Adapting an Ergosterol Extraction Method with Marine Yeasts for the Quantification of Oceanic Fungal Biomass
Abstract
1. Introduction
2. Materials and Methods
2.1. Testing a Method to Allow Detecting Low Concentrations of Ergosterol Using a Cultured Fungal Strain
2.1.1. Fungal Culture and Dilutions Preparation
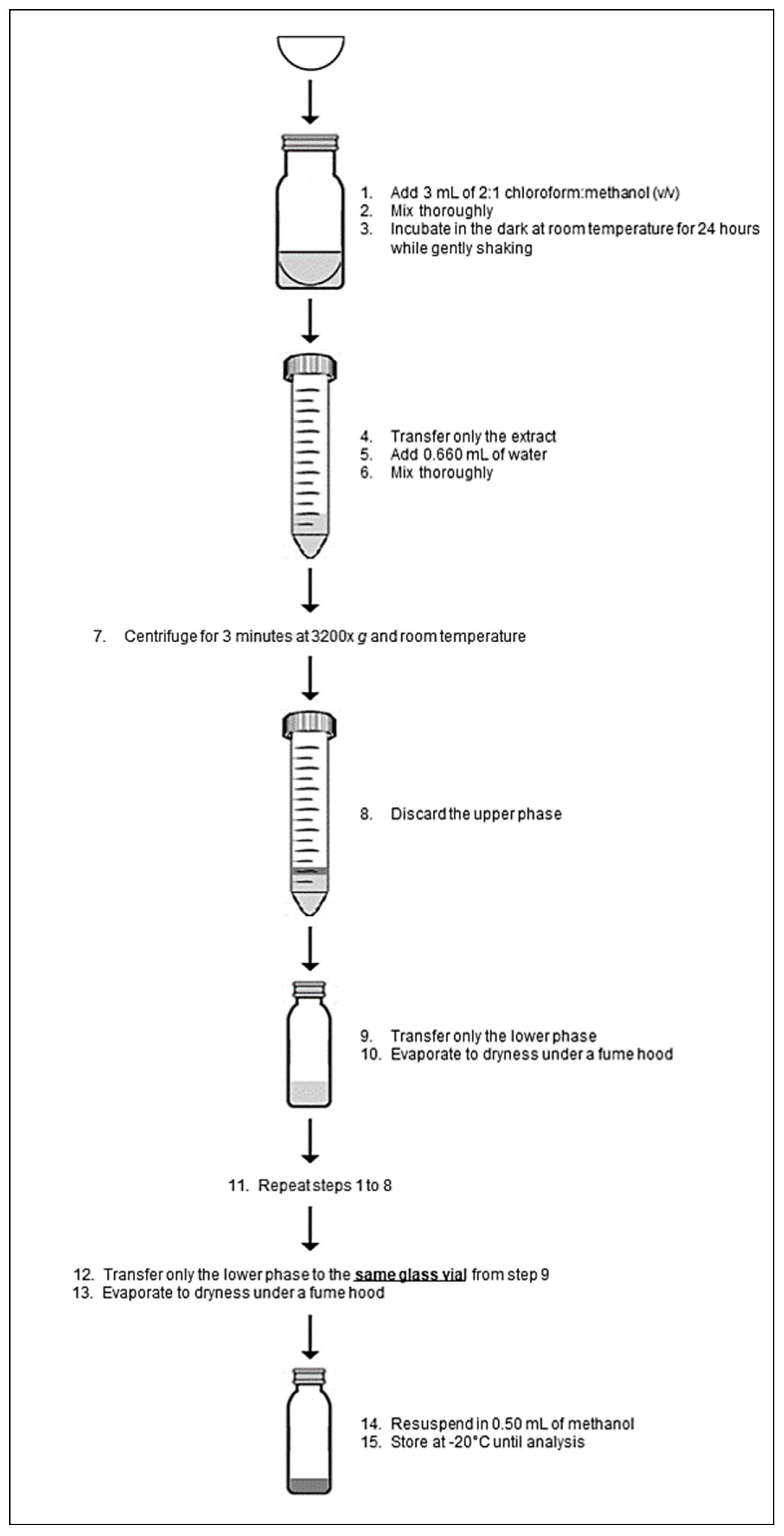
2.1.2. Ergosterol Extraction
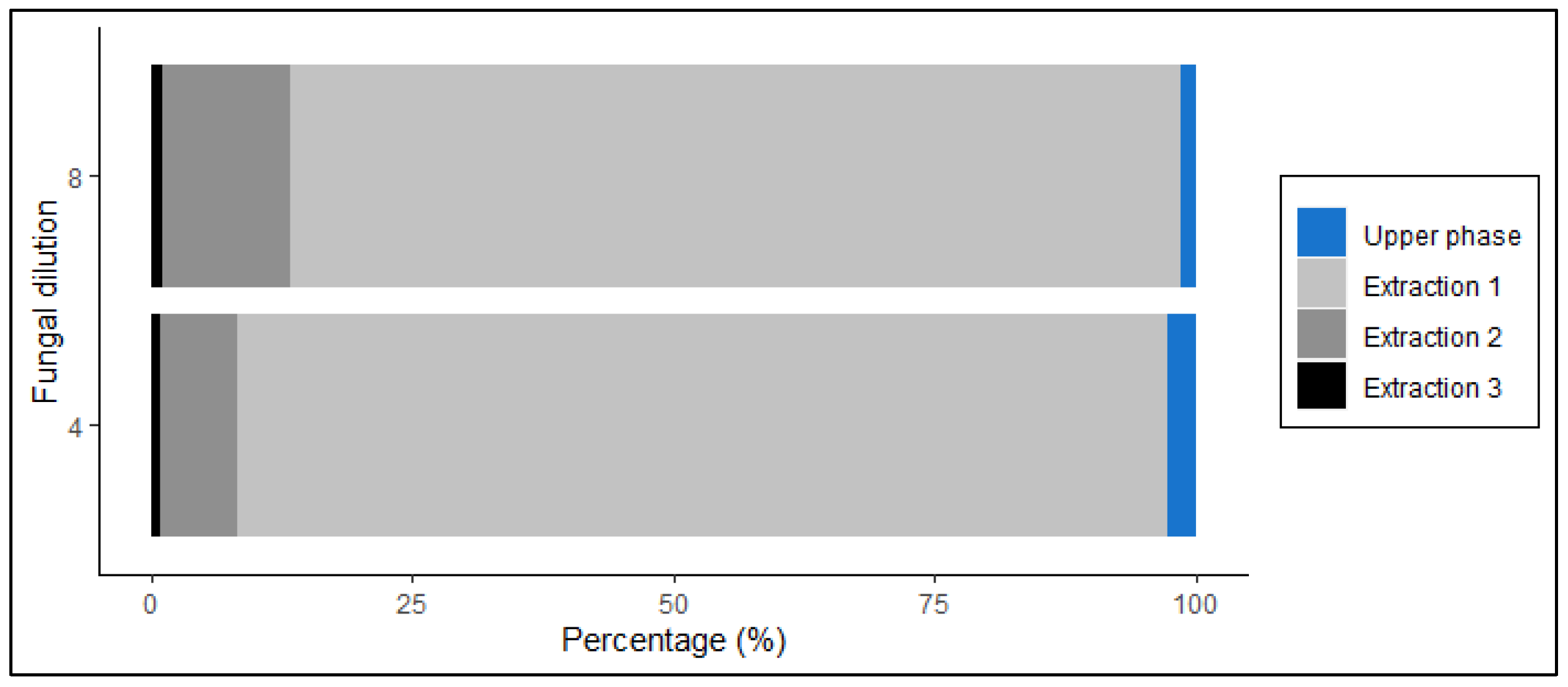
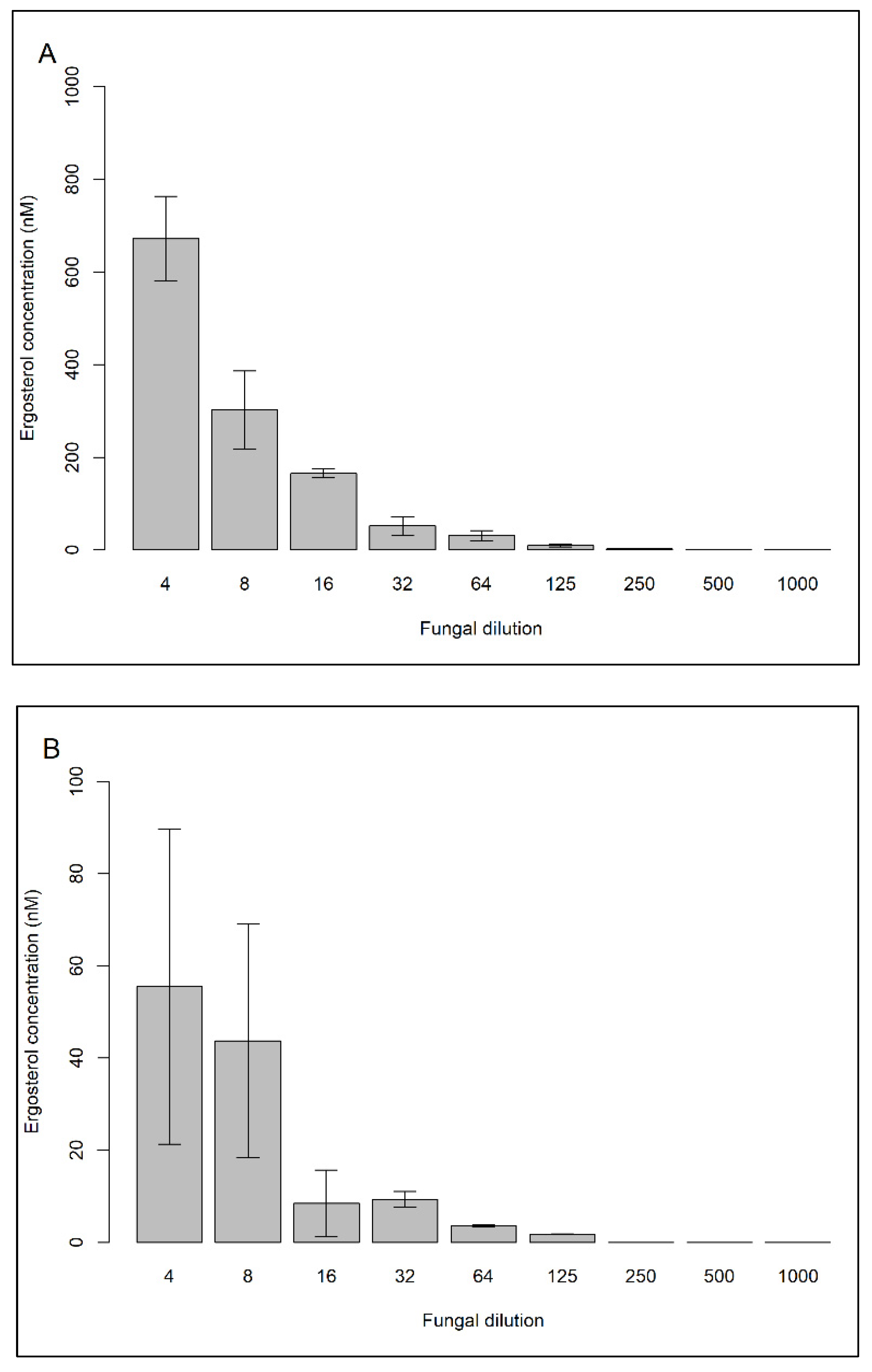
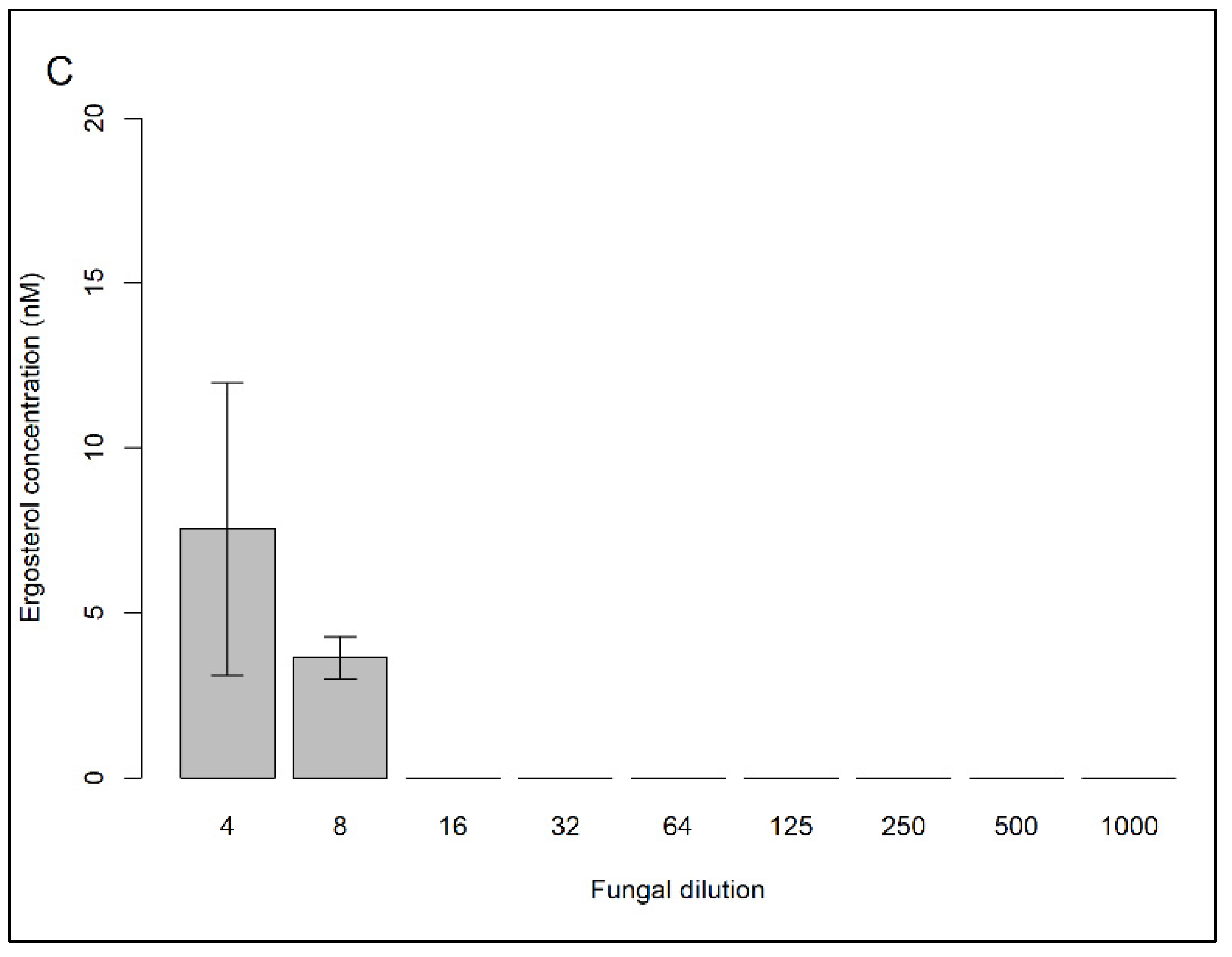
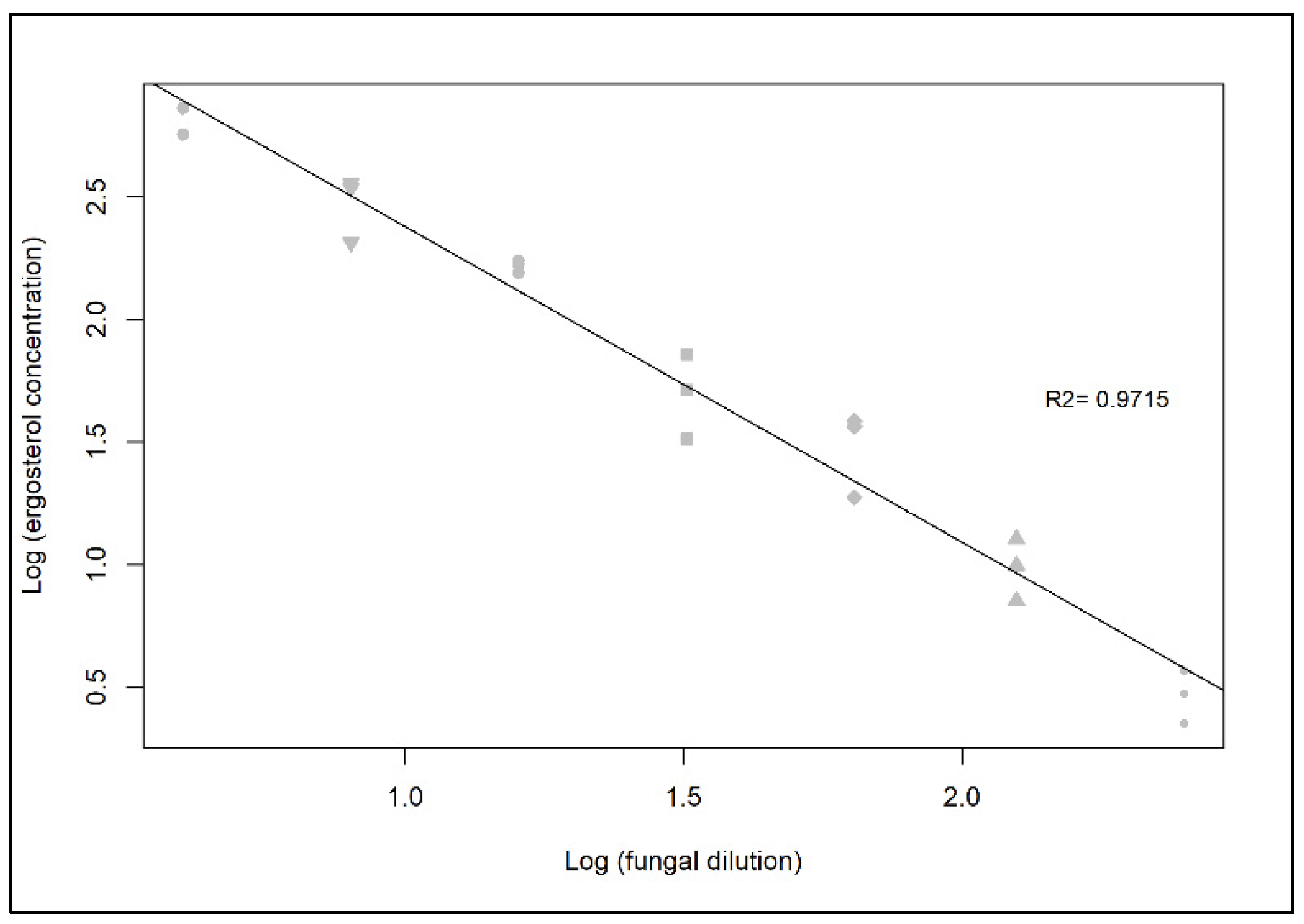
2.1.3. Determining the Extraction Efficiency of Ergosterol
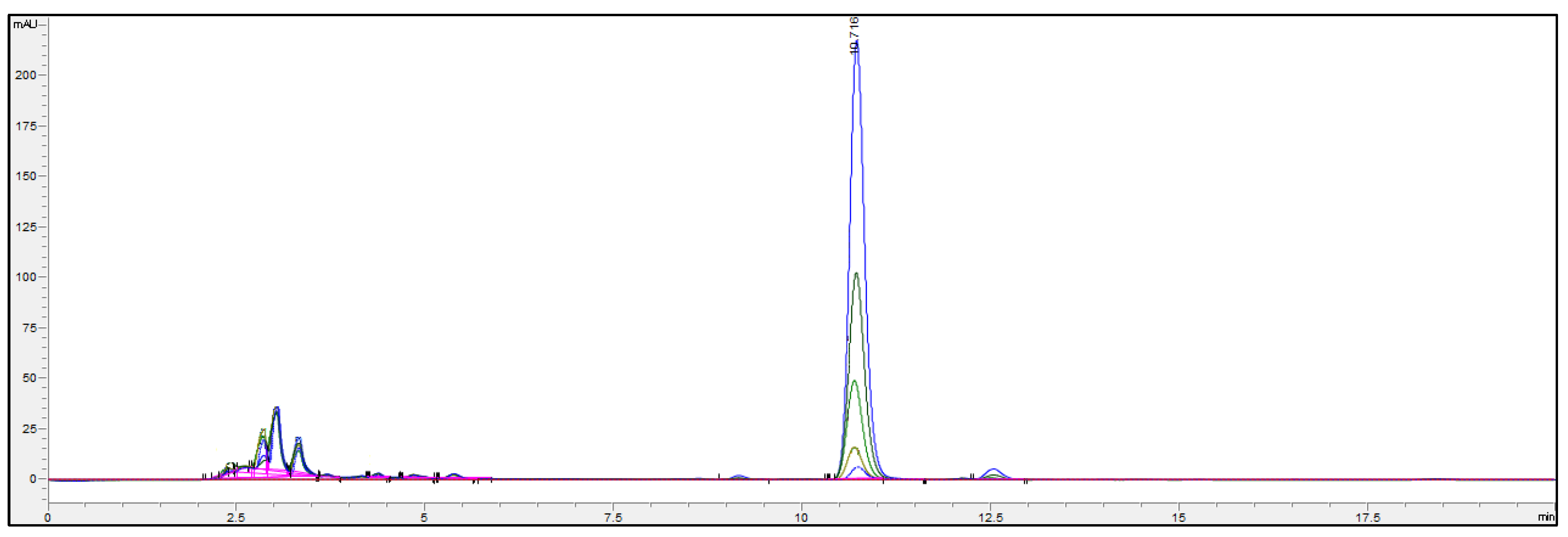
2.1.4. Ergosterol Quantification by High-Performance Liquid Chromatography (HPLC-UV) Analysis
2.1.5. Limit of Detection (LOD) and Limit of Quantitation (LOQ) of Ergosterol by HPLC Analysis
2.2. Measuring Ergosterol Concentrations in Samples Collected throughout the Water Column of the Atlantic Ocean
2.2.1. Sampling
2.2.2. Ergosterol Extraction and Concentration
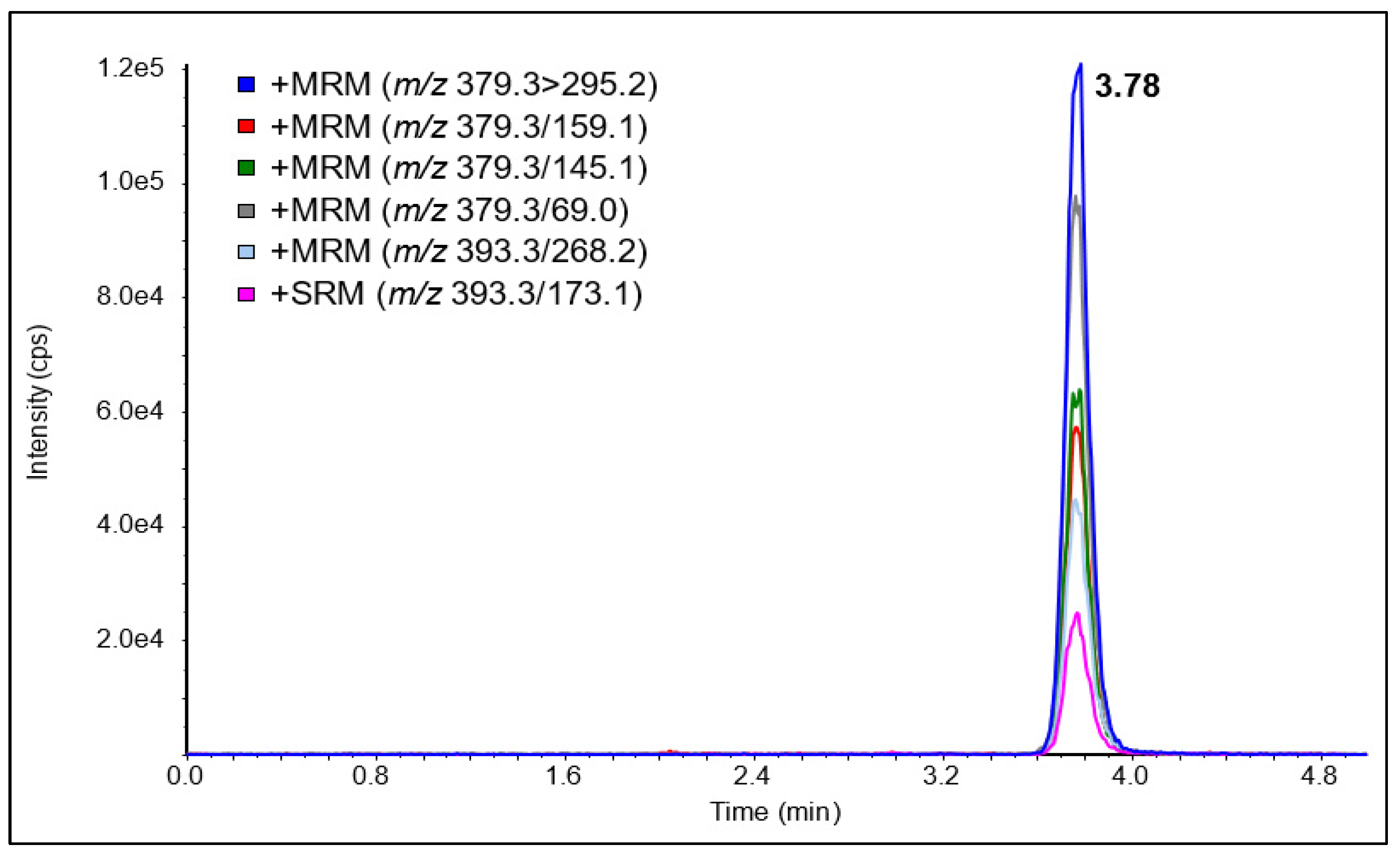
2.2.3. Ergosterol Quantification via Liquid Chromatography–Mass Spectrometry/Mass Spectrometry (LC-MS/MS) Analysis
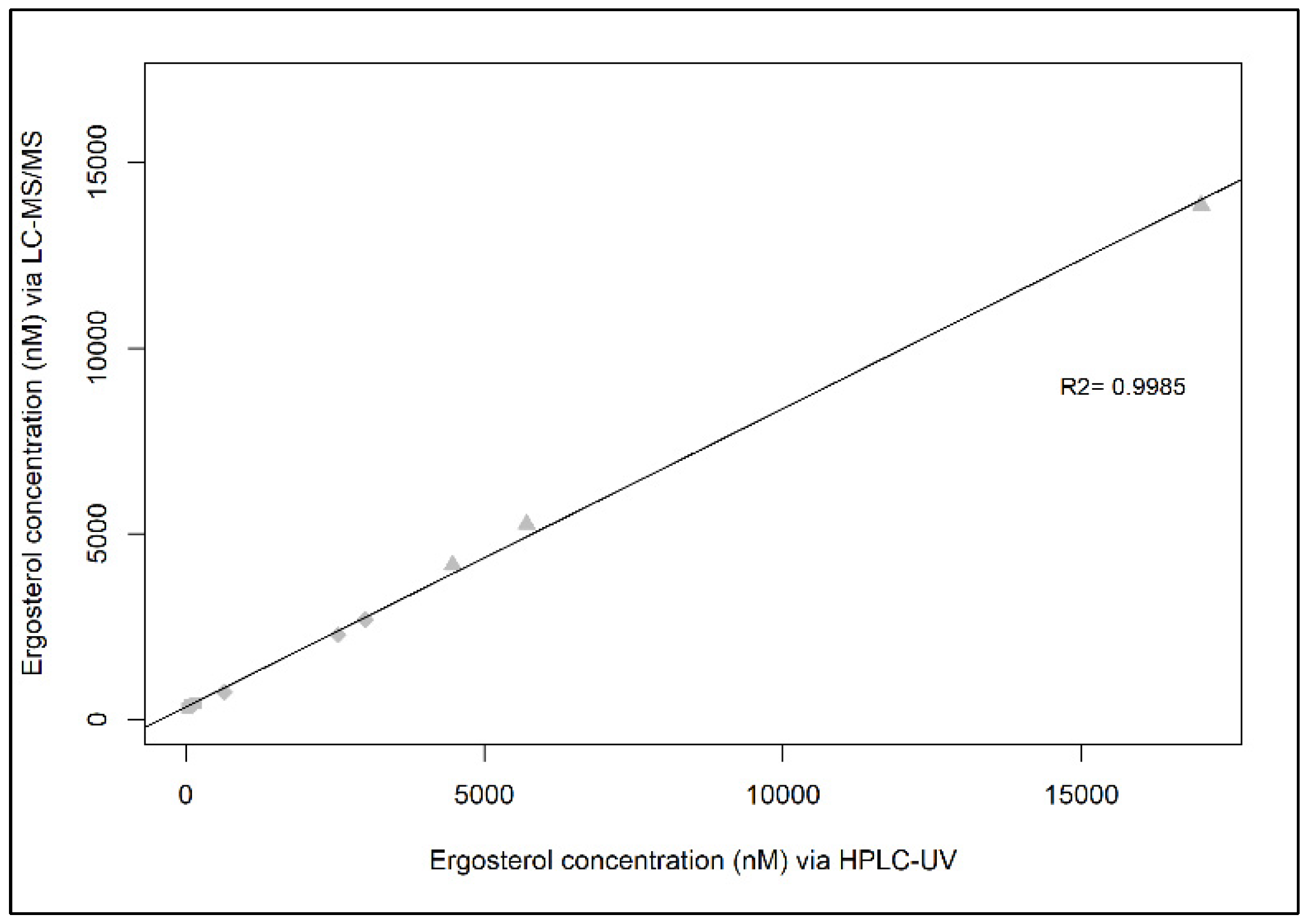
2.3. Correlation between HPLC-UV and LC-MS/MS
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomson, P.G.; Davidson, A.T.; van den Enden, R.; Pearce, I.; Seuront, L.; Paterson, J.S.; Williams, G.D. Distribution and abundance of marine microbes in the Southern Ocean between 30 and 80 E. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 815–827. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Milo, R. The biomass composition of the oceans: A blueprint of our blue planet. Cell 2019, 179, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Bochdansky, A.B.; Clouse, M.A.; Herndl, G.J. Eukaryotic microbes, principally fungi and labyrinthulomycetes, dominate biomass on bathypelagic marine snow. ISME J. 2017, 11, 362–373. [Google Scholar] [CrossRef]
- Grant, W.; West, A. Measurement of ergosterol, diaminopimelic acid and glucosamine in soil: Evaluation as indicators of microbial biomass. J. Microbiol. Methods 1986, 6, 47–53. [Google Scholar] [CrossRef]
- Stahl, P.D.; Parkin, T.B. Relationship of soil ergosterol concentration and fungal biomass. Soil Biol. Biochem. 1996, 28, 847–855. [Google Scholar] [CrossRef]
- Grattan, R.; Suberkropp, K. Effects of nutrient enrichment on yellow poplar leaf decomposition and fungal activity in streams. J. North Am. Benthol. Soc. 2001, 20, 33–43. [Google Scholar] [CrossRef]
- Baldy, V.; Gessner, M.O.; Chauvet, E. Bacteria, fungi and the breakdown of leaf litter in a large river. Oikos 1995, 74, 93–102. [Google Scholar] [CrossRef]
- Gessner, M.O.; Chauvet, E. Ergosterol-to-biomass conversion factors for aquatic hyphomycetes. Appl. Environ. Microbiol. J. 1993, 59, 502–507. [Google Scholar] [CrossRef]
- Newell, S.Y.; Arsuffi, T.L.; Fallon, R.D. Fundamental procedures for determining ergosterol content of decaying plant material by liquid chromatography. Appl. Environ. Microbiol. J. 1988, 54, 1876–1879. [Google Scholar] [CrossRef]
- Grossart, H.P.; Massana, R.; McMahon, K.D.; Walsh, D.A. Linking metagenomics to aquatic microbial ecology and biogeochemical cycles. Limnol. Oceanogr. 2019, 269, 103–108. [Google Scholar] [CrossRef]
- Morales, S.E.; Biswas, A.; Herndl, G.J.; Baltar, F. Global structuring of phylogenetic and functional diversity of pelagic fungi by depth and temperature. Front. Mar. Sci. 2019, 6, 1–12. [Google Scholar] [CrossRef]
- Baltar, F.; Zhao, Z.; Herndl, G.J. Potential and expression of carbohydrate utilization by marine fungi in the global ocean. Microbiome 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Mille-Lindblom, C.; von Wachenfeldt, E.; Tranvik, L.J. Ergosterol as a measure of living fungal biomass: Persistence in environmental samples after fungal death. J. Microbiol. Methods 2004, 59, 253–262. [Google Scholar] [CrossRef]
- Seitz, L.; Mohr, H.; Burroughs, R.; Sauer, D. Ergosterol as an indicator of fungal invasion in grains. Cereal Chem. 1977, 54, 1207–1217. [Google Scholar] [CrossRef]
- Hassett, B.T.; Borrego, E.J.; Vonnahme, T.R.; Rama, T.; Kolomiets, M.V.; Gradinger, R. Arctic marine fungi: Biomass, functional genes, and putative ecological roles. ISME J. 2019, 13, 1484–1496. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Lemetais, G.; Ferreira, T.; Cayot, P.; Gervais, P.; Beney, L. Ergosterol biosynthesis: A fungal pathway for life on land? Evolution 2012, 66, 2961–2968. [Google Scholar] [CrossRef] [PubMed]
- Ravelet, C.; Grosset, C.; Alary, J. Quantitation of ergosterol in river sediment by liquid chromatography. J. Chromatogr. Sci. 2001, 39, 239–242. [Google Scholar] [CrossRef][Green Version]
- Verma, B.; Robarts, R.D.; Headley, J.V.; Peru, K.; Christofi, N. Extraction efficiencies and determination of ergosterol in a variety of environmental matrices. Commun. Soil Sci. Plant Anal. 2002, 33, 3261–3275. [Google Scholar] [CrossRef]
- Gutiérrez, M.H.; Vera, J.; Srain, B.; Quiñones, R.A.; Wörmer, L.; Hinrichs, K.U.; Pantoja-Gutiérrez, S. Biochemical fingerprints of marine fungi: Implications for trophic and biogeochemical studies. Aquat. Microb. Ecol. 2020, 84, 75–90. [Google Scholar] [CrossRef]
- Newell, S. Fungal biomass and productivity. Methods Microbiol. 2001, 30, 357–372. [Google Scholar] [CrossRef]
- Raghukumar, S. Fungi in Coastal and Oceanic Marine Ecosystems; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Akihisa, T.; Hori, T.; Suzuki, H.; Sakoh, T.; Yokota, T.; Tamura, T. 24β-methyl-5α-cholest-9 (11)-en-3β-ol, two 24β-alkyl-Δ5, 7, 9 (11)-sterols and other 24β-alkylsterol from Chlorella vulgaris. Phytochemistry 1992, 31, 1769–1772. [Google Scholar] [CrossRef]
- Taylor, J.D.; Cunliffe, M. Multi-year assessment of coastal planktonic fungi reveals environmental drivers of diversity and abundance. ISME J. 2016, 10, 2118–2128. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Wickerham, J. A taxonomic study of Monilia albicans with special emphasis on morphology and morphological variation. J. Trop. Med. Hyg. 1939, 42, 174–179. [Google Scholar]
- Wickerham, L. Taxonomy of Yeasts; Technical Bulletin; US Department of Agriculture: Washington, DC, USA, 1951; Volume 1029, pp. 1–56. [Google Scholar]
- Gulis, V.; Bärlocher, F. Fungi: Biomass, production, and community structure. Methods Stream Ecol. 2017, 1, 177–192. [Google Scholar] [CrossRef]
- Wenzl, T.; Haedrich, J.; Schaechtele, A.; Robouch, P.; Stroka, J. Guidance document on the estimation of LOD and LOQ for measurements in the field of contaminants in feed and food; Publications Office of the European Union: Luxembourg, 2016; Volume 58. [Google Scholar] [CrossRef]
- Ory, L.; Gentil, E.; Kumla, D.; Kijjoa, A.; Nazih, E.H.; Roullier, C. Detection of ergosterol using liquid chromatography/electrospray ionization mass spectrometry: Investigation of unusual in-source reactions. Rapid Commun. Mass Spectrom. 2020, 34, e8780. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L. The multifunctional fungal ergosterol. mBio 2018, 9, e01755-18. [Google Scholar] [CrossRef]
- Roose, P.; Smedes, F. Evaluation of the results of the QUASIMEME lipid intercomparison: The Bligh & Dyer total lipid extraction method. Mar. Pollut. Bull. 1996, 32, 674–680. [Google Scholar] [CrossRef]
| Coordinates | Pelagic Zone | Depth (m) | Ergosterol Concentration (pM) | Ergosterol Concentration (ng/L) | |
|---|---|---|---|---|---|
| Latitude | Longitude | ||||
| 13°35.748 | −29°42.830 | Epipelagic | 5 | 0.306 | 0.121 |
| Mesopelagic | 950 | 0.120 | 0.048 | ||
| Bathypelagic | 4000 | 0.000 | 0.000 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar Alekseyeva, K.; Mähnert, B.; Berthiller, F.; Breyer, E.; Herndl, G.J.; Baltar, F. Adapting an Ergosterol Extraction Method with Marine Yeasts for the Quantification of Oceanic Fungal Biomass. J. Fungi 2021, 7, 690. https://doi.org/10.3390/jof7090690
Salazar Alekseyeva K, Mähnert B, Berthiller F, Breyer E, Herndl GJ, Baltar F. Adapting an Ergosterol Extraction Method with Marine Yeasts for the Quantification of Oceanic Fungal Biomass. Journal of Fungi. 2021; 7(9):690. https://doi.org/10.3390/jof7090690
Chicago/Turabian StyleSalazar Alekseyeva, Katherine, Barbara Mähnert, Franz Berthiller, Eva Breyer, Gerhard J. Herndl, and Federico Baltar. 2021. "Adapting an Ergosterol Extraction Method with Marine Yeasts for the Quantification of Oceanic Fungal Biomass" Journal of Fungi 7, no. 9: 690. https://doi.org/10.3390/jof7090690
APA StyleSalazar Alekseyeva, K., Mähnert, B., Berthiller, F., Breyer, E., Herndl, G. J., & Baltar, F. (2021). Adapting an Ergosterol Extraction Method with Marine Yeasts for the Quantification of Oceanic Fungal Biomass. Journal of Fungi, 7(9), 690. https://doi.org/10.3390/jof7090690







