Tocilizumab Induces IL-10-Mediated Immune Tolerance in Invasive Candidiasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Stimuli
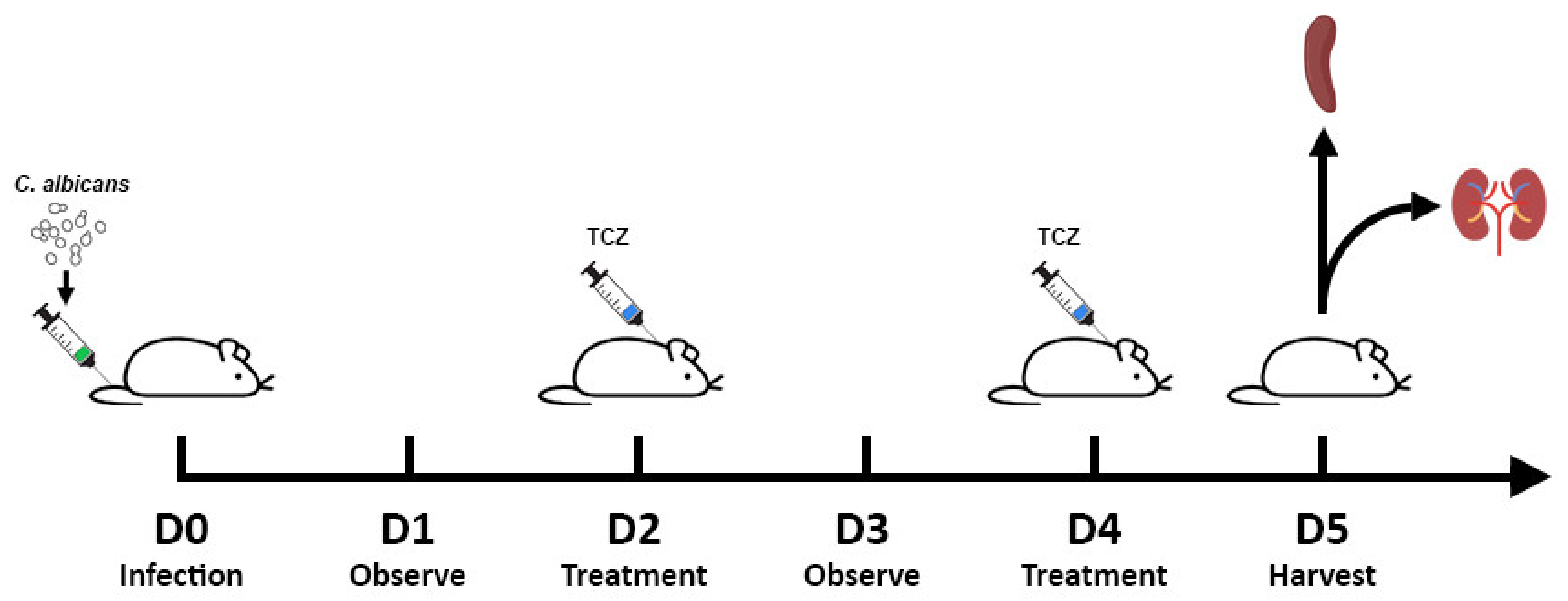
2.2. Disseminated Candidiasis Mice Model
2.3. Infection
2.4. Fungal Load Quantification
2.5. Histology
2.6. Stimulation and Cytokine Measurement
2.7. Statistics
2.8. Flow Cytometry
3. Results
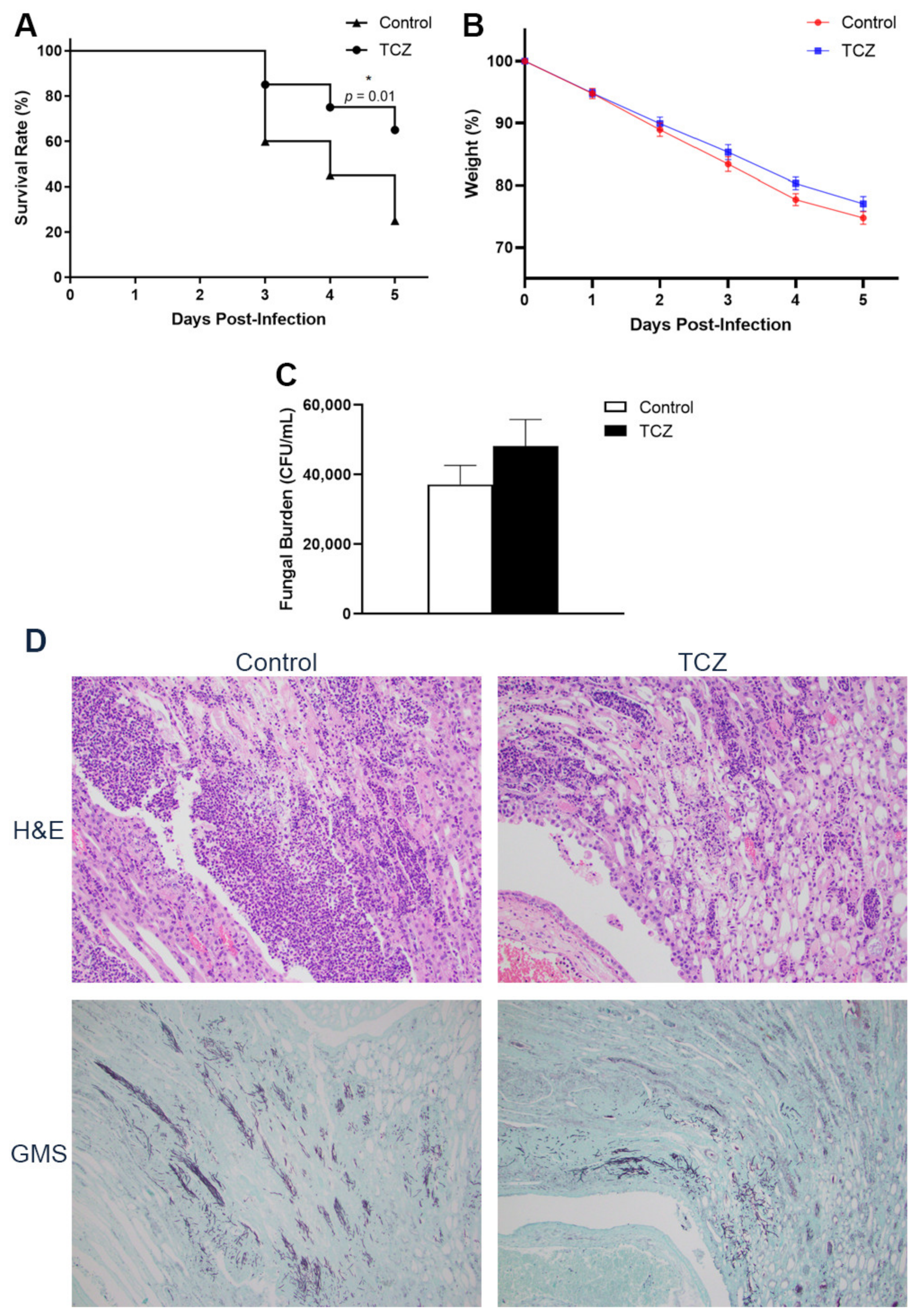
3.1. Tocilizumab-Treated Mice Showed Lower Short-Term Mortality despite No Reduction in Fungal Burden
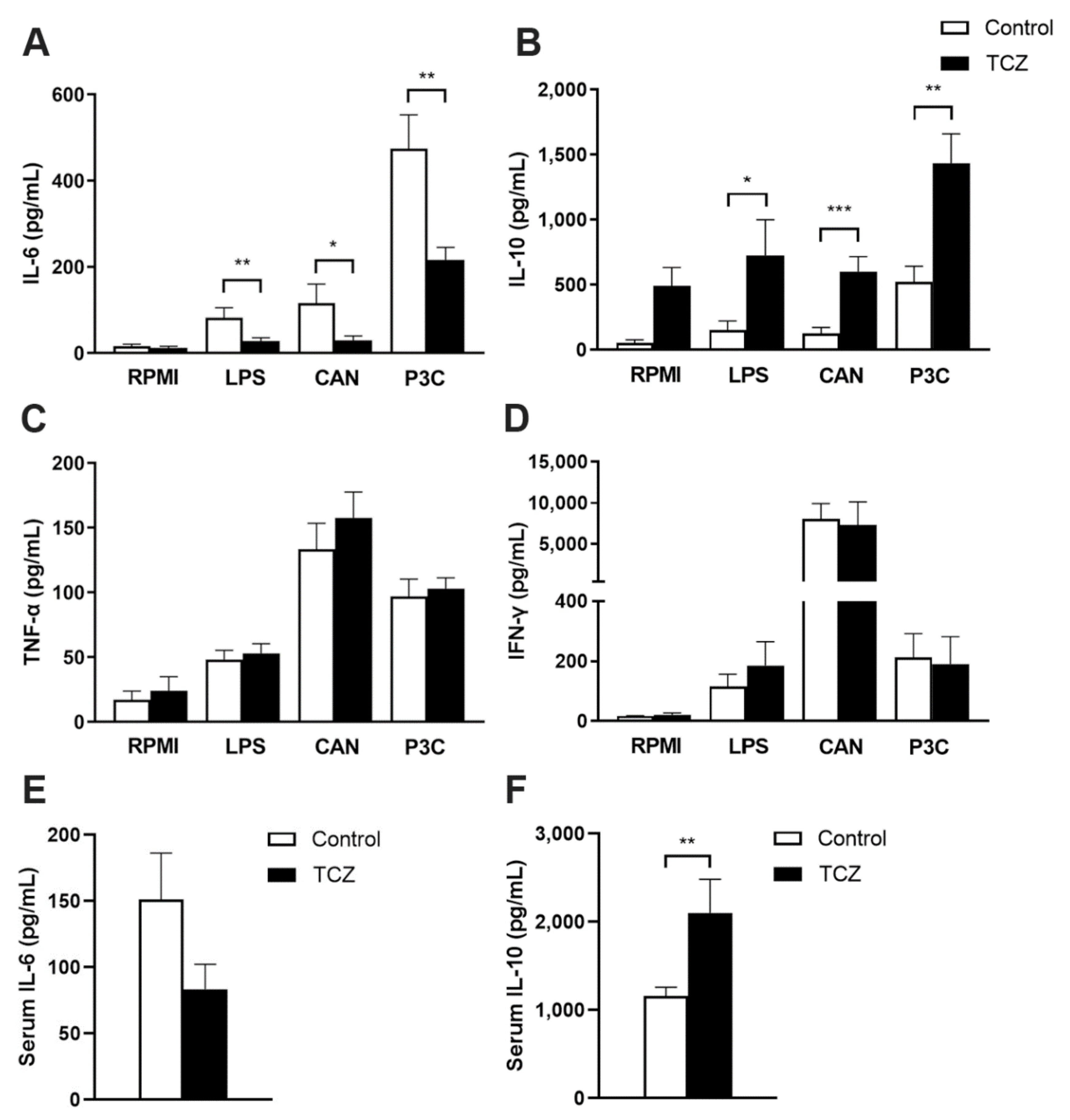
3.2. Tocilizumab Treatment Results in Elevated Interleukin-10 Response
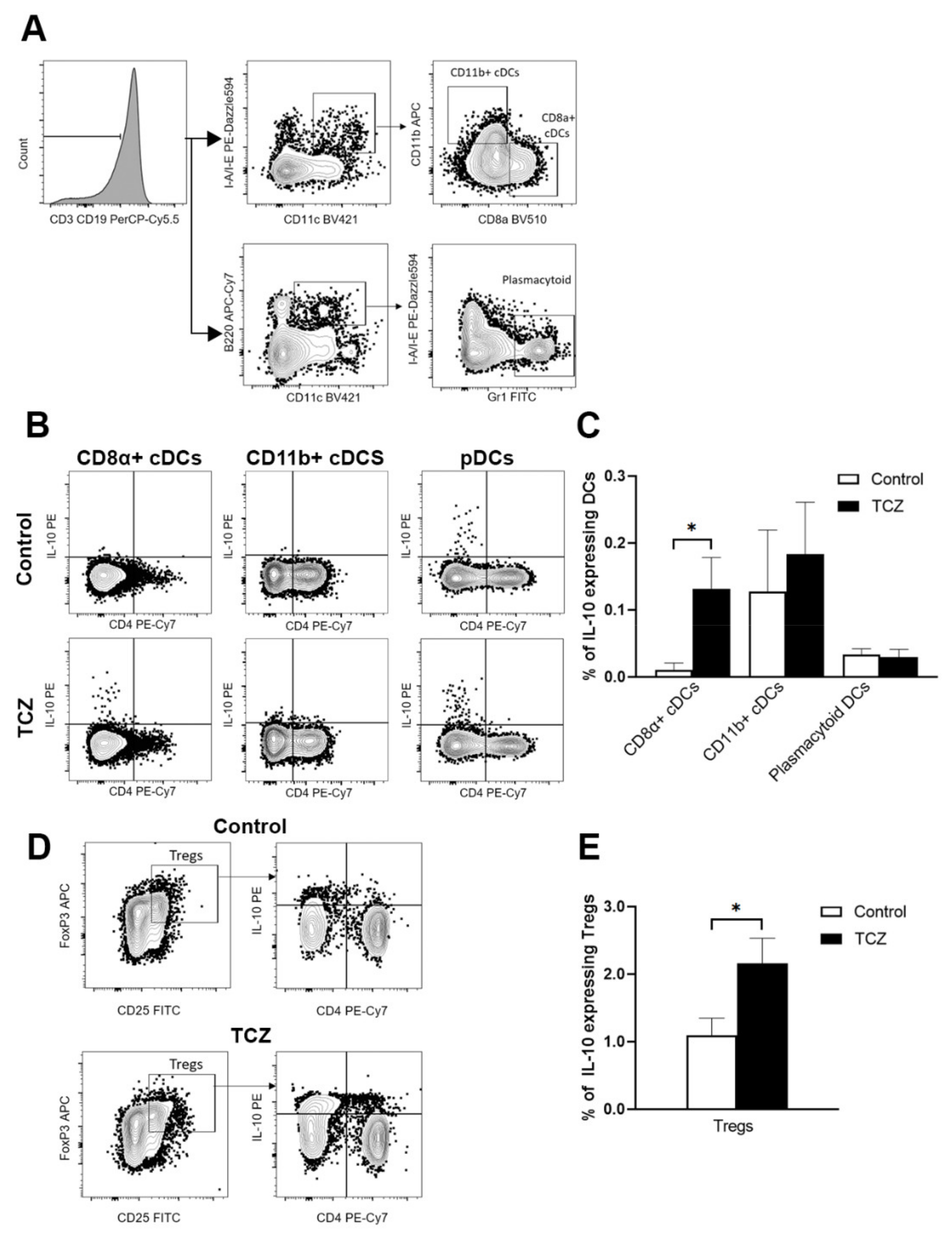
3.3. Tocilizumab Induced IL-10-Secreting CD8α+ Dendritic Cells and Peripheral T Regulatory Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Neofytos, D.; Horn, D.; Anaissie, E.; Steinbach, W.; Olyaei, A.; Fishman, J.; Pfaller, M.; Chang, C.; Webster, K.; Marr, K. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: Analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin. Infect. Dis. 2009, 48, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.Y.; Vonk, A.G.; Kullberg, B.J.; Netea, M.G. Immune response to Aspergillus fumigatus in compromised hosts: From bedside to bench. Future Microbiol. 2011, 6, 73–83. [Google Scholar] [CrossRef]
- Wong, A.Y.W.; Fric, J.; Zelante, T. Learning to control tissue damage while fighting Aspergillus. Med. Mycol. 2019, 57, S189–s195. [Google Scholar] [CrossRef]
- Chai, L.; Netea, M.G.; Teerenstra, S.; Earnest, A.; Vonk, A.G.; Schlamm, H.T.; Herbrecht, R.; Troke, P.F.; Kullberg, B.J. Early proinflammatory cytokines and C-reactive protein trends as predictors of outcome in invasive Aspergillosis. J. Infect. Dis. 2010, 202, 1454–1462. [Google Scholar] [CrossRef]
- Miceli, M.H.; Maertens, J.; Buvé, K.; Grazziutti, M.; Woods, G.; Rahman, M.; Barlogie, B.; Anaissie, E.J. Immune reconstitution inflammatory syndrome in cancer patients with pulmonary aspergillosis recovering from neutropenia: Proof of principle, description, and clinical and research implications. Cancer 2007, 110, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Dellière, S.; Guery, R.; Candon, S.; Rammaert, B.; Aguilar, C.; Lanternier, F.; Chatenoud, L.; Lortholary, O. Understanding Pathogenesis and Care Challenges of Immune Reconstitution Inflammatory Syndrome in Fungal Infections. J. Fungi 2018, 4, 139. [Google Scholar] [CrossRef]
- Mihara, M.; Ohsugi, Y.; Kishimoto, T. Tocilizumab, a humanized anti-interleukin-6 receptor antibody, for treatment of rheumatoid arthritis. Open Access Rheumatol. 2011, 3, 19–29. [Google Scholar] [CrossRef]
- Choy, E.H.; De Benedetti, F.; Takeuchi, T.; Hashizume, M.; John, M.R.; Kishimoto, T. Translating IL-6 biology into effective treatments. Nat. Rev. Rheumatol. 2020, 16, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Kovács, R.; Czudar, A.; Horváth, L.; Szakács, L.; Majoros, L.; Kónya, J. Serum interleukin-6 levels in murine models of Candida albicans infection. Acta Microbiol. Immunol. Hung. 2014, 61, 61–69. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Tesmer, L.A.; Lundy, S.K.; Sarkar, S.; Fox, D.A. Th17 cells in human disease. Immunol. Rev. 2008, 223, 87–113. [Google Scholar] [CrossRef] [PubMed]
- Yuk, C.M.; Park, H.J.; Kwon, B.I.; Lah, S.J.; Chang, J.; Kim, J.Y.; Lee, K.M.; Park, S.H.; Hong, S.; Lee, S.H. Basophil-derived IL-6 regulates T(H)17 cell differentiation and CD4 T cell immunity. Sci. Rep. 2017, 7, 41744. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Tocilizumab: A Review in Rheumatoid Arthritis. Drugs 2017, 77, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Lokau, J.; Kleinegger, F.; Garbers, Y.; Waetzig, G.H.; Grötzinger, J.; Rose-John, S.; Haybaeck, J.; Garbers, C. Tocilizumab does not block interleukin-6 (IL-6) signaling in murine cells. PLoS ONE 2020, 15, e0232612. [Google Scholar] [CrossRef] [PubMed]
- Meley, D.; Héraud, A.; Gouilleux-Gruart, V.; Ivanes, F.; Velge-Roussel, F. Tocilizumab Contributes to the Inflammatory Status of Mature Dendritic Cells through Interleukin-6 Receptor Subunits Modulation. Front. Immunol. 2017, 8, 926. [Google Scholar] [CrossRef]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef]
- Dudziak, D.; Kamphorst, A.O.; Heidkamp, G.F.; Buchholz, V.R.; Trumpfheller, C.; Yamazaki, S.; Cheong, C.; Liu, K.; Lee, H.W.; Park, C.G.; et al. Differential antigen processing by dendritic cell subsets in vivo. Science 2007, 315, 107–111. [Google Scholar] [CrossRef]
- Audiger, C.; Rahman, M.J.; Yun, T.J.; Tarbell, K.V.; Lesage, S. The Importance of Dendritic Cells in Maintaining Immune Tolerance. J. Immunol. 2017, 198, 2223–2231. [Google Scholar] [CrossRef] [PubMed]
- Schnorrer, P.; Behrens, G.M.; Wilson, N.S.; Pooley, J.L.; Smith, C.M.; El-Sukkari, D.; Davey, G.; Kupresanin, F.; Li, M.; Maraskovsky, E.; et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc. Natl. Acad. Sci. USA 2006, 103, 10729–10734. [Google Scholar] [CrossRef] [PubMed]
- Heath, W.R.; Belz, G.T.; Behrens, G.M.; Smith, C.M.; Forehan, S.P.; Parish, I.A.; Davey, G.M.; Wilson, N.S.; Carbone, F.R.; Villadangos, J.A. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol. Rev. 2004, 199, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Das, R.; Komorowski, R.; Beres, A.; Hessner, M.J.; Mihara, M.; Drobyski, W.R. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood 2009, 114, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Sakai, R.; Ito, M.; Yoshimoto, K.; Chikuma, S.; Kurasawa, T.; Kondo, T.; Suzuki, K.; Takeuchi, T.; Amano, K.; Yoshimura, A. Tocilizumab monotherapy uncovered the role of the CCL22/17-CCR4(+) Treg axis during remission of crescentic glomerulonephritis. Clin. Transl. Immunol. 2020, 9, e1203. [Google Scholar] [CrossRef]
- Antonelli, L.R.; Junqueira, C.; Vinetz, J.M.; Golenbock, D.T.; Ferreira, M.U.; Gazzinelli, R.T. The immunology of Plasmodium vivax malaria. Immunol. Rev. 2020, 293, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Losikoff, P.T.; Self, A.A.; Gregory, S.H. Dendritic cells, regulatory T cells and the pathogenesis of chronic hepatitis C. Virulence 2012, 3, 610–620. [Google Scholar] [CrossRef]
- Self, A.A.; Losikoff, P.T.; Gregory, S.H. Divergent contributions of regulatory T cells to the pathogenesis of chronic hepatitis C. Hum. Vaccines Immunother. 2013, 9, 1569–1576. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tleyjeh, I.M.; Kashour, Z.; Damlaj, M.; Riaz, M.; Tlayjeh, H.; Altannir, M.; Altannir, Y.; Al-Tannir, M.; Tleyjeh, R.; Hassett, L.; et al. Efficacy and safety of tocilizumab in COVID-19 patients: A living systematic review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Beardsley, J.; Wolbers, M.; Kibengo, F.M.; Ggayi, A.B.; Kamali, A.; Cuc, N.T.; Binh, T.Q.; Chau, N.V.; Farrar, J.; Merson, L.; et al. Adjunctive Dexamethasone in HIV-Associated Cryptococcal Meningitis. N. Engl. J. Med. 2016, 374, 542–554. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Z.; Mok, M.M.H.; Mar Soe, W.; Thamboo, T.P.; Goh, J.G.; Sam, Q.H.; Osato, M.; Ravikumar, S.; Chai, L.Y.A. Tocilizumab Induces IL-10-Mediated Immune Tolerance in Invasive Candidiasis. J. Fungi 2021, 7, 656. https://doi.org/10.3390/jof7080656
Tan Z, Mok MMH, Mar Soe W, Thamboo TP, Goh JG, Sam QH, Osato M, Ravikumar S, Chai LYA. Tocilizumab Induces IL-10-Mediated Immune Tolerance in Invasive Candidiasis. Journal of Fungi. 2021; 7(8):656. https://doi.org/10.3390/jof7080656
Chicago/Turabian StyleTan, Zhaohong, Michelle Meng Huang Mok, Win Mar Soe, Thomas Paulraj Thamboo, Jessamine Geraldine Goh, Qi Hui Sam, Motomi Osato, Sharada Ravikumar, and Louis Yi Ann Chai. 2021. "Tocilizumab Induces IL-10-Mediated Immune Tolerance in Invasive Candidiasis" Journal of Fungi 7, no. 8: 656. https://doi.org/10.3390/jof7080656
APA StyleTan, Z., Mok, M. M. H., Mar Soe, W., Thamboo, T. P., Goh, J. G., Sam, Q. H., Osato, M., Ravikumar, S., & Chai, L. Y. A. (2021). Tocilizumab Induces IL-10-Mediated Immune Tolerance in Invasive Candidiasis. Journal of Fungi, 7(8), 656. https://doi.org/10.3390/jof7080656





