.; Arenas, S.; Pronty, D.; Gershengorn, H.B.; Ferreira, T.; Arias, C.A.; Shukla, B.S. Candida auris Invasive Infections During a COVID-19 Case Surge. Antimicrob. Agents Chemother. 2021, AAC-01146. [Google Scholar] [CrossRef]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet. Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Nasrullah, A.; Javed, A.; Malik, K. Coronavirus Disease-Associated Pulmonary Aspergillosis: A Devastating Complication of COVID-19. Cureus 2021, 31, e13004. [Google Scholar]
- Arastehfar, A.; Carvalho, A.; van de Veerdonk, F.L.; Jenks, J.D.; Koehler, P.; Krause, R.; Cornely, O.A.; Perlin, D.S.; Lass-Flörl, C.; Hoenigl, M. COVID-19 Associated Pulmonary Aspergillosis (CAPA)—From Immunology to Treatment. J. Fungi 2020, 6, 91. [Google Scholar] [CrossRef]
- Dupont, D.; Menotti, J.; Turc, J.; Miossec, C.; Wallet, F.; Richard, J.-C.; Argaud, L.; Paulus, S.; Wallon, M.; Ader, F.; et al. Pulmonary aspergillosis in critically ill patients with Coronavirus Disease 2019 (COVID-19). Med. Mycol. 2020, 59, 110–114. [Google Scholar] [CrossRef]
- Wu, S.; Yang, S.; Chen, R.; Chen, H.; Xu, Y.; Lin, B. Dynamic Immune Response Profiles and Recovery of a COVID-19 Patient with Coinfection of Aspergillus fumigatus and Other Baseline Diseases: A Case Report. OMICS A J. Integr. Biol. 2020, 24, 615–618. [Google Scholar] [CrossRef]
- Armstrong-James, D.; Youngs, J.; Bicanic, T.; Abdolrasouli, A.; Denning, D.W.; Johnson, E.; Mehra, V.; Pagliuca, T.; Patel, B.; Rhodes, J.; et al. Confronting and mitigating the risk of COVID-19 associated pulmonary aspergillosis. Eur. Respir. J. 2020, 56, 2002554. [Google Scholar] [CrossRef]
- Brown, L.-A.K.; Ellis, J.; Gorton, R.; De, S.; Stone, N. Surveillance for COVID-19-associated pulmonary aspergillosis. Lancet Microbe 2020, 1, e152. [Google Scholar] [CrossRef]
- Schein, F.; Munoz-Pons, H.; Mahinc, C.; Grange, R.; Cathébras, P.; Flori, P. Fatal aspergillosis complicating severe SARS-CoV-2 infection: A case report. J. Mycol. Med. 2020, 30, 101039. [Google Scholar] [CrossRef]
- De Lamballerie, C.N.; Pizzorno, A.; Fouret, J.; Szpiro, L.; Padey, B.; Dubois, J.; Julien, T.; Traversier, A.; Dulière, V.; Brun, P.; et al. Transcriptional Profiling of Immune and Inflammatory Responses in the Context of SARS-CoV-2 Fungal Superinfection in a Human Airway Epithelial Model. Microorganisms 2020, 8, 1974. [Google Scholar] [CrossRef] [PubMed]
- Wheat, L.J.; Azar, M.M.; Bahr, N.C.; Spec, A.; Relich, R.F.; Hage, C. Histoplasmosis. Infect. Dis. Clin. North Am. 2016, 30, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Azar, M.M.; Hage, C.A. Clinical Perspectives in the Diagnosis and Management of Histoplasmosis. Clin. Chest Med. 2017, 38, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Basso, R.P.; Poester, V.R.; Benelli, J.L.; Stevens, D.A.; Zogbi, H.E.; da Vasconcellos, I.C.S.; Pasqualotto, A.C.; Xavier, M.O. COVID-19 associated histoplasmosis in an AIDS patient. Mycopathologia 2020, 186, 109–112. [Google Scholar] [CrossRef]
- De Macedo, P.M.; Freitas, A.D.; Bártholo, T.P.; Bernardes-Engemann, A.R.; de Abreu Almeida, M.; Almeida-Silva, F.; Zancopé-Oliveira, R.M.; Almeida-Paes, R. Acute Pulmonary Histoplasmosis Following COVID-19: Novel Laboratorial Methods Aiding Diagnosis. J. Fungi 2021, 7, 346. [Google Scholar] [CrossRef]
- Stasiak, C.E.S.; Nigri, D.H.; Cardoso, F.R.; de Almeida Rezende d Mattos, R.S.; Martins, P.A.G.; Carvalho, A.R.S.; de Almeida, S.A.; Rodrigues, R.S.; Rosado-de-Castro, P.H. Case Report: Incidental Finding of COVID-19 Infection after Positron Emission Tomography/CT Imaging in a Patient with a Diagnosis of Histoplasmosis and Recurring Fever. Am. J. Trop. Med. Hyg. 2021, 104, 1651–1654. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Mutti, M.F.; Barletta, J.A.E.; Falak, A.; Cuatz, D.; Sisto, A.; Ragusa, M.A.; Claros, N.O.F.; Rolón, M.J. COVID-19 associated with AIDS-related disseminated histoplasmosis: A case report. Int. J. STD AIDS 2020, 31, 1222–1224. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Denning, D.W.; Ferguson, B.J.; Ponikau, J.; Buzina, W.; Kita, H.; Marple, B.; Panda, N.; Vlaminck, S.; Kauffmann-Lacroix, C.; et al. Fungal rhinosinusitis. Laryngoscope 2009, 119, 1809–1818. [Google Scholar] [CrossRef]
- Ferguson, B.J. Definitions of fungal rhinosinusitis. Otolaryngol. Clin. North Am. 2000, 33, 227–235. [Google Scholar] [CrossRef]
- Hallur, V.; Prakash, H.; Sable, M.; Preetam, C.; Purushotham, P.; Senapati, R.; Shankarnarayan, S.A.; Bag, N.D.; Rudramurthy, S.M. Cunninghamella arunalokei a New Species of Cunninghamella from India Causing Disease in an Immunocompetent Individual. J. Fungi 2021, 7, 670. [Google Scholar] [CrossRef] [PubMed]
- Scheckenbach, K.; Cornely, O.; Hoffmann, T.K.; Engers, R.; Bier, H.; Chaker, A.; Greve, J.; Schipper, J.; Wagenmann, M. Emerging therapeutic options in fulminant invasive rhinocerebral mucormycosis. Auris Nasus Larynx 2010, 37, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Vairaktaris, E.; Moschos, M.M.; Vassiliou, S.; Baltatzis, S.; Kalimeras, E.; Avgoustidis, D.; Pappas, Z.; Moschos, M.N. Orbital cellulitis, orbital subperiosteal and intraorbital abscess. Report of three cases and review of the literature. J. Cranio-Maxillofac. Surg. 2009, 37, 132–136. [Google Scholar] [CrossRef]
- Mohindra, S.; Mohindra, S.; Gupta, R.; Bakshi, J.; Gupta, S.K. Rhinocerebral mucormycosis: The disease spectrum in 27 patients. Mycoses 2007, 50, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Munir, N.; Jones, N.S. Rhinocerebral mucormycosis with orbital and intracranial extension: A case report and review of optimum management. J. Laryngol. Otol. 2006, 121, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Deshazo, R.D. Fungal Sinusitis. Am. J. Med. Sci. 1998, 316, 39–45. [Google Scholar] [PubMed]
- Ballester, D.G.; González-García, R.; García, C.M.; Ruiz-Laza, L.; Gil, F.M. Mucormycosis of the head and neck: Report of five cases with different presentations. J. Cranio-Maxillofac. Surg. 2012, 40, 584–591. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Hanley, B.; Naresh, K.N.; Roufosse, C.; Nicholson, A.G.; Weir, J.; Cooke, G.S.; Thursz, M.; Manousou, P.; Corbett, R.; Goldin, R.; et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: A post-mortem study. Lancet Microbe 2020, 1, e245–e253. [Google Scholar] [CrossRef]
- Werthman-Ehrenreich, A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am. J. Emerg. Med. 2021, 42, 264.e5–264. [Google Scholar] [CrossRef]
- Mehta, S.; Pandey, A. Rhino-Orbital Mucormycosis Associated with COVID-19. Cureus 2020, 12, e10726. [Google Scholar] [CrossRef]
- Do Monte Junior, E.S.; dos Santos, M.E.L.; Ribeiro, I.B.; de Oliveira Luz, G.; Baba, E.R.; Hirsch, B.S.; Funari, M.P.; de Moura, E.G.H. Rare and Fatal Gastrointestinal Mucormycosis (Zygomycosis) in a COVID-19 Patient: A Case Report. Clin. Endosc. 2020, 53, 746–749. [Google Scholar] [CrossRef]
- Placik, D.A.; Taylor, W.L.; Wnuk, N.M. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiol. Case Rep. 2020, 15, 2378–2381. [Google Scholar] [CrossRef]
- Mekonnen, Z.K.; Ashraf, D.C.; Jankowski, T.; Grob, S.R.; Vagefi, M.R.; Kersten, R.C.; Simko, J.P.; Winn, B.J. Acute Invasive Rhino-Orbital Mucormycosis in a Patient with COVID-19-Associated Acute Respiratory Distress Syndrome. Ophthalmic Plast. Reconstr. Surg. 2020, 37, e40–e80. [Google Scholar] [CrossRef]
- Pasero, D.; Sanna, S.; Liperi, C.; Piredda, D.; Pietro Branca, G.; Casadio, L.; Simeo, R.; Buselli, A.; Rizzo, D.; Bussu, F.; et al. A challenging complication following SARS-CoV-2 infection: A case of pulmonary mucormycosis. Infection 2020, 1–6. [Google Scholar] [CrossRef]
- Garg, D.; Muthu, V.; Sehgal, I.S.; Ramachandran, R.; Kaur, H.; Bhalla, A.; Puri, G.D.; Chakrabarti, A.; Agarwal, R. Coronavirus Disease (Covid-19) Associated Mucormycosis (CAM): Case Report and Systematic Review of Literature. Mycopathologia 2021, 186, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, M.; Reddy, R.; Vincent, M.J. Title of the Article: Paranasal Mucormycosis in COVID-19 Patient. Indian J. Otolaryngol. Head Neck Surg. 2021, 1–4. [Google Scholar] [CrossRef]
- Krishna, D.S.; Raj, H.; Kurup, P.; Juneja, M. Maxillofacial Infections in Covid-19 Era—Actuality or the Unforeseen: 2 Case Reports. Indian J. Otolaryngol. Head Neck Surg. 2021, 1–4. [Google Scholar] [CrossRef]
- Selarka, L.; Sharma, A.K.; Rathod, G.; Saini, D.; Patel, S.; Sharma, V.K. Mucormycosis—A Dreaded Complication of Covid-19. QJM An Int. J. Med. 2021, hcab166. [Google Scholar] [CrossRef]
- Johnson, A.K.; Ghazarian, Z.; Cendrowski, K.D.; Persichino, J.G. Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med. Mycol. Case Rep. 2021, 32, 64–67. [Google Scholar] [CrossRef]
- Waizel-Haiat, S.; Guerrero-Paz, J.A.; Sanchez-Hurtado, L.; Calleja-Alarcon, S.; Romero-Gutierrez, L. A Case of Fatal Rhino-Orbital Mucormycosis Associated with New Onset Diabetic Ketoacidosis and COVID-19. Cureus 2021, 13, e13163. [Google Scholar]
- Topcu, O.; Ozaslan, M.; Kılıc, İ.H.; Oguzkan, S.B.; Kurt, B.S.; Cay, M.; Tonus, S.S.; Bayram, A. Susceptibility of severe COVID-19 patients to rhino-orbital mucormycosis fungal infection in different clinical manifestations. Jpn. J. Ophthalmol. 2021, 65, 515–525. [Google Scholar]
- Fouad, Y.A.; Abdelaziz, T.T.; Askoura, A.; Saleh, M.I.; Mahmoud, M.S.; Ashour, D.M.; Ashour, M.M. Spike in Rhino-Orbital-Cerebral Mucormycosis Cases Presenting to a Tertiary Care Center During the COVID-19 Pandemic. Front. Med. 2021, 8, 645270. [Google Scholar] [CrossRef]
- Zurl, C.; Hoenigl, M.; Schulz, E.; Hatzl, S.; Gorkiewicz, G.; Krause, R.; Eller, P.; Prattes, J. Autopsy Proven Pulmonary Mucormycosis Due to Rhizopus microsporus in a Critically Ill COVID-19 Patient with Underlying Hematological Malignancy. J. Fungi 2021, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, A.; Gaikwad, R.; Krishna, S.; Hegde, R.; Tripathi, K.K.; Kale, P.G.; Rao, P.S.; Haldipur, D.; Bonanthaya, K. SARS-CoV-2, Uncontrolled Diabetes and Corticosteroids—An Unholy Trinity in Invasive Fungal Infections of the Maxillofacial Region? A Retrospective, Multi-centric Analysis. J. Maxillofac. Oral Surg. 2021, 20, 418–425. [Google Scholar] [CrossRef]
- Pakdel, F.; Ahmadikia, K.; Salehi, M.; Tabari, A.; Jafari, R.; Mehrparvar, G.; Rezaie, Y.; Rajaeih, S.; Alijani, N.; Barac, A.; et al. Mucormycosis in patients with COVID-19: A cross-sectional descriptive multicenter study from Iran. Mycoses 2021. [Google Scholar] [CrossRef]
- Veisi, A.; Bagheri, A.; Eshaghi, M.; Rikhtehgar, M.H.; Kanavi, M.R.; Farjad, R. Rhino-orbital mucormycosis during steroid therapy in COVID-19 patients: A case report. Eur. J. Ophthalmol. 2021, 112067212110094. [Google Scholar] [CrossRef] [PubMed]
- Alekseyev, K.; Didenko, L.; Chaudhry, B. Rhinocerebral Mucormycosis and COVID-19 Pneumonia. J. Med. Cases 2021, 12, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.M.; Abdelaziz, T.T.; Ashour, D.M.; Askoura, A.; Saleh, M.I.; Mahmoud, M.S. Imaging spectrum of acute invasive fungal rhino-orbital-cerebral sinusitis in COVID-19 patients: A case series and a review of literature. J. Neuroradiol. 2021, in press. [Google Scholar] [CrossRef]
- Revannavar, S.M.; Supriya, S.P.; Samaga, L.; Vineeth, V.K. COVID-19 triggering mucormycosis in a susceptible patient: A new phenomenon in the developing world? BMJ Case Rep. 2021, 14, e241663. [Google Scholar] [CrossRef] [PubMed]
- Maini, A.; Tomar, G.; Khanna, D.; Kini, Y.; Mehta, H.; Bhagyasree, V. Sino-orbital mucormycosis in a COVID-19 patient: A case report. Int. J. Surg. Case Rep. 2021, 82, 105957. [Google Scholar] [CrossRef]
- Buil, J.B.; van Zanten, A.R.H.; Bentvelsen, R.G.; Rijpstra, T.A.; Goorhuis, B.; van der Voort, S.; Wammes, L.J.; Janson, J.A.; Melchers, M.; Heusinkveld, M.; et al. Case series of four secondary mucormycosis infections in COVID-19 patients, the Netherlands, December 2020 to May 2021. Eurosurveillance 2021, 26, 2100510. [Google Scholar] [CrossRef]
- Arana, C.; Ramirez, R.E.C.; Xipell, M.; Casals, J.; Moreno, A.; Herrera, S.; Bodro, M.; Cofan, F.; Diekmann, F.; Esforzado, N. Mucormycosis associated with COVID-19 in two kidney transplant~patients. Transpl. Infect. Dis. 2021, e13652. [Google Scholar] [CrossRef]
- Sharma, S.; Grover, M.; Bhargava, S.; Samdani, S.; Kataria, T. Post coronavirus disease mucormycosis: A deadly addition to the pandemic spectrum. J. Laryngol. Otol. 2021, 135, 442–447. [Google Scholar] [CrossRef]
- Honavar, S.; Sen, M.; Lahane, S.; Lahane, T.; Parekh, R. Mucor in a Viral Land: A Tale of Two Pathogens. Indian J. Ophthalmol. 2021, 69, 244. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Galougahi, M.; Arastou, S.; Haseli, S. Fulminant mucormycosis complicating coronavirus disease 2019 (COVID-19). Int. Forum Allergy Rhinol. 2021, 11, 1029–1030. [Google Scholar] [CrossRef]
- Kanwar, A.; Jordan, A.; Olewiler, S.; Wehberg, K.; Cortes, M.; Jackson, B.R. A Fatal Case of Rhizopus azygosporus Pneumonia Following COVID-19. J. Fungi 2021, 7, 174. [Google Scholar] [CrossRef] [PubMed]
- Khatri, A.; Chang, K.-M.; Berlinrut, I.; Wallach, F. Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient—Case report and review of literature. J. Med. Mycol. 2021, 31, 101125. [Google Scholar] [CrossRef]
- Nehara, H.R.; Puri, I.; Singhal, V.; IH, S.; Bishnoi, B.R.; Sirohi, P. Rhinocerebral mucormycosis in COVID-19 patient with diabetes a deadly trio: Case series from the north-western part of India. Indian J. Med. Microbiol. 2021, 39, 180–383. [Google Scholar] [CrossRef]
- Khatib, M.; Ahmed, A.; Shaat, S.; soliman Mohamed, A.; Nashwan, A. Cryptococcemia in a Patient with COVID-19: A Case Report. Clin. Case Rep. 2020, 9, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Mang, S.; Kaddu-Mulindwa, D.; Metz, C.; Becker, A.; Seiler, F.; Smola, S.; Maßmann, A.; Becker, S.L.; Papan, C.; Bals, R.; et al. Pneumocystis jirovecii Pneumonia and Severe Acute Respiratory Syndrome Coronavirus 2 Coinfection in a Patient with Newly Diagnosed HIV-1 Infection. Clin. Infect. Dis. 2020, 72, 1487–1489. [Google Scholar] [CrossRef] [PubMed]
- Viceconte, G.; Buonomo, A.R.; Lanzardo, A.; Pinchera, B.; Zappulo, E.; Scotto, R.; Moriello, N.S.; Vargas, M.; Iacovazzo, C.; Servillo, G.; et al. Pneumocystis jirovecii pneumonia in an immunocompetent patient recovered from COVID-19. Infect. Dis. 2021, 53, 382–385. [Google Scholar] [CrossRef]
- Jeican, I.I.; Inișca, P.; Gheban, D.; Tuaran, F.; Aluaș, M.; Trombitas, V.; Cristea, V.; Crivii, C.; Junie, L.M.; Albu, S. COVID-19 and Pneumocystis jirovecii Pulmonary Coinfection—The First Case Confirmed through Autopsy. Medicina 2021, 57, 302. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, V.C.; Perosa, A.H.; de Souza Luna, L.K.; Conte, D.D.; Nascimento, O.A.; Ota-Arakaki, J.; Bellei, N. Detected SARS-CoV-2 in Ascitic Fluid Followed by Cryptococcemia: A Case Report. Compr. Clin. Med. 2020, 2, 2414–2418. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.J.C.; Montenegro-Idrogo, J.J.; Vadillo, A.R.V.; Torres, M.S.; Matos, I.V.; Delgado, C.P.R. Hospital-acquired SARS-CoV-2 pneumonia in a person living with HIV. Int. J. 2020, 31, 1320–1322. [Google Scholar]
- Passerini, M.; Terzi, R.; Piscaglia, M.; Passerini, S.; Piconi, S. Disseminated Cryptococcosis in a Patient with Metastatic Prostate Cancer Who Died in the Coronavirus Disease 2019 (COVID-19) Outbreak. Cureus 2020, 12, e8254. [Google Scholar] [CrossRef] [PubMed]
- Krauth, D.S.; Jamros, C.M.; Rivard, S.C.; Olson, N.H.; Maves, R.C. Accelerated Progression of Disseminated Coccidioidomycosis Following SARS-CoV-2 Infection: A Case Report. Mil. Med. 2021, usab132. [Google Scholar] [CrossRef]
- Chang, C.C.; Senining, R.; Kim, J.; Goyal, R. An Acute Pulmonary Coccidioidomycosis Coinfection in a Patient Presenting with Multifocal Pneumonia with COVID-19. J. Investig. Med. High Impact Case Rep. 2020, 8, 232470962097224. [Google Scholar] [CrossRef]
- Cai, S.; Sun, W.; Li, M.; Dong, L. A complex COVID-19 case with rheumatoid arthritis treated with tocilizumab. Clin. Rheumatol. 2020, 39, 2797–2802. [Google Scholar] [CrossRef] [PubMed]
- Menon, A.A.; Berg, D.D.; Brea, E.J.; Deutsch, A.J.; Kidia, K.K.; Thurber, E.G.; Polsky, S.B.; Yeh, T.; Duskin, J.A.; Holliday, A.M.; et al. A Case of COVID-19 and Pneumocystis jirovecii Coinfection. Am. J. Respir. Crit. Care Med. 2020, 202, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Alanio, A.; Dellière, S.; Voicu, S.; Bretagne, S.; Mégarbane, B. The presence of Pneumocystis jirovecii in critically ill patients with COVID-19. J. Infect. 2021, 82, 84–123. [Google Scholar] [CrossRef]
- Coleman, H.; Snell, L.B.; Simons, R.; Douthwaite, S.T.; Lee, M.J. Coronavirus disease 2019 and Pneumocystis jirovecii pneumonia: A diagnostic dilemma in HIV. AIDS 2020, 34, 1258–1260. [Google Scholar] [CrossRef]
- Kelly, S.; Waters, L.; Cevik, M.; Collins, S.; Lewis, J.; Wu, M.-S.; Blanchard, T.J.; Geretti, A.M. Pneumocystispneumonia, a COVID-19 mimic, reminds us of the importance of HIV testing in COVID-19. Clin. Med. 2020, 20, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Ventoulis, I.; Sarmourli, T.; Amoiridou, P.; Mantzana, P.; Exindari, M.; Gioula, G.; Vyzantiadis, T.A. Bloodstream Infection by Saccharomyces cerevisiae in Two COVID-19 Patients after Receiving Supplementation of Saccharomyces in the ICU. J. Fungi 2020, 6, 98. [Google Scholar] [CrossRef]
- Poignon, C.; Blaize, M.; Vezinet, C.; Lampros, A.; Monsel, A.; Fekkar, A. Invasive pulmonary fusariosis in an immunocompetent critically ill patient with severe COVID-19. Clin. Microbiol. Infect. 2020, 26, 1582–1584. [Google Scholar] [CrossRef]
- Kirkland, T.N.; Fierer, J. Innate Immune Receptors and Defense Against Primary Pathogenic Fungi. Vaccines 2020, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Shoham, S.; Levitz, S.M. The immune response to fungal infections. Br. J. Haematol. 2005, 129, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Lewis, R.E.; Walsh, T.J.; Kontoyiannis, D.P. Navigating the uncertainties of COVID-19 associated aspergillosis (CAPA): A comparison with influenza associated aspergillosis (IAPA). J. Infect. Dis. 2021, jiab163. [Google Scholar] [CrossRef]
- Stanzani, M.; Vianelli, N.; Cavo, M.; Kontoyiannis, D.P.; Lewis, R.E. Development and internal validation of a model for predicting 60-day risk of invasive mould disease in patients with haematological malignancies. J. Infect. 2019, 78, 484–490. [Google Scholar] [CrossRef]
- Netea, M.G.; Giamarellos-Bourboulis, E.J.; Domínguez-Andrés, J.; Curtis, N.; van Crevel, R.; van de Veerdonk, F.L.; Bonten, M. Trained immunity: A tool for reducing susceptibility to and the severity of SARS-CoV-2 infection. Cell 2020, 181, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Ahmadikia, K.; Badali, H.; Khodavaisy, S. Opportunistic fungal infections in the epidemic area of COVID-19: A clinical and diagnostic perspective from Iran. Mycopathologia 2020, 185, 607–611. [Google Scholar] [CrossRef]
- Hocková, B.; Riad, A.; Valky, J.; Šulajová, Z.; Stebel, A.; Slávik, R.; Bečková, Z.; Pokorná, A.; Klugarová, J.; Klugar, M. Oral complications of ICU patients with COVID-19: Case-series and review of two hundred ten cases. J. Clin. Med. 2021, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Shaban, T.; Zarrinfar, H.; Roudbary, M.; Ghazanfari, M.; Hedayati, M.-T.; Sedaghat, A.; Ilkit, M.; Najafzadeh, M.J.; Perlin, D.S. Candidemia among iranian patients with severe COVID-19 admitted to ICUs. J. Fungi 2021, 7, 280. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Rogers, T.R.; Talento, A.F. COVID-19 associated invasive pulmonary aspergillosis: Diagnostic and therapeutic challenges. J. Fungi 2020, 6, 115. [Google Scholar] [CrossRef]
- Costantini, C.; van de Veerdonk, F.L.; Romani, L. Covid-19-Associated Pulmonary Aspergillosis: The Other Side of the Coin. Vaccines 2020, 8, 713. [Google Scholar] [CrossRef] [PubMed]
- Van de Veerdonk, F.L.; Netea, M.G. Blocking IL-1 to prevent respiratory failure in COVID-19. Crit. Care 2020, 24, 1–6. [Google Scholar] [CrossRef]
- Borger, P.; Koeter, G.H.; Timmerman, J.A.B.; Vellenga, E.; Tomee, J.F.C.; Kauffman, H.F. Proteases from Aspevgillus fumigatus Induce Interleukin (IL)-6 and IL-8 Production in Airway Epithelial Cell Lines by Transcriptional Mechanisms. J. Infect. Dis. 1999, 180, 1267–1274. [Google Scholar] [CrossRef] [Green Version]
- Toniati, P.; Piva, S.; Cattalini, M.; Garrafa, E.; Regola, F.; Castelli, F.; Franceschini, F.; Focà, E.; Andreoli, L.; Latronico, N. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020, 19, 102568. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guaraldi, G.; Meschiari, M.; Cozzi-Lepri, A.; Milic, J.; Tonelli, R.; Menozzi, M.; Franceschini, E.; Cuomo, G.; Orlando, G.; Borghi, V. Tocilizumab in patients with severe COVID-19: A retrospective cohort study. Lancet Rheumatol. 2020, 2, e474–e484. [Google Scholar] [CrossRef]
- Romani, L.; Bistoni, F.; Perruccio, K.; Montagnoli, C.; Gaziano, R.; Bozza, S.; Bonifazi, P.; Bistoni, G.; Rasi, G.; Velardi, A. Thymosin α1 activates dendritic cell tryptophan catabolism and establishes a regulatory environment for balance of inflammation and tolerance. Blood 2006, 108, 2265–2274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romani, L.; Oikonomou, V.; Moretti, S.; Iannitti, R.G.; D’Adamo, M.C.; Villella, V.R.; Pariano, M.; Sforna, L.; Borghi, M.; Bellet, M.M. Thymosin α1 represents a potential potent single-molecule-based therapy for cystic fibrosis. Nat. Med. 2017, 23, 590–600. [Google Scholar] [CrossRef] [Green Version]
- Renga, G.; Bellet, M.M.; Pariano, M.; Gargaro, M.; Stincardini, C.; D’Onofrio, F.; Mosci, P.; Brancorsini, S.; Bartoli, A.; Goldstein, A.L. Thymosin α1 protects from CTLA-4 intestinal immunopathology. Life Sci. Alliance 2020, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Romani, L.; Tomino, C.; Puccetti, P.; Garaci, E. Off-label therapy targeting pathogenic inflammation in COVID-19. Cell Death Discov. 2020, 6, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; He, J.; Wu, Y.; Xie, B.; Liu, X.; Wei, B.; Zhou, H.; Lin, B.; Zuo, Z.; Wen, W. Dysregulated adaptive immune response contributes to severe COVID-19. Cell Res. 2020, 30, 814–816. [Google Scholar] [CrossRef]
- Liu, Y.; Pang, Y.; Hu, Z.; Wu, M.; Wang, C.; Feng, Z.; Mao, C.; Tan, Y.; Liu, Y.; Chen, L. Thymosin alpha 1 (Tα1) reduces the mortality of severe COVID-19 by restoration of lymphocytopenia and reversion of exhausted T cells. Clin. Infect. Dis. 2020, 71, 2150–2157. [Google Scholar]
- Aouba, A.; Baldolli, A.; Geffray, L.; Verdon, R.; Bergot, E.; Martin-Silva, N.; Justet, A. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: Case series. Ann. Rheum. Dis. 2020, 79, 1381–1382. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.H.; Calabrese, C. Cytokine release syndrome and the prospects for immunotherapy with COVID-19. Part 2: The role of interleukin 1. Clevel. Clin. J. Med. 2020. [Google Scholar] [CrossRef]
- Iannitti, R.G.; Napolioni, V.; Oikonomou, V.; De Luca, A.; Galosi, C.; Pariano, M.; Massi-Benedetti, C.; Borghi, M.; Puccetti, M.; Lucidi, V. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat. Commun. 2016, 7, 1–16. [Google Scholar] [CrossRef]
- De Luca, A.; Smeekens, S.P.; Casagrande, A.; Iannitti, R.; Conway, K.L.; Gresnigt, M.S.; Begun, J.; Plantinga, T.S.; Joosten, L.A.B.; van der Meer, J.W.M. IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 3526–3531. [Google Scholar] [CrossRef] [Green Version]
- Puccetti, M.; Paolicelli, G.; Oikonomou, V.; De Luca, A.; Renga, G.; Borghi, M.; Pariano, M.; Stincardini, C.; Scaringi, L.; Giovagnoli, S. Towards targeting the aryl hydrocarbon receptor in cystic fibrosis. Mediators Inflamm. 2018, 2018, 1601486. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockinger, B.; Di Meglio, P.; Gialitakis, M.; Duarte, J.H. The aryl hydrocarbon receptor: Multitasking in the immune system. Annu. Rev. Immunol. 2014, 32, 403–432. [Google Scholar] [CrossRef]
- Jawhara, S. Could intravenous immunoglobulin collected from recovered coronavirus patients protect against COVID-19 and strengthen the immune system of new patients? Int. J. Mol. Sci. 2020, 21, 2272. [Google Scholar] [CrossRef] [Green Version]
- Hossain, K.S.; Hossain, M.G.; Moni, A.; Rahman, M.M.; Rahman, U.H.; Alam, M.; Kundu, S.; Rahman, M.M.; Hannan, M.A.; Uddin, M.J. Prospects of honey in fighting against COVID-19: Pharmacological insights and therapeutic promises. Heliyon 2020, 6, e05798. [Google Scholar] [CrossRef]
- Geller, A.; Yan, J. Could the Induction of Trained Immunity by β -Glucan Serve as a Defense Against COVID-19 ? Front. Immunol. 2020, 11, 1782. [Google Scholar] [CrossRef] [PubMed]
- Lucien, M.A.B.; Canarie, M.F.; Kilgore, P.E.; Jean-Denis, G.; Fénélon, N.; Pierre, M.; Cerpa, M.; Joseph, G.A.; Maki, G.; Zervos, M.J.; et al. Antibiotics and antimicrobial resistance in the COVID-19 era: Perspective from resource-limited settings. Int. J. Infect. Dis. 2021, 104, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Segal, J.P.; Mak, J.W.Y.; Mullish, B.H.; Alexander, J.L.; Ng, S.C.; Marchesi, J.R. The gut microbiome: An under-recognised contributor to the COVID-19 pandemic? Therap. Adv. Gastroenterol. 2020, 13, 1756284820974914. [Google Scholar] [CrossRef]
- Zuo, T.; Zhan, H.; Zhang, F.; Liu, Q.; Tso, E.Y.K.; Lui, G.C.Y.; Chen, N.; Li, A.; Lu, W.; Chan, F.K.L.; et al. Alterations in Fecal Fungal Microbiome of Patients with COVID-19 During Time of Hospitalization until Discharge. Gastroenterology 2020, 159, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: Implications for pandemic influenza preparedness. J. Infect. Dis. 2008, 198, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Cave, E. COVID-19 Super-spreaders: Definitional Quandaries and Implications. Asian Bioeth. Rev. 2020, 12, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.; Green, J.A.; Paton, N.I. Hospitalized patients with bacterial infections: A potential focus of SARS transmission during an outbreak. Epidemiol. Infect. 2004, 132, 407–408. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Heard, K.L.; Hughes, S.; Mughal, N.; Moore, L.S.P. COVID-19 and fungal superinfection. Lancet Microbe 2020, 1, e107. [Google Scholar] [CrossRef]
- Rezasoltani, S.; Yadegar, A.; Hatami, B.; Asadzadeh Aghdaei, H.; Zali, M.R. Antimicrobial Resistance as a Hidden Menace Lurking Behind the COVID-19 Outbreak: The Global Impacts of Too Much Hygiene on AMR. Front. Microbiol. 2020, 11, 590683. [Google Scholar] [CrossRef] [PubMed]
- Meijer, E.F.J.; Dofferhoand, A.S.M.; Meis, J.F.; Hoiting, O.; Buil, J.B. Azole-resistant COVID-19-associated pulmonary aspergillosis in an immunocompetent host: A case report. J. Fungi 2020, 6, 79. [Google Scholar] [CrossRef]
- Ghelfenstein-Ferreira, T.; Saade, A.; Alanio, A.; Bretagne, S.; Araujo de Castro, R.; Hamane, S.; Azoulay, E.; Bredin, S.; Dellière, S. Recovery of a triazole-resistant Aspergillus fumigatus in respiratory specimen of COVID-19 patient in ICU – A case report. Med. Mycol. Case Rep. 2021, 31, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Ahmadikia, K.; Mahmoudi, S.; Kalantari, S.; Jamalimoghadamsiahkali, S.; Izadi, A.; Kord, M.; Dehghan Manshadi, S.A.; Seifi, A.; Ghiasvand, F.; et al. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses 2020, 63, 771–778. [Google Scholar] [CrossRef]
- Nadagir, S.D. Significance of isolation and drug susceptibility testing of non-Candida albicans species causing oropharyngeal candidiasis in HIV patients. Southeast Asian J. Trop. Med. Public Health 2008, 39, 492. [Google Scholar]
- Chowdhary, A.; Tarai, B.; Singh, A.; Sharma, A. Emerging Infectious Diseases Emerg. Infect. Dis. 2020, 26, 2694–2696. [Google Scholar]
- Nikoomanesh, F.; Roudbarmohammadi, S.; Khoobi, M.; Haghighi, F.; Roudbary, M. Design and synthesis of mucoadhesive nanogel containing farnesol: Investigation of the effect on HWP1, SAP6 and Rim101 genes expression of Candida albicans in vitro. Artif. Cells Nanomed. Biotechnol. 2019, 47, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, I.; Sharma, M.; Mehta, M.; Badyal, S.; Sharma, V.; Sharma, I.; Singh, H.; Sistla, S. Bioactive compounds and probiotics—A ray of hope in COVID-19 management. Food Sci. Hum. Wellness 2021, 10, 131–140. [Google Scholar] [CrossRef]
- Pandey, A.T.; Pandey, I.; Zamboni, P.; Gemmati, D.; Kanase, A.; Singh, A.V.; Singh, M.P. Traditional herbal remedies with a multifunctional therapeutic approach as an implication in COVID-19 associated co-infections. Coatings 2020, 10, 761. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Cekanova, M.; LeMieux, M.; Greer, B.; Moustaid-Moussa, N. Modulation of adipose tissue inflammation by bioactive food compounds. J. Nutr. Biochem. 2013, 24, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Capella, J.M.; Buniowska, M.; Esteve, M.J.; Frígola, A. Effect of Stevia rebaudiana addition on bioaccessibility of bioactive compounds and antioxidant activity of beverages based on exotic fruits mixed with oat following simulated human digestion. Food Chem. 2015, 184, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Attar, A.; Papizadeh, S.; Salimi Jeda, A.; Reza Hosseini-Fard, S.; Jamasbi, E.; Kazemi, S.; Amerkani, S.; Reza Talei, G.; Moradi, P.; et al. The emerging role of probiotics as a mitigation strategy against coronavirus disease 2019 (COVID-19). Arch. Virol. 2021, 166, 1819–1840. [Google Scholar] [CrossRef] [PubMed]
- Oria, R.B.; Liu, Z.; Chifiriuc, M.C.; Stavropoulou, E.; Bezirtzoglou, E. Probiotics in Medicine: A Long Debate. Front. Immunol. 2020, 11, 2192. [Google Scholar]
- Andrade, J.C.; Kumar, S.; Kumar, A.; Černáková, L.; Rodrigues, C.F. Application of probiotics in candidiasis management. Crit. Rev. Food Sci. Nutr. 2021, 1–16. [Google Scholar] [CrossRef]
- Salehi, B.; Dimitrijević, M.; Aleksić, A.; Neffe-Skocińska, K.; Zielińska, D.; Kołożyn-Krajewska, D.; Sharifi-Rad, J.; Stojanović-Radić, Z.; Prabu, S.M.; Rodrigues, C.F.; et al. Human microbiome and homeostasis: Insights into the key role of prebiotics, probiotics, and symbiotics. Crit. Rev. Food Sci. Nutr. 2020, 61, 1415–1428. [Google Scholar] [CrossRef]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, L.-H.; Dadar, M.; Li, W.; Ng, S.C.; Zuo, T. The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Front. Microbiol. 2018, 9, 2247. [Google Scholar]
- Berg, G.; Suchodolski, J.S.; Vital, M.; Shellito, J.E.; Samuelson, D.R.; Welsh, D.A. Regulation of lung immunity and host defense by the intestinal microbiota. Front. Microbiol. 2015, 6, 1085. [Google Scholar]
- Olaimat, A.N.; Aolymat, I.; Al-Holy, M.; Ayyash, M.; Abu Ghoush, M.; Al-Nabulsi, A.A.; Osaili, T.; Apostolopoulos, V.; Liu, S.-Q.; Shah, N.P. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. NPJ Sci. Food 2020, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lopez, A.; Behnsen, J.; Nuccio, S.P.; Raffatellu, M. Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 2016, 16, 135–148. [Google Scholar] [CrossRef]
- Bachem, A.; Makhlouf, C.; Binger, K.J.; de Souza, D.P.; Tull, D.; Hochheiser, K.; Whitney, P.G.; Fernandez-Ruiz, D.; Dähling, S.; Kastenmüller, W.; et al. Microbiota-Derived Short-Chain Fatty Acids Promote the Memory Potential of Antigen-Activated CD8+ T Cells. Immunity 2019, 51, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.; Haynes, M.; Hanson, N.; Angly, F.E.; Heath, A.C.; Rohwer, F.; Gordon, J.I. Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature 2010, 466, 334–338. [Google Scholar] [CrossRef] [PubMed]
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, Y.; Takeshita, T. The oral microbiome and human health. J. Oral Sci. 2017, 59, 201–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehtoranta, L.; Pitkäranta, A.; Korpela, R. Probiotics in respiratory virus infections. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1289–1302. [Google Scholar] [CrossRef]
- Behnsen, J.; Deriu, E.; Sassone-Corsi, M.; Raffatellu, M. Probiotics: Properties, examples, and specific applications. Cold Spring Harb. Perspect. Biol. 2013, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Wang, C.; Zhang, Y.; Lei, G.; Xu, K.; Zhao, N.; Lu, J.; Meng, F.; Yu, L.; Yan, J.; et al. Integrated gut virome and bacteriome dynamics in COVID-19 patients. Gut Microbes 2021, 13, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Wang, H.; Cui, G.; Lu, H.; Wang, L.; Luo, H.; Chen, X.; Ren, H.; Sun, R.; Liu, W.; et al. Alterations in the human oral and gut microbiomes and lipidomics in COVID-19. Gut 2021, 70, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Polk, D.B. Probiotics and immune health. Curr. Opin. Gastroenterol. 2011, 27, 496–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, Y.; Uematsu, S.; Kiyono, H. Epithelial glycosylation in gut homeostasis and inflammation. Nat. Immunol. 2016, 17, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef] [Green Version]
- Oliver He, Y.; Sun, X.; Prakash Dubey, G.; Pasteur, I.; Hong Yu, F.; Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; et al. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 9. [Google Scholar]
- Sencio, V.; Barthelemy, A.; Tavares, L.P.; Machado, M.G.; Soulard, D.; Cuinat, C.; Queiroz-Junior, C.M.; Noordine, M.L.; Salomé-Desnoulez, S.; Deryuter, L.; et al. Gut Dysbiosis during Influenza Contributes to Pulmonary Pneumococcal Superinfection through Altered Short-Chain Fatty Acid Production. Cell Rep. 2020, 30, 2934–2947. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.Y.; Chen, Y.X.; Fang, J.Y. 2019 Novel coronavirus infection and gastrointestinal tract. J. Dig. Dis. 2020, 21, 125–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, K.; Cai, H.; Shen, Y.; Ni, Q.; Chen, Y.; Hu, S.; Li, J.; Wang, H.; Yu, L.; Huang, H.; et al. Management of COVID-19: The Zhejiang experience. Zhejiang Da Xue Xue Bao Yi Xue Ban 2020, 49, 147–157. [Google Scholar] [PubMed]
- Kumar, J.R.V.; Seo, B.J.; Mun, M.R.; Kim, C.-J.; Lee, I.; Kim, H.; Park, Y.-H. Putative probiotic Lactobacillus spp. from porcine gastrointestinal tract inhibit transmissible gastroenteritis coronavirus and enteric bacterial pathogens. Trop. Anim. Health Prod. 2010, 42, 1855–1860. [Google Scholar] [CrossRef] [Green Version]
- Salman, J.A.S.; Mahmood, N.N.; Abdulsattar, B.O.; Abid, H.A. The effectiveness of probiotics against viral infections: A rapid review with focus on SARS-CoV-2 infection. Open Access Maced. J. Med. Sci. 2020, 8, 496–508. [Google Scholar] [CrossRef]
- Eguchi, K.; Fujitani, N.; Nakagawa, H.; Miyazaki, T. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci. Rep. 2019, 9, 4812. [Google Scholar] [CrossRef]
- Jayawardena, R.; Sooriyaarachchi, P.; Chourdakis, M.; Jeewandara, C.; Ranasinghe, P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L.; Nosanchuk, J.D. Fungal diseases as neglected pathogens: A wake-up call to public health officials. PLoS Negl. Trop. Dis. 2020, 14, e0007964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Anon COVID-19 Clinical Management: Living Guidance; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Silva, L.N.; de Mello, T.P.; de Souza Ramos, L.; Branquinha, M.H.; Roudbary, M.; dos Santos, A.L.S. Fungal Infections in COVID-19-Positive Patients: A Lack of Optimal Treatment Options. Curr. Top. Med. Chem. 2020, 20, 1951–1957. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).