First Characterization and Description of Aspergillus Series Versicolores in French Bioaerosols
Abstract
1. Introduction
2. Material and Methods
2.1. Sampling
2.2. Molecular Characterization
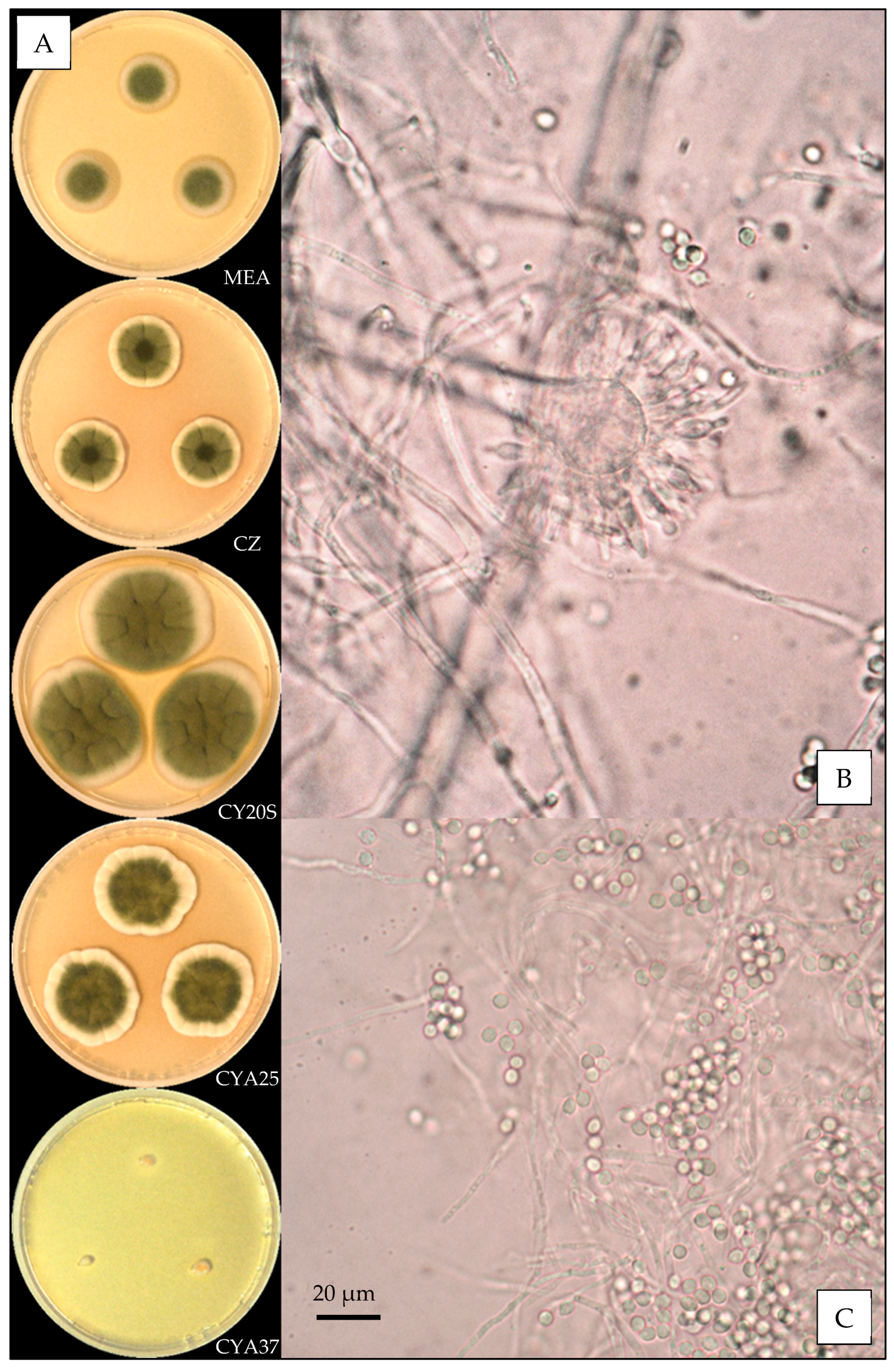
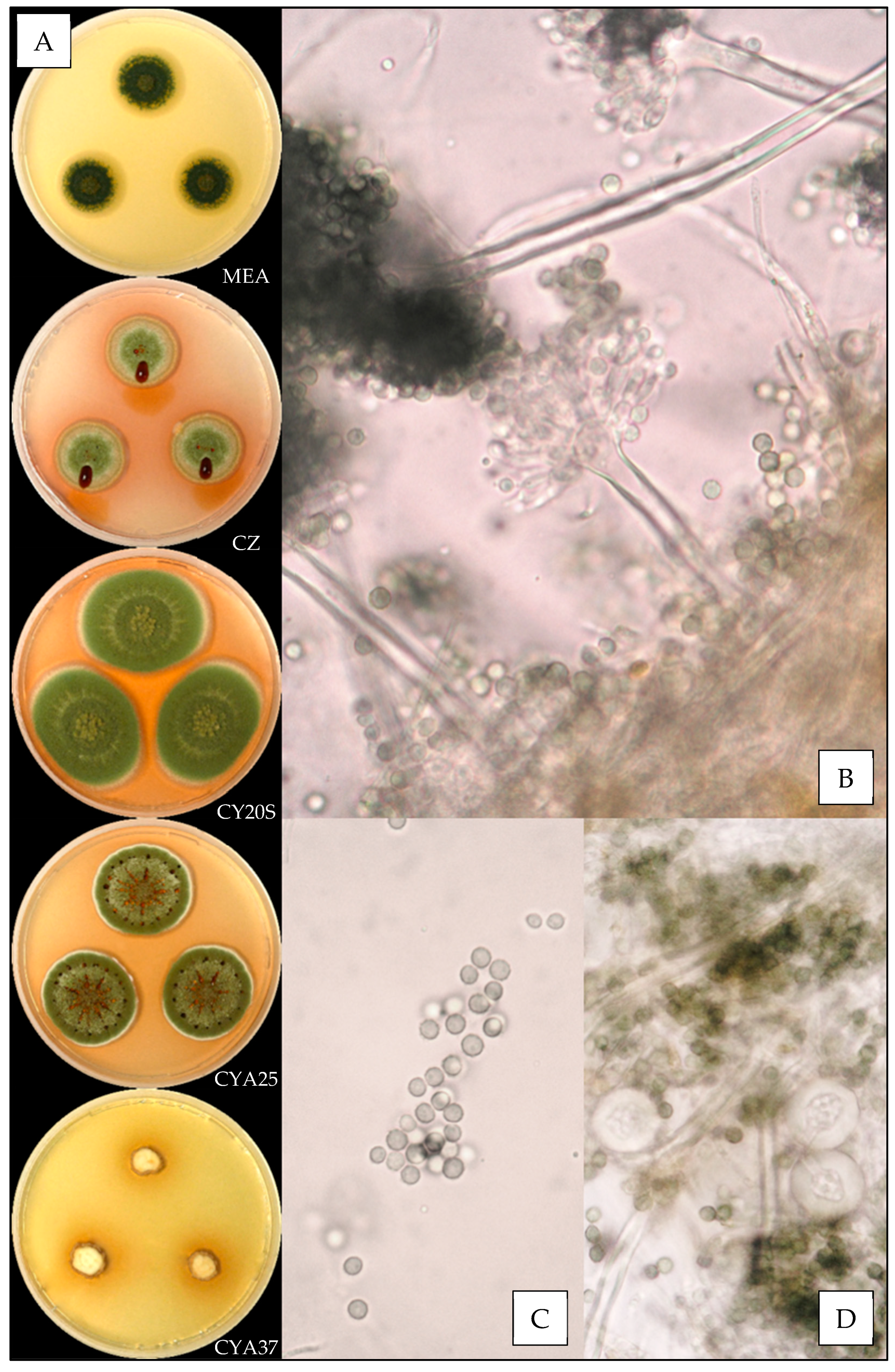
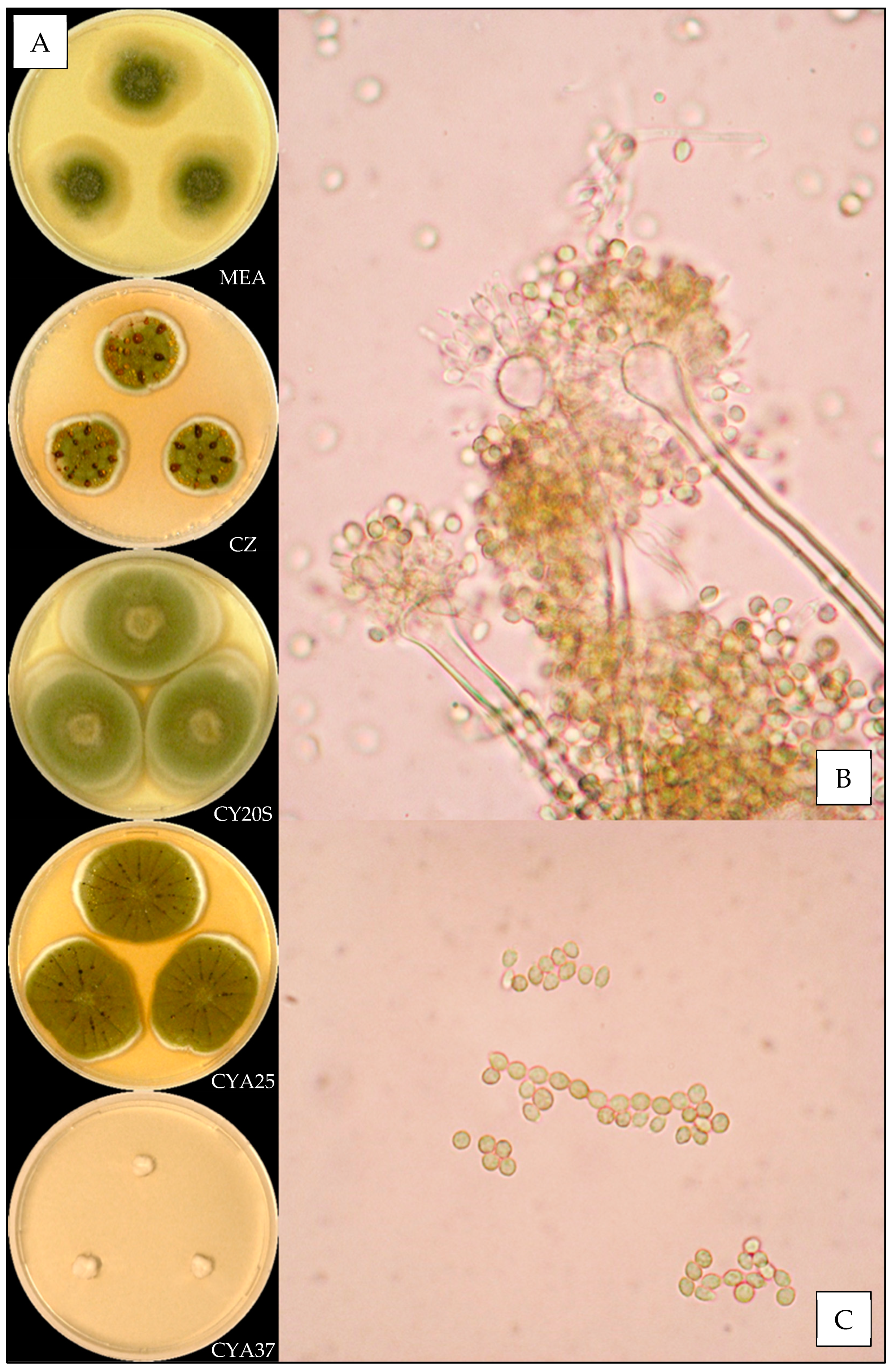
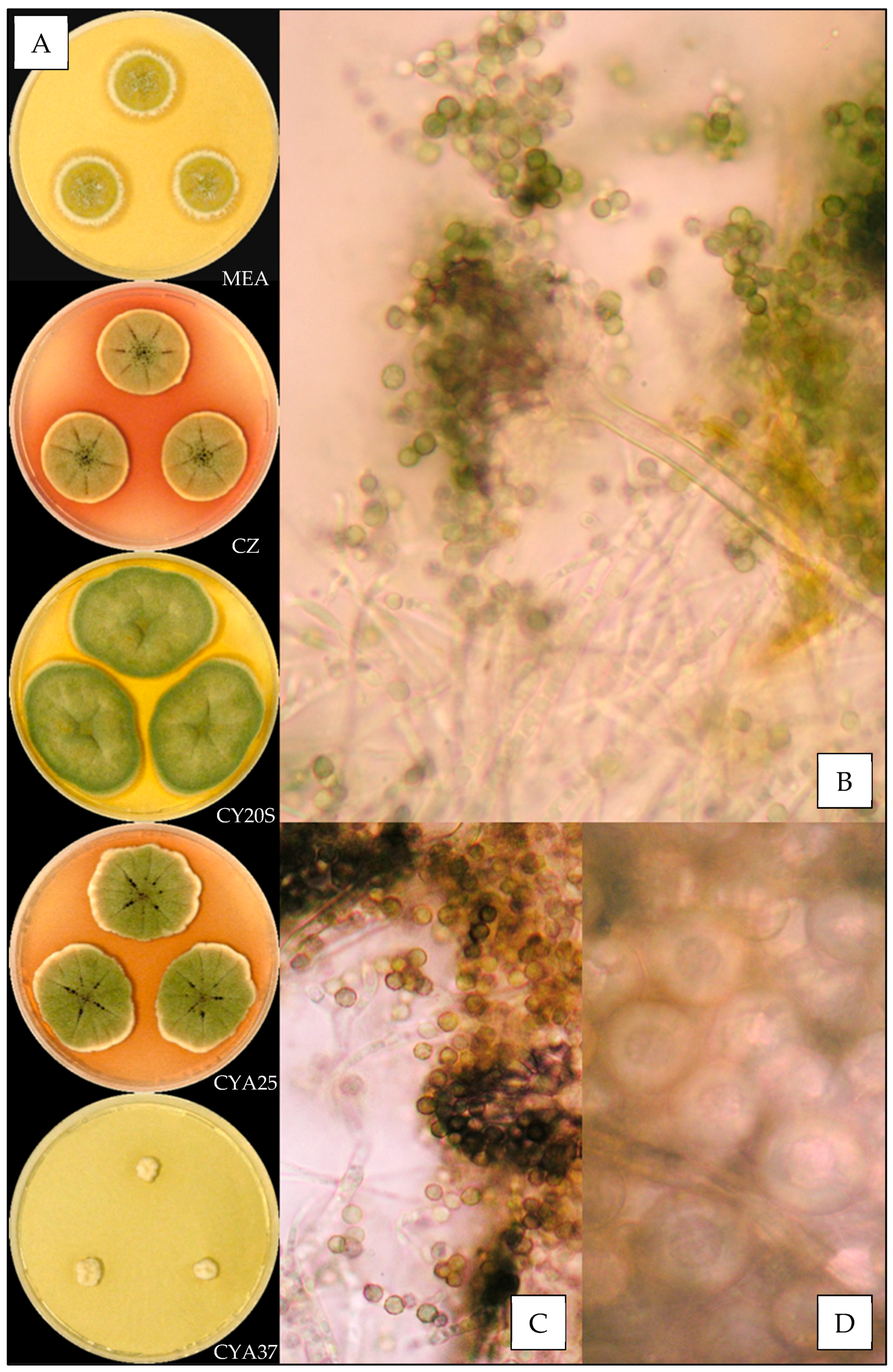
2.3. Macroscopic Characterization
2.4. Microscopic Characterization
2.5. Statistical Analysis
3. Results
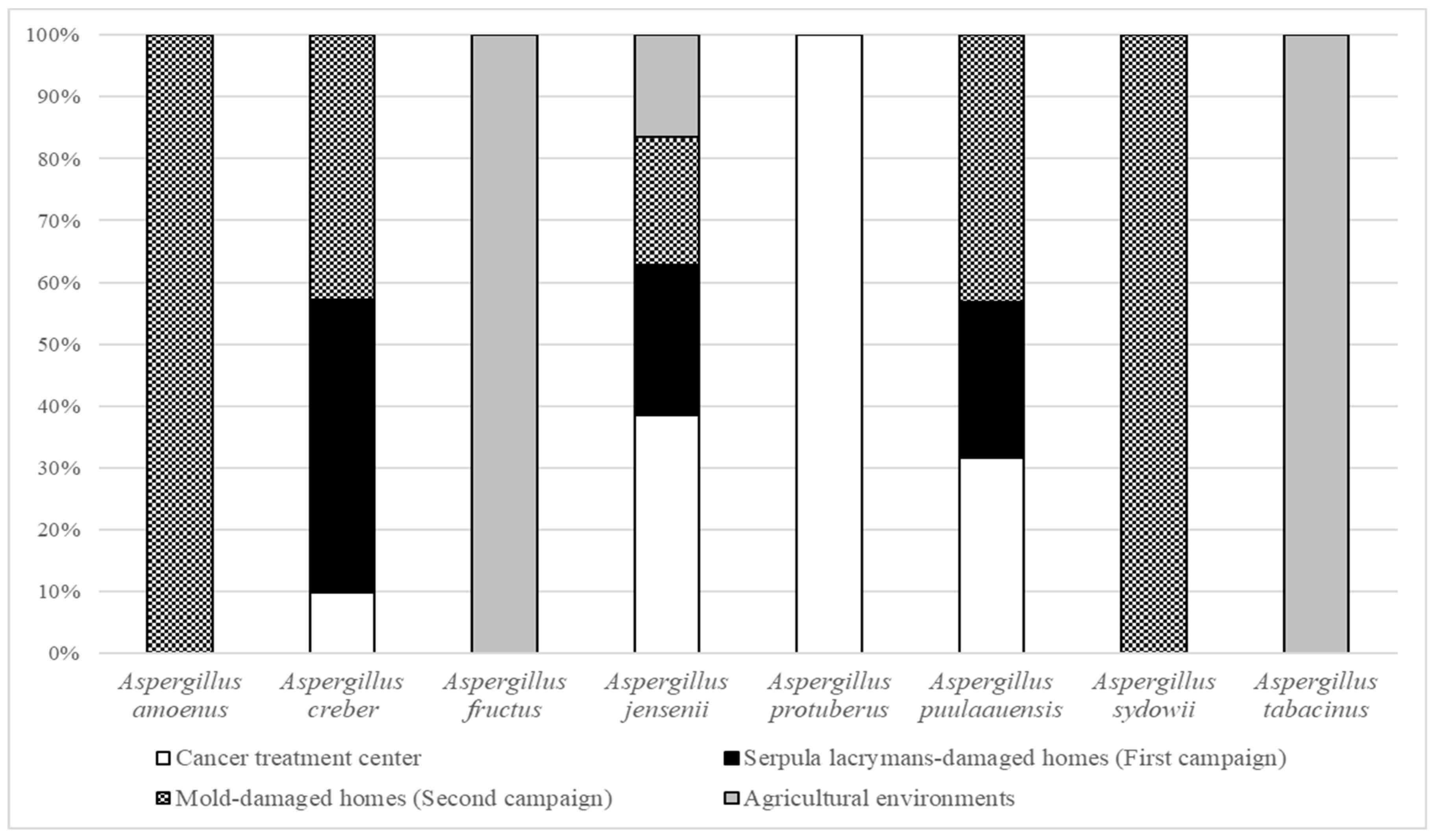
3.1. Relative Abundance in French Bioaerosols
3.2. Environmental Data
3.3. Description of the Versicolores Series Species
3.3.1. Aspergillus amoenus
3.3.2. Aspergillus creber
3.3.3. Aspergillus fructus
3.3.4. Aspergillus jensenii
3.3.5. Aspergillus protuberus
3.3.6. Aspergillus puulaauensis
3.3.7. Aspergillus sydowii
3.3.8. Aspergillus tabacinus
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (Ed.) Indoor Air Pollutants: Exposure and Health Effects: Report on a WHO Meeting, Nördlingen, 8–11 June 1982; EURO Reports and Studies; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 1983; ISBN 978-92-890-1244-7. [Google Scholar]
- Boulanger, G.; Bayeux, T.; Mandin, C.; Kirchner, S.; Vergriette, B.; Pernelet-Joly, V.; Kopp, P. Socio-Economic Costs of Indoor Air Pollution: A Tentative Estimation for Some Pollutants of Health Interest in France. Environ. Int. 2017, 104, 14–24. [Google Scholar] [CrossRef]
- Who Regional Office For Europe. WHO Guidelines for Indoor Air Quality: Dampness and Mould; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2009; ISBN 978-92-890-4168-3. [Google Scholar]
- Andersen, B.; Frisvad, J.C.; Søndergaard, I.; Rasmussen, I.S.; Larsen, L.S. Associations between Fungal Species and Water-Damaged Building Materials. Appl. Environ. Microbiol. 2011, 77, 4180–4188. [Google Scholar] [CrossRef]
- ANSES. Moisissures Dans Le Bâti; ANSES Editions: Maisons-Alfort, France, 2016; p. 374. [Google Scholar]
- Kopp, P.; Kirchner, S.; Boulanger, G.; Pernelet-Joly, V.; Bayeux, T.; Vergriette, B.; Mandin, C. Étude Exploratoire Du Coût Socio-Économique Des Polluants de l’air Intérieur; ANSES Editions: Maisons-Alfort, France, 2014. [Google Scholar]
- Tiotiu, A.I.; Novakova, P.; Nedeva, D.; Chong-Neto, H.J.; Novakova, S.; Steiropoulos, P.; Kowal, K. Impact of Air Pollution on Asthma Outcomes. Int. J. Environ. Res. Public. Health 2020, 17, 6212. [Google Scholar] [CrossRef]
- Rick, E.; Woolnough, K.; Pashley, C.; Wardlaw, A. Allergic Fungal Airway Disease. J. Investig. Allergol. Clin. Immunol. 2016, 26, 344–354. [Google Scholar] [CrossRef]
- Gletsou, E.; Ioannou, M.; Liakopoulos, V.; Tsiambas, E.; Ragos, V.; Stefanidis, I. Aspergillosis in Immunocompromised Patients with Haematological Malignancies. J. BUON Off. J. Balk. Union Oncol. 2018, 23, 7–10. [Google Scholar]
- Spellberg, B.; Edwards, J.; Ibrahim, A. Novel Perspectives on Mucormycosis: Pathophysiology, Presentation, and Management. Clin. Microbiol. Rev. 2005, 18, 556–569. [Google Scholar] [CrossRef]
- Bailey, J.R.; Breton, J.; Panic, G.; Cogan, T.A.; Bailey, M.; Swann, J.R.; Lee, M.R. The Mycotoxin Deoxynivalenol Significantly Alters the Function and Metabolism of Bovine Kidney Epithelial Cells In Vitro. Toxins 2019, 11, 554. [Google Scholar] [CrossRef]
- Schmutz, C.; Cenk, E.; Marko, D. The Alternaria Mycotoxin Alternariol Triggers the Immune Response of IL-1β-stimulated, Differentiated Caco-2 Cells. Mol. Nutr. Food Res. 2019, 63, 1900341. [Google Scholar] [CrossRef] [PubMed]
- Benkerroum, N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef]
- Kis-Papo, T.; Kirzhner, V.; Wasser, S.P.; Nevo, E. Evolution of Genomic Diversity and Sex at Extreme Environments: Fungal Life under Hypersaline Dead Sea Stress. Proc. Natl. Acad. Sci. USA 2003, 100, 14970–14975. [Google Scholar] [CrossRef]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi; Academic Press: London, UK; New York, NY, USA, 1980; ISBN 978-0-12-220401-2. [Google Scholar]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: New York, NY, USA, 2009; ISBN 978-0-387-92207-2. [Google Scholar]
- Delanoë, A.; Heutte, N.; Gente, S.; Séguin, V.; Garon, D. Relationships between Exposure to Bioaerosols, Moldy Surface and Symptoms in French Mold-Damaged Homes. Atmosphere 2020, 11, 223. [Google Scholar] [CrossRef]
- Vincent, M.; Percier, P.; De Prins, S.; Huygen, K.; Potemberg, G.; Muraille, E.; Romano, M.; Michel, O.; Denis, O. Investigation of Inflammatory and Allergic Responses to Common Mold Species: Results from in Vitro Experiments, from a Mouse Model of Asthma, and from a Group of Asthmatic Patients. Indoor Air 2017, 27, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Mintz-Cole, R.A.; Gibson, A.M.; Bass, S.A.; Budelsky, A.L.; Reponen, T.; Hershey, G.K.K. Dectin-1 and IL-17A Suppress Murine Asthma Induced by Aspergillus Versicolor but Not Cladosporium Cladosporioides Due to Differences in β-Glucan Surface Exposure. J. Immunol. 2012, 189, 3609–3617. [Google Scholar] [CrossRef] [PubMed]
- Norbäck, D.; Hashim, J.H.; Cai, G.-H.; Hashim, Z.; Ali, F.; Bloom, E.; Larsson, L. Rhinitis, Ocular, Throat and Dermal Symptoms, Headache and Tiredness among Students in Schools from Johor Bahru, Malaysia: Associations with Fungal DNA and Mycotoxins in Classroom Dust. PLoS ONE 2016, 11, e0147996. [Google Scholar] [CrossRef]
- Torres-Rodríguez, J.M.; Madrenys-Brunet, N.; Siddat, M.; López-Jodra, O.; Jimenez, T. Aspergillus Versicolor as Cause of Onychomycosis: Report of 12 Cases and Susceptibility Testing to Antifungal Drugs. J. Eur. Acad. Dermatol. Venereol. JEADV 1998, 11, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Charles, M.P. Invasive Pulmonary Aspergillosis Caused by Aspergillus Versicolor in a Patient on Mechanical Ventilation. Australas. Med. J. 2011, 4, 632–634. [Google Scholar] [CrossRef]
- Kubosaki, A.; Kobayashi, N.; Watanabe, M.; Yoshinari, T.; Takatori, K.; Kikuchi, Y.; Hara-Kudo, Y.; Terajima, J.; Sugita-Konishi, Y. A New Protocol for the Detection of Sterigmatocystin-Producing Aspergillus Section Versicolores Using a High Discrimination Polymerase. Biocontrol Sci. 2020, 25, 113–118. [Google Scholar] [CrossRef]
- IARC Monographs On The Identification of Carcinogenic Hazards To Humans. Available online: https://monographs.iarc.who.int/ (accessed on 23 June 2021).
- Jurjevic, Z.; Peterson, S.W.; Horn, B.W. Aspergillus Section Versicolores: Nine New Species and Multilocus DNA Sequence Based Phylogeny. IMA Fungus 2012, 3, 59–79. [Google Scholar] [CrossRef]
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.-C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and Related Genera (Eurotiales): An Overview of Families, Genera, Subgenera, Sections, Series and Species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef]
- Visagie, C.M.; Hirooka, Y.; Tanney, J.B.; Whitfield, E.; Mwange, K.; Meijer, M.; Amend, A.S.; Seifert, K.A.; Samson, R.A. Aspergillus, Penicillium and Talaromyces Isolated from House Dust Samples Collected around the World. Stud. Mycol. 2014, 78, 63–139. [Google Scholar] [CrossRef] [PubMed]
- Jakšić, D.; Sertić, M.; Kifer, D.; Kocsubè, S.; Mornar Turk, A.; Nigović, B.; Šarkanj, B.; Krska, R.; Sulyok, M.; Šegvić Klarić, M. Fungi and Their Secondary Metabolites in Water-damaged Indoors after a Major Flood Event in Eastern Croatia. Indoor Air 2021, 31, 730–744. [Google Scholar] [CrossRef]
- Kobayashi, N.; Kubosaki, A.; Takahashi, Y.; Yanai, M.; Konuma, R.; Uehara, S.; Chiba, T.; Watanabe, M.; Terajima, J.; Sugita-Konishi, Y. Distribution of Sterigmatocystin-Producing Aspergilli in Japan. Food Saf. 2018, 6, 67–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siqueira, J.P.Z.; Sutton, D.A.; García, D.; Gené, J.; Thomson, P.; Wiederhold, N.; Guarro, J. Species Diversity of Aspergillus Section Versicolores in Clinical Samples and Antifungal Susceptibility. Fungal Biol. 2016, 120, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Heutte, N.; André, V.; Bonhomme, J.; Dubos Arvis, C.; Kientz-Bouchart, V.; Lemarié, F.; Legendre, P.; Louis, M.-Y.; Madelaine, S.; Gente, S.; et al. Suivi de la Qualité de L’air Dans un Centre de Lutte Contre le Cancer: Evaluation et Caractérisation de L’exposition aux Contaminants Fongiques (Biohospitalair). Available online: https://www.hygienes.net/boutique/hygienes-2/suivi-de-qualite-de-lair-centre-de-lutte-contre-cancer-evaluation-caracterisation-de-lexposition-aux-contaminants-fongiques-biohospitalair/ (accessed on 24 June 2021).
- Pottier, D.; Andre, V.; Rioult, J.; Bourreau, A.; Duhamel, C.; Bouchart, V.K.; Richard, E.; Guibert, M.; Verite, P.; Garon, D. Airborne Molds and Mycotoxins in Serpula Lacrymans-Damaged Homes. Atmos. Pollut. Res. 2014, 5, 325–334. [Google Scholar] [CrossRef]
- Lanier, C.; Richard, E.; Heutte, N.; Picquet, R.; Bouchart, V.; Garon, D. Airborne Molds and Mycotoxins Associated with Handling of Corn Silage and Oilseed Cakes in Agricultural Environment. Atmos. Environ. 2010, 44, 1980–1986. [Google Scholar] [CrossRef]
- Séguin, V.; Lemauviel-Lavenant, S.; Garon, D.; Bouchart, V.; Gallard, Y.; Blanchet, B.; Diquelou, S.; Personeni, E.; Gauduchon, P.; Ourry, A. Effect of Agricultural and Environmental Factors on the Hay Characteristics Involved in Equine Respiratory Disease. Agric. Ecosyst. Environ. 2010, 135, 206–215. [Google Scholar] [CrossRef]
- Desjardins, P.; Conklin, D. NanoDrop Microvolume Quantitation of Nucleic Acids. J. Vis. Exp. 2010, 45, 2565. [Google Scholar] [CrossRef]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.-B.; Hubka, V.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, Identification and Nomenclature of the Genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef] [PubMed]
- Klich, M.A. Identification of Common Aspergillus Species; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2002. [Google Scholar]
- Micheluz, A.; Sulyok, M.; Manente, S.; Krska, R.; Varese, G.C.; Ravagnan, G. Fungal Secondary Metabolite Analysis Applied to Cultural Heritage: The Case of a Contaminated Library in Venice. World Mycotoxin J. 2016, 9, 397–407. [Google Scholar] [CrossRef]
- Jakšić, D.; Kocsubé, S.; Bencsik, O.; Kecskeméti, A.; Szekeres, A.; Vágvölgyi, C.; Varga, J.; Klarić, M.Š. Species Diversity and Cytotoxic Potency of Airborne Sterigmatocystin-Producing Aspergilli from the Section Versicolores. Sci. Total Environ. 2016, 562, 296–304. [Google Scholar] [CrossRef]
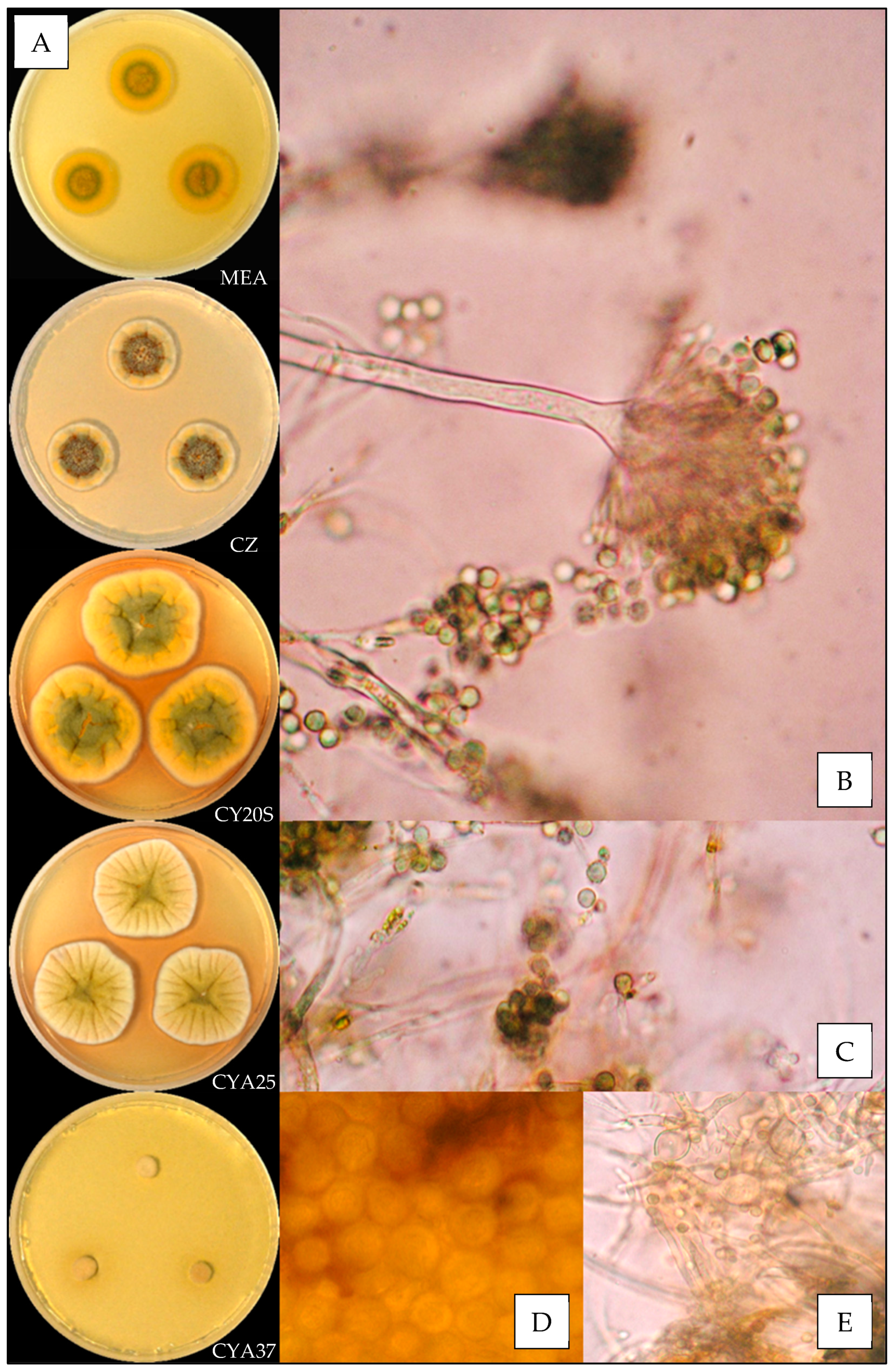
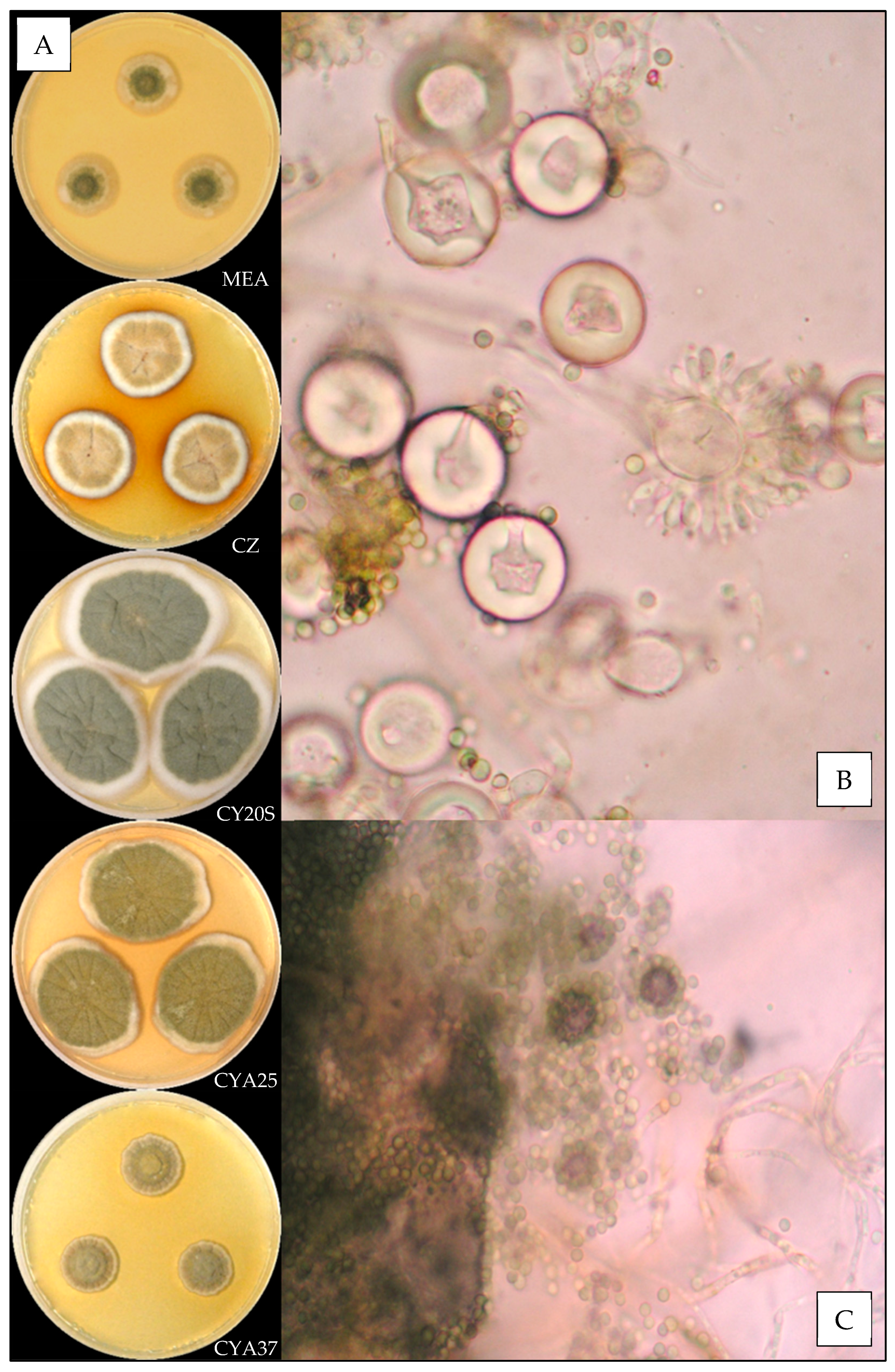
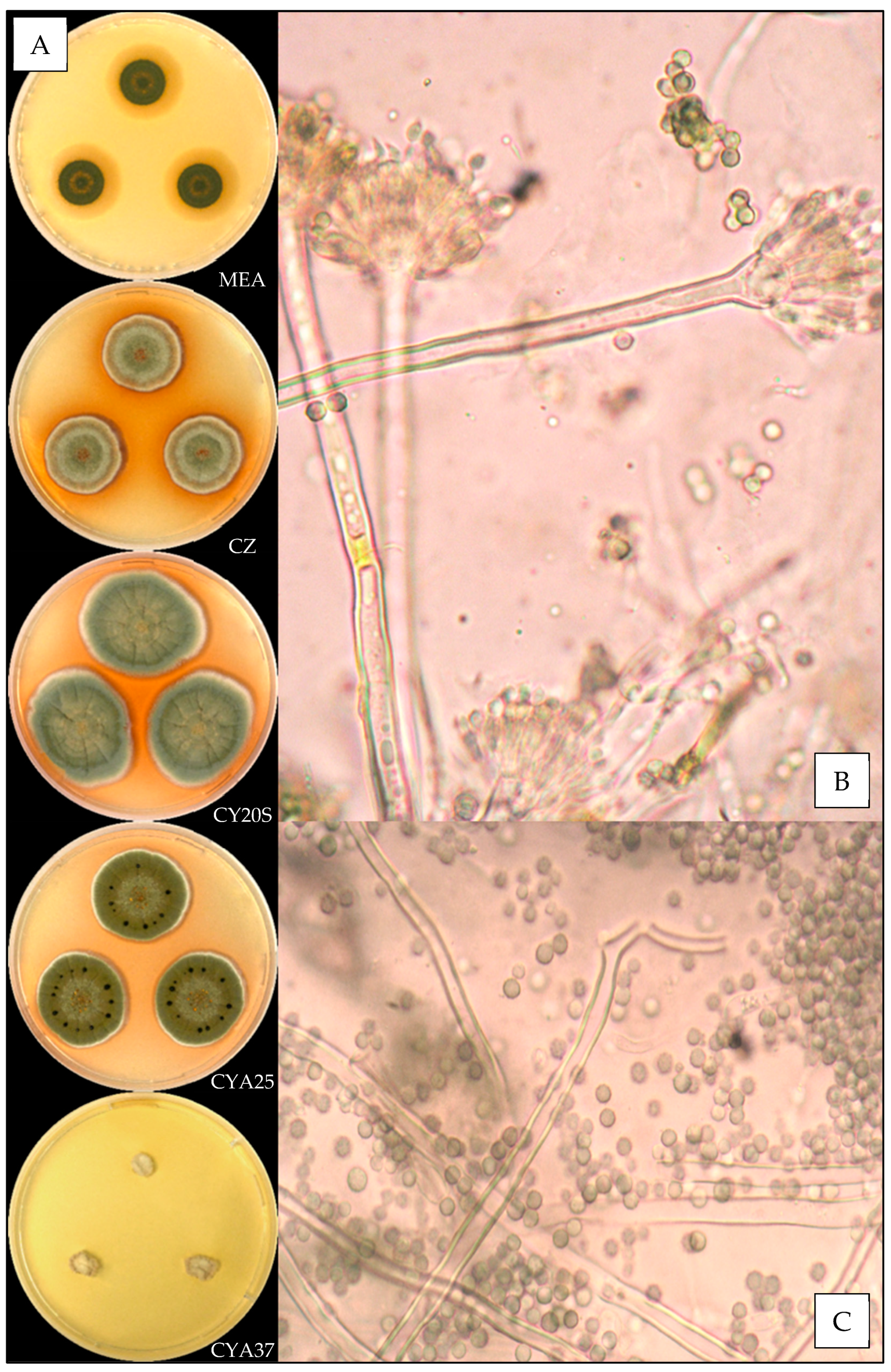
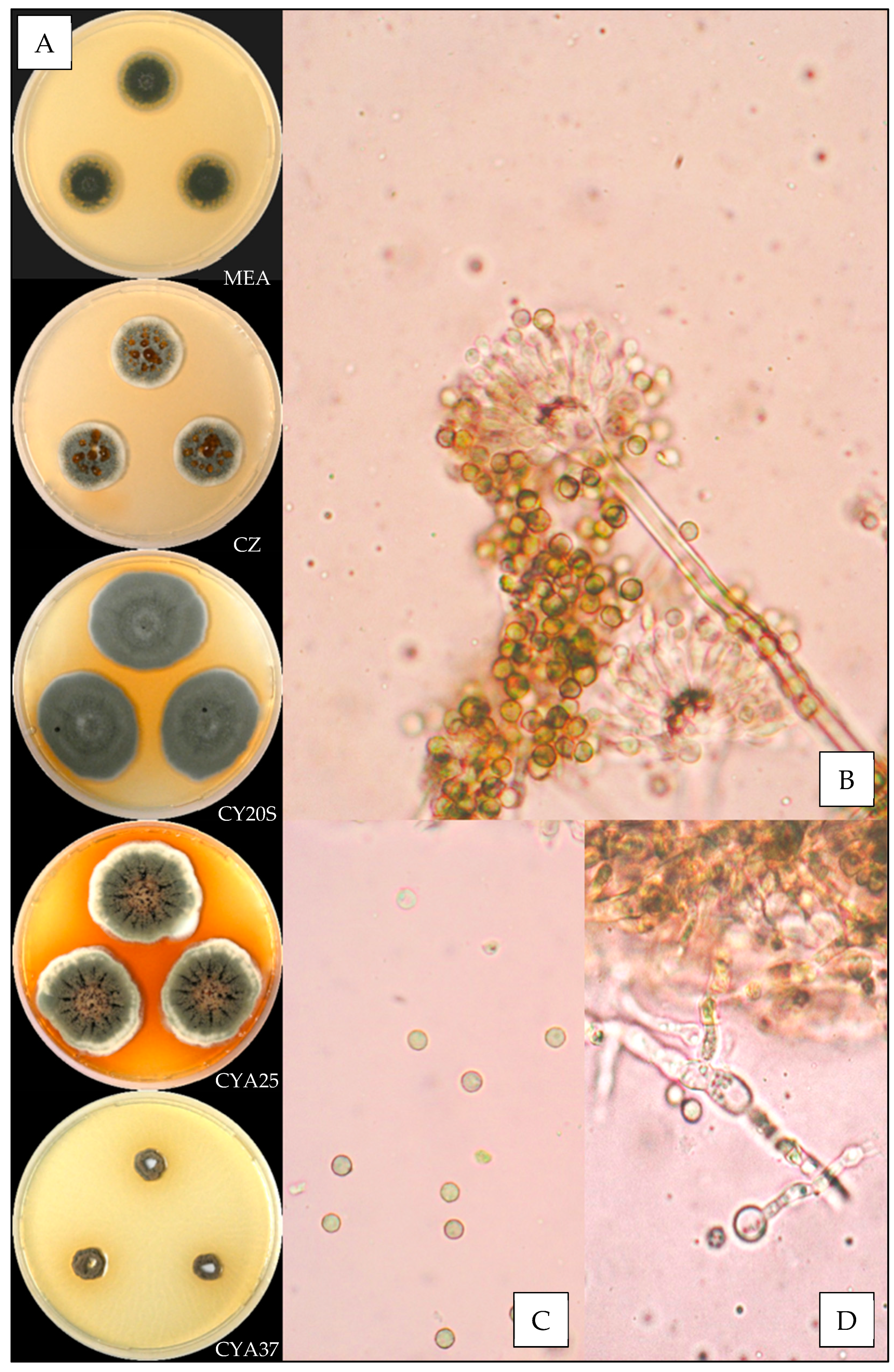
| Species | N | Temperature (°C) | Relative Humidity (%) | ||||
|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Min | Max | Mean | ||
| A. amoenus | 1 | - | - | 19.9 | - | - | 66.1 |
| A. creber | 40 | 9.4 | 26.1 | 20.7 | 23.8 | 74.3 | 57.5 |
| A. fructus | 1 | - | - | 18.9 | - | - | 41.2 |
| A. jensenii | 37 | 10.0 | 24.7 | 20.8 | 23.8 | 70.8 | 50.2 |
| A. protuberus | 6 | 22.9 | 24.3 | 23.7 | 26.1 | 45.7 | 34.6 |
| A. puulaauensis | 4 | 10.3 | 23.9 | 20.2 | 23.8 | 66.9 | 53.0 |
| A. sydowii | 2 | 22.8 | 26.1 | 24.5 | 46.7 | 69.6 | 58.2 |
| A. tabacinus | 2 | 18.4 | 20.8 | 19.6 | 32.4 | 49.2 | 40.8 |
| Aspergilllus amoenus | Aspergillus creber | Aspergillus fructus | Aspergillus jensenii | Aspergillus protuberus | Aspergillus puulaauensis | Aspergillus sydowii | Aspergillus tabacinus | |
|---|---|---|---|---|---|---|---|---|
| Conidial head (µm) | 12–15 | (3–) 5–13 (–15) | 12–13 | (3–) 7–14 | 11–16 | 9–15 | 6–12 (–14) | 9–14 |
| Metulae (µm) | 4–6 | (3–) 4–6 (–7) | 4–6 | 4–6 (–7) | 3–5 | 4–6 | 4–7 | 4–6 |
| Phialides (µm) | 5–6 | 4–6 (–8) | 5–6 | 4–7 (–8) | 4–7 | 5–6 | 5–7 (–8) | 5–7 |
| Conidia size (µm) | 3–4 | 2.5–4.5 | 3–4 | (2–) 3–4 (–5) | 2.5–3.5 | 3–4.5 | 3–4 (–5) | 2.5–3.5 |
| Conidial wall ornementation | finely or distinctly roughened | finely or distinctly roughened | smooth or finely roughened | roughened or finely roughened | smooth or finely roughened | roughened | roughened to distinctly roughened | distinctly roughened |
| Hülle cells | − | + | − | + | + | − | − | + |
| Chlamydospores | − | − | − | + | − | − | + | − |
| Diminutive conidial head | − | + | − | + | − | − | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Géry, A.; Rioult, J.-P.; Heutte, N.; Séguin, V.; Bonhomme, J.; Garon, D. First Characterization and Description of Aspergillus Series Versicolores in French Bioaerosols. J. Fungi 2021, 7, 676. https://doi.org/10.3390/jof7080676
Géry A, Rioult J-P, Heutte N, Séguin V, Bonhomme J, Garon D. First Characterization and Description of Aspergillus Series Versicolores in French Bioaerosols. Journal of Fungi. 2021; 7(8):676. https://doi.org/10.3390/jof7080676
Chicago/Turabian StyleGéry, Antoine, Jean-Philippe Rioult, Natacha Heutte, Virginie Séguin, Julie Bonhomme, and David Garon. 2021. "First Characterization and Description of Aspergillus Series Versicolores in French Bioaerosols" Journal of Fungi 7, no. 8: 676. https://doi.org/10.3390/jof7080676
APA StyleGéry, A., Rioult, J.-P., Heutte, N., Séguin, V., Bonhomme, J., & Garon, D. (2021). First Characterization and Description of Aspergillus Series Versicolores in French Bioaerosols. Journal of Fungi, 7(8), 676. https://doi.org/10.3390/jof7080676






