Mycopharmaceuticals and Nutraceuticals: Promising Agents to Improve Human Well-Being and Life Quality
Abstract
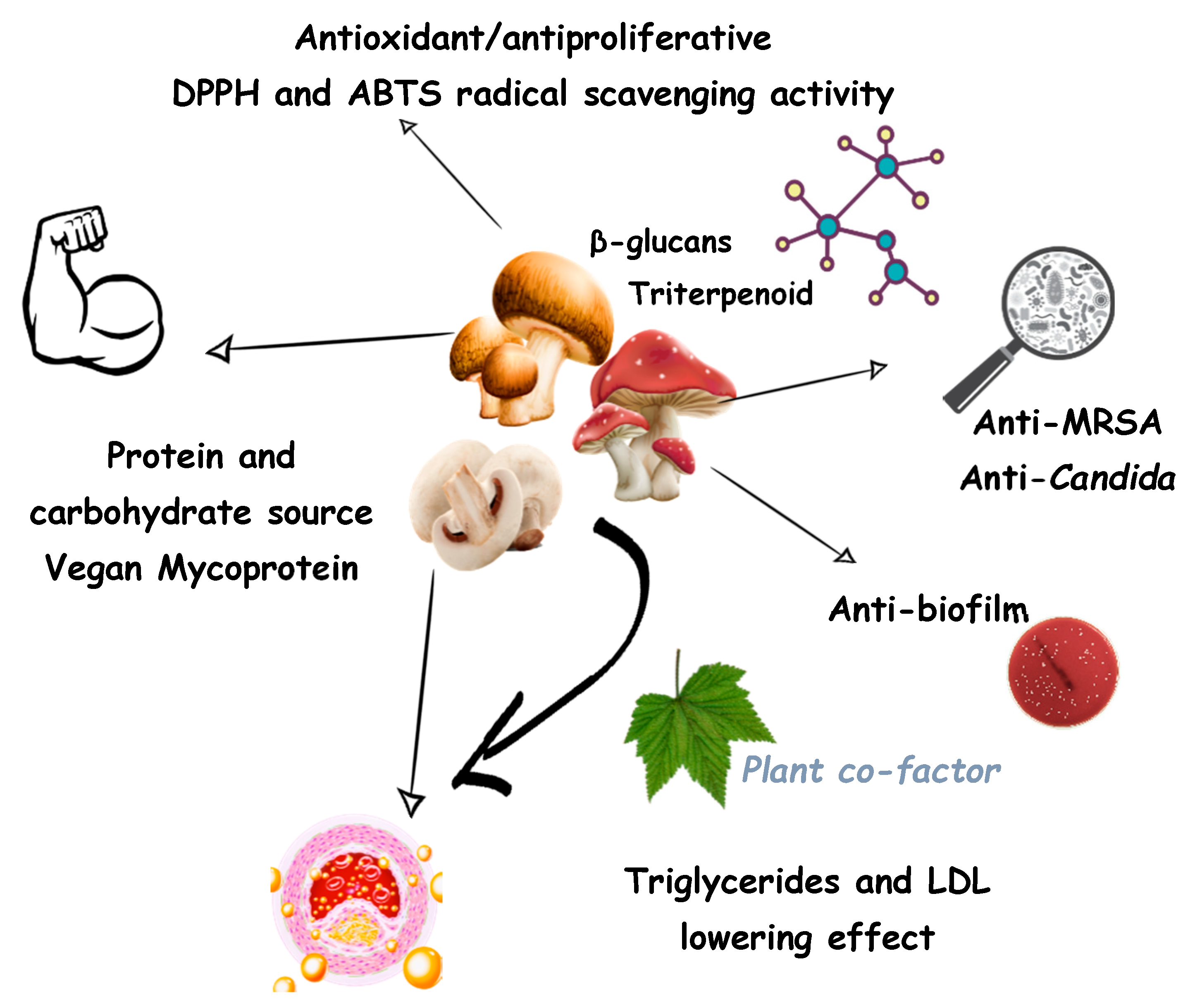
:1. Introduction
2. Fungi Contribution as Antioxidant and Anticancer Products
3. Fungi as Protein and Carbohydrate Source
| Fungal Species | Wild or Cultivated | Location | Composition | References |
|---|---|---|---|---|
| 24 Chilean wild and commercial edible mushrooms from genera Agaricus, Agrocybe, Boletus, Cortinarius, Cyttaria, Flammulina, Grifola, Lactarius, Lentinus, Macrolepiota, Morchella, Pleurotus, Ramaria, Suillus, Tricholoma, and Xeroco-mus | Wild and cultivated mushrooms | Ñuble and Bio-Bio Regions, Chile | Crude protein content: 8.56–23.88 g/100 g d.w. (Highest in Cortinarius lebre (Chilean endemic mushroom)); Carbohydrate content: 62.97–83.65 g/100 g d.w. (highest in Marcolepiota procera); | [124] |
| Volvariella volvacea | Cultivated mushroom | Solan, India | Protein content: 32%; Carbohydrate content: 52.2% | [125] |
| Clavaria rosea, Ganoderma sp., Geastrum triplex, Hygrocybe parvula, Schleroderma bermudense | Wild mushrooms | Shivamogga District, Karnataka, India | Protein contents: 25.71–36.51% (highest in Hygrocybe parvula); Carbohydrate contents:37.38–48.63% (highest in Ganoderma sp.) | [126] |
| Pleurotus pulmonarius RN2, P. djamor RN81 and RN82 | Cultivated mushrooms (cultivated on rice straw (Oryza sativa L.), corn stubble and husk (Zea maize L.)) | USA and Panama | Protein contents: 23.54–43.07% (highest in RN82 cultivated on corn husk); carbohydrate contents: 27.39–52.44% (highest in RN2 cultivated on corn stubbles) | [127] |
| Lentinus sajor-caju and Lentinus torulosus | Wild mushrooms | Similipal Biosphere Reserve, India | protein content: 27. 31–28. 36 g/100 g; carbohydrate content: 64. 95–68. 24 g/100 g. | [128] |
| Amanita crocea (Quél. in Bourd.) Singer ex Singer, Amanita mairei (Foley), Boletus porosporus (Imler ex Bon & G. Moreno), Boletus regius (Krombh.), Gyromitra esculenta (Pers. ex Pers.) Fr., Helvella lacunose (Afzel.), Russula aurea Pers., Russula virescens (Schaeff.)Fr. | Wild mushrooms | Bragança (Northeast Portugal) | Protein content: 4.40–21.85 g/100 g d.w. (highest in Rusula virenscens); Carbohydrate content: 49.64–88.79 g/100 g d.w. (highest in Boletus regius). | [129] |
| Agaricus bohusii Bon | Wild mushroom | Jabučki rid, Northern Serbia | Protein content: 18.06 g/100 g dw; carbohydrate content: 69.79 g/100 g d.w. | [130] |
| Fistulina hepatica, Infundibulicybe geotropa, Laetiporus sulphureus, Macrolepiota procera var. procera and Suillus granulatus | Wild mushrooms | Sicily, Southern Italy | Protein contents: 1.31–4.37 g% (highest in L. sulphureus); carbohydrate contents: 2.08–4.57 g% (highest in I. geotropa) | [131] |
| Cantharellus isabellinus, C. cibarius var. longipes, C. rhodophyllus, C. miniatescens, C. appalachiensis, C. cibarius, C. natarajanii, C. fibrillosus, C. lateritius, C. applanatus, Cr. cibarius var. intermedius C. himalayensis, C. elongatipes, C. cibarius var. multiramis, C. indicus, C. pseudoformosus, C. umbonatus, C. minor | Wild mushrooms | Northwestern Himalayas, India | Protein: 21.6–43.2 mg/g (highest in C. miniatescens); carbohydrate: 9.94–26.5 mg/g (highest in C. minor) | [132] |
| Armillaria mellea (Vahl) P. Kumm., Calocybe gambosa (Fr.) Donk, Clitocybe odora (Fr.) P. Kumm., Coprinus comatus (O.F. Müll.) Pers. | Wild mushrooms | Bragança, Northeast Portugal | Protein: 15.46–17.33 g/100 g dw (highest in Clitocybe odora); carbohydrates: 69.83–71.28 g/100 g dw (highest in Armillaria mellea) | [133] |
| Pleurotus florida, P. sajor-caju and P. ostreatus | Cultivated mushrooms (cultivated on bean straw) | Pantnagar, India | Protein contents: 30.92–36.75% db (highest in Pleurotus sajor-caju); carbohydrate contents: 0.49–31.59% db (highest in Pleurotus florida) | [134] |
| Agaricus campestris, Boletus edulis, Calocybe gambosa, Cantharelluscibarius, Calocybe cornucopioides, Entoloma clypeatum, Flammulina velutipes, Macroleptiotaprocera, M. elata, Pleurotus ostreatus | Wild mushrooms | Croatian regions of Istria (northwest) and Slavonia (northeast) | Protein: 24.22–47.21 g/100 g dw (highest in C. cornucopioides); carbohydrates: 24.6–66.78 g/100 g (highest in Macroleptiota procera) | [135] |
| Boletus aereus Bull., Boletus edulis Bull., Boletus reticulatus Schaeff. | Wild mushrooms | Bragança, Northeast Portugal | Protein: 17.86–22.57 g/100 g (highest in Boletus reticulatus); carbohydrates: 55.16–72.83 g/100 g (highest in Boletus aereus Bull. | [136] |
| Candida valida | Edible yeast isolated from babies’ weaning food produced from fermented corn (Ogi) and grown on synthetic medium and cane molasses | Japan | Protein: 42.6–44.3% (highest when cultured using cane molasses); carbohydrate: 26.9–28.8% (highest when cultured using synthetic medium) | [137] |
| Polyporus tenuiculus | Cultivated mushroom (cultivated in supplemented and nonsupplemented wheat straw and willow sawdust) | Argentina | Protein: 15.1–22.5% (highest when cultivated using wheat straw supplemented with soybean flour (5%) and wheat brand (15%)); carbohydrate: 47.2–51.6% (highest when cultivated using willow sawdust) | [138] |
| Terfezia boudieri | Wild desert truffle | Ben Guerdane, Southeast Tunisia | Protein: 10.5%, 15.4% total sugars | [139] |
| Terfezia boudieri | Wild desert truffle | Hilvan- Sanliurfa, Yenice/Ceylanpinar/Sanliurfa, Polatlı/Ceylanpinar/Sanliurfa, Kiziltepe-Mardin and Malatya from Southeast of Turkey | Protein 1.40–2.73 g/100 g carbohydrate: 4.84–12.30 g/100 g (highest from Kiziltepe/Mardin) | [140] |
| Astraeus hygromatricus | Wild edible fungus | South-west India | 11.71% and 4.66% protein from inner and outer part of the fruit bodies, 29.48% and 35.41% carbohydrate from inner and outer fruit bodies | [141] |
| Pleurotus ostreatus | Cultivated mushroom (cultivated on oat straw (control), blank paper scraps and printed paper scraps) | Portugal | Protein contents: 9.29–14.7 g/100 g (highest when cultivated on oat straw; Carbohydrate contents: 73.2–78.6 g/100 g (highest when cultivated in printed paper) | [142] |
| Pleurotus florida and P. eous | Cultivated mushrooms (cultivated on paddy straw that has been added with either chicken manure, rice bran, wheat bran, black gram, green gram, or horse gram.) | Tamil-Nadu, India | Protein contents: 3.4–35.2% dwt. (highest when cultivated on paddy straw with chicken manure); carbohydrate contents: 31–63.8% dwt. (highest when cultivated on paddy straw with green gram) | [143] |
| Boletus edulis, Boletus mirabilis, and Lactarius deliciosus | Wild mushrooms | KwaZulu-Natal, South Africa | Protein contents: 17.5–39.0% (highest in B. edulis); carbohydrate content: 51.7–76.0% (highest in L. deliciosus) | [144] |
| Pleurotus pulmonarius | Cultivated mushroom | Sao Paolo, Brazil | Protein contents: 31% in Basodioma, 32% in Mycelium; Carbohydrate contents: 30% of the aqueous solution | [145] |
| Pleurotus eryngii, Dictyophora indusiata (Vent. ex Pers) Fisch, Agrocybe aegerita, Ganoderma lucidum (Leyss. ex Fr.) Karst., Yanshan Agaric, Pholiota nameko Ito ex Imai., Hericium erinaceus, Copyinds comatus (MUII. Fr) Gray, Tremella, Cordyceps militaris, Lentinus edodes (Berk.) Sing, Auricularia auricula (L.ex Hook.) under wood, Agaricus blazei Murrill, Volvariella volvacea (Bull.:Fr.) Sing., Morchella esculenta, Griflola frondosa, Arimillaria mellea, Boletus, Russula vinosa Lindblad, and Sparassis crispa. | - | China | Protein contents: 9.31–37.23% (highest in Tricholoma Shiitake); Carbohydrate contents: 0.54–37.23% (highest in Pleurotus eryngii); Ganoderma lucidum (Leyss ex Fr.) Karst. Yashan has the lowest protein and carbohydrate contents. | [146] |
4. Antiobesity and Antidiabetic Abilities of Fungi
5. Fungi as a Biocontrol Agent against Human Pathogen
6. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L. Valuable Secondary Metabolites from Fungi. In Biosynthesis and Molecular Genetics of Fungal Secondary Metabolites; Martín, J.-F., García-Estrada, C., Zeilinger, S., Eds.; Springer: New York, NY, USA, 2014; pp. 1–15. [Google Scholar]
- Calvo, A.M.; Cary, J.W. Association of fungal secondary metabolism and sclerotial biology. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinina, S.A.; Jagels, A.; Cramer, B.; Geisen, R.; Humpf, H.-U. Influence of Environmental Factors on the Production of Penitrems A-F by Penicillium crustosum. Toxins 2017, 9, 210. [Google Scholar] [CrossRef]
- Richter, L.; Wanka, F.; Boecker, S.; Storm, D.; Kurt, T.; Vural, Ö.; Süßmuth, R.; Meyer, V. Engineering of Aspergillus niger for the production of secondary metabolites. Fung.. Biol. Biotechnol. 2014, 1, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bills, G.F.; Gloer, J.B. Biologically active secondary metabolites from the fungi. Microbiol. Spectr. 2017, 4, 1087–1119. [Google Scholar] [CrossRef]
- Wang, J.B.; St. Leger, R.J.; Wang, C. Chapter Three-Advances in Genomics of Entomopathogenic Fungi. Adv. Genet. 2016, 94, 67–105. [Google Scholar] [PubMed]
- Smith, H.; Doyle, S.; Murphy, R. Filamentous fungi as a source of natural antioxidants. Food Chem. 2015, 185, 389–397. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gupta, V.K.; Dahms, T.E.S.; Silva, R.N.; Singh, H.B.; Upadhyay, R.S.; Gomes, E.V.; Tsui, C.K.-M.; Nayak, S.C. Friends or foes? Emerging insights from fungal interactions with plants. FEMS Microbiol. Rev. 2016, 40, 182–207. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Dannert, C. Biocatalytic portfolio of Basidiomycota. Curr. Opin. Chem. Biol. 2016, 31, 40–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L. Secondary metabolites and bioactivities from higher fungi in China. Mini. Rev. Med. Chem. 2015, 15, 157–177. [Google Scholar] [CrossRef]
- Kalač, P. Chapter 1-Introduction. In Edible Mushrooms; Kalač, P., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 1–6. [Google Scholar]
- Sabaratnam, V.; Kah-Hui, W.; Naidu, M.; David, P.R. Neuronal Health–Can Culinary and Medicinal Mushrooms Help? J. Tradit. Complem. Med. 2013, 3, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Thatoi, H.; Singdevsachan, S.K.; Patra, J.K. Chapter 5-Prebiotics and Their Production From Unconventional Raw Materials (Mushrooms). In Therapeutic, Probiotic, and Unconventional Food; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 79–99. [Google Scholar]
- Miles, P.G.; Chang, S.-T. Mushrooms: Cultivation, Utritional Value, Medicinal Effect, and Environmental Impact; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Larmkie, H.L.; Torgbor, J.P.-N.; Yaa, B.A.M.; Christopher, S.; Matilda, D. Sensory Attributes of Three Edible Tropical Mushrooms and Their Use in Formulating Food Products for Children 2–5 Years Old. Int. J. Nutr. Food. Sci. 2018, 7, 100–109. [Google Scholar] [CrossRef]
- Gupta, S.; Summuna, B.; Gupta, M.; Annepu, S.K. Edible Mushrooms: Cultivation, Bioactive Molecules, and Health Benefits. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–33. [Google Scholar]
- Al-Obaidi, J.R. Proteomics of edible mushrooms: A mini-review. Electrophoresis 2016, 37, 1257–1263. [Google Scholar] [CrossRef]
- Lee, S.; Ryoo, R.; Choi, J.H.; Kim, J.-H.; Kim, S.-H.; Kim, K.H. Trichothecene and tremulane sesquiterpenes from a hallucinogenic mushroom Gymnopilus junonius and their cytotoxicity. Arch. Pharm. Res. 2020, 43, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Prados-Rosales, R.; Toriola, S.; Nakouzi, A.; Chatterjee, S.; Stark, R.; Gerfen, G.; Tumpowsky, P.; Dadachova, E.; Casadevall, A. Structural Characterization of Melanin Pigments from Commercial Preparations of the Edible Mushroom Auricularia auricula. J. Agric. Food Chem. 2015, 63, 7326–7332. [Google Scholar] [CrossRef] [Green Version]
- De Souza, R.A.; Kamat, N.M.; Nadkarni, V.S. Purification and characterisation of a sulphur rich melanin from edible mushroom Termitomyces albuminosus Heim. Mycology 2018, 9, 296–306. [Google Scholar] [CrossRef] [Green Version]
- Lindequist, U.; Niedermeyer, T.H.; Jülich, W.-D. The pharmacological potential of mushrooms. Evid. Based Complement. Altern. Med. 2005, 2, 285–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Choi, M.-H.; Li, J.; Yang, H.; Shin, H.-J. Mushroom cosmetics: The present and future. Cosmetics 2016, 3, 22. [Google Scholar] [CrossRef]
- Nakalembe, I.; Kabasa, J.D.; Olila, D. Comparative nutrient composition of selected wild edible mushrooms from two agro-ecological zones, Uganda. SpringerPlus 2015, 4, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I. An Investigation of Potential Marketing Strategies for Entry into the Shiitake Mushroom Industry in Utah. Master’s Thesis, Utah State University, Logan, UT, USA, 2011. [Google Scholar]
- Ma, G.; Yang, W.; Zhao, L.; Pei, F.; Fang, D.; Hu, Q. A critical review on the health promoting effects of mushrooms nutraceuticals. Food. Sci. Hum. Well. 2018, 7, 125–133. [Google Scholar] [CrossRef]
- Al-Obaidi, J.R.; Alobaidi, K.H.; Al-Taie, B.S.; Wee, D.H.-S.; Hussain, H.; Jambari, N.N.; Ahmad-Kamil, E.I.; Ariffin, N.S. Uncovering Prospective Role and Applications of Existing and New Nutraceuticals from Bacterial, Fungal, Algal and Cyanobacterial, and Plant Sources. Sustainability 2021, 13, 3671. [Google Scholar] [CrossRef]
- Newman, D.J. Predominately Uncultured Microbes as Sources of Bioactive Agents. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Andersen, M.R.; Nielsen, J.B.; Klitgaard, A.; Petersen, L.M.; Zachariasen, M.; Hansen, T.J.; Blicher, L.H.; Gotfredsen, C.H.; Larsen, T.O.; Nielsen, K.F. Accurate prediction of secondary metabolite gene clusters in filamentous fungi. Proc. Natl. Acad. Sci. USA 2013, 110, E99-E107. [Google Scholar] [CrossRef] [Green Version]
- Künzler, M. How fungi defend themselves against microbial competitors and animal predators. PLoS Pathog. 2018, 14, 1–10. [Google Scholar] [CrossRef]
- Goyal, S.; Ramawat, K.G.; Mérillon, J.-M. Different Shades of Fungal Metabolites: An Overview. In Fungal Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switerland, 2017; pp. 1–29. [Google Scholar]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Major ecological adaptations and evolutionary transitions. Biol. Rev. 2019, 94, 1443–1476. [Google Scholar] [CrossRef] [Green Version]
- Soldatou, S.; Eldjarn, G.H.; Huerta-Uribe, A.; Rogers, S.; Duncan, K.R. Linking biosynthetic and chemical space to accelerate microbial secondary metabolite discovery. FEMS Microbiol. Lett. 2019, 366. [Google Scholar] [CrossRef]
- Beekman, A.M.; Barrow, R.A. Fungal metabolites as pharmaceuticals. Aust. J. Chem. 2014, 67, 827–843. [Google Scholar] [CrossRef]
- Wasser, S. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 2014, 37, 345–356. [Google Scholar] [CrossRef]
- Qin, D.-W.; Han, C. Medicinal and edible fungi as an alternative medicine for treating age-related disease. Evid. Based Complement. Altern. Med. eCAM 2014, 2014, 638561. [Google Scholar] [CrossRef]
- Sánchez, C. Bioactives from Mushroom and Their Application. In Food Bioactives: Extraction and Biotechnology Applications; Puri, M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 23–57. [Google Scholar]
- Mahmood, R.I.; Abbass, A.K.; Al-Saffar, A.Z.; Al-Obaidi, J.R. An in vitro cytotoxicity of a novel pH-Sensitive lectin loaded-cockle shell-derived calcium carbonate nanoparticles against MCF-7 breast tumour cell. J. Drug Deliv. Sci. Technol. 2021, 61, 102230. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- Phan, C.-W.; Tan, E.Y.-Y.; Sabaratnam, V. Bioactive Molecules in Edible and Medicinal Mushrooms for Human Wellness. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–24. [Google Scholar]
- Chowdhury, M.M.H.; Kubra, K.; Ahmed, S.R. Screening of antimicrobial, antioxidant properties and bioactive compounds of some edible mushrooms cultivated in Bangladesh. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 8. [Google Scholar] [CrossRef] [Green Version]
- Kumaran, R.S.; Jung, H.; Kim, H.J. In vitro screening of taxol, an anticancer drug produced by the fungus, Colletotrichum capsici. Eng. Life Sci. 2011, 11, 264–271. [Google Scholar] [CrossRef]
- Gao, P.; Hirano, T.; Chen, Z.; Yasuhara, T.; Nakata, Y.; Sugimoto, A. Isolation and identification of C-19 fatty acids with anti-tumor activity from the spores of Ganoderma lucidum (reishi mushroom). Fitoterapia 2012, 83, 490–499. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Houbraken, J.; Popma, S.; Samson, R.A. Two new Penicillium species Penicillium buchwaldii and Penicillium spathulatum, producing the anticancer compound asperphenamate. FEMS Microbiol. Lett. 2013, 339, 77–92. [Google Scholar] [CrossRef] [Green Version]
- Akanbi, M.H.J.; Post, E.; van Putten, S.M.; de Vries, L.; Smisterova, J.; Meter-Arkema, A.H.; Wösten, H.A.B.; Rink, R.; Scholtmeijer, K. The antitumor activity of hydrophobin SC3, a fungal protein. Appl. Microbiol. Biotechnol. 2013, 97, 4385–4392. [Google Scholar] [CrossRef]
- Chakravarthi, B.V.S.K.; Sujay, R.; Kuriakose, G.C.; Karande, A.A.; Jayabaskaran, C. Inhibition of cancer cell proliferation and apoptosis-inducing activity of fungal taxol and its precursor baccatin III purified from endophytic Fusarium solani. Cancer Cell Int. 2013, 13, 105. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-C.; Hsiao, Y.-M.; Wu, M.-F.; Ou, C.-C.; Lin, Y.-W.; Lue, K.-H.; Ko, J.-L. Interruption of Lung Cancer Cell Migration and Proliferation by Fungal Immunomodulatory Protein FIP-fve from Flammulina velutipes. J. Agric. Food Chem. 2013, 61, 12044–12052. [Google Scholar] [CrossRef]
- Ding, X.; Hou, Y.; Zhu, Y.; Wang, P.; Fu, L.; Zhu, H.; Zhang, N.; Qin, H.; Qu, W.; Wang, F.; et al. Structure elucidation, anticancer and antioxidant activities of a novel polysaccharide from Gomphus clavatus Gray. Oncol. Rep. 2015, 33, 3162–3170. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Kong, Y.-Y.; Chen, X.; Guo, M.-Y.; Bai, X.-H.; Lu, Y.-J.; Li, W.; Zhou, X.-W. Recombinant FIP-gat, a Fungal Immunomodulatory Protein from Ganoderma atrum, Induces Growth Inhibition and Cell Death in Breast Cancer Cells. J. Agric. Food Chem. 2016, 64, 2690–2698. [Google Scholar] [CrossRef]
- Pushparajah, V.; Fatima, A.; Chong, C.H.; Gambule, T.Z.; Chan, C.J.; Ng, S.T.; Tan, C.S.; Fung, S.Y.; Lee, S.S.; Tan, N.H.; et al. Characterisation of a New Fungal Immunomodulatory Protein from Tiger Milk mushroom, Lignosus rhinocerotis. Sci. Rep. 2016, 6, 30010. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Compton, C.; Rankin, G.O.; Cutler, S.J.; Rojanasakul, Y.; Tu, Y.; Chen, Y.C. 3-Hydroxyterphenyllin, a natural fungal metabolite, induces apoptosis and S phase arrest in human ovarian carcinoma cells. Int. J. Oncol. 2017, 50, 1392–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, R.; Han, Y.-J.; Zhang, M.-H.; Zhang, K.-R.; Ng, T.B.; Liu, F. Purification and characterization of a novel ubiquitin-like antitumour protein with hemagglutinating and deoxyribonuclease activities from the edible mushroom Ramaria botrytis. AMB Express 2017, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Li, S.; Sun, L.; Liu, S.; Wang, F.; Wen, B.; Sun, L.; Fang, X.; Chai, Y.; Cao, H.; et al. Fungal Immunomodulatory Protein from Nectria haematococca Suppresses Growth of Human Lung Adenocarcinoma by Inhibiting the PI3K/Akt Pathway. Int. J. Mol. Sci. 2018, 19, 3429. [Google Scholar] [CrossRef] [Green Version]
- Matuszewska, A.; Jaszek, M.; Stefaniuk, D.; Ciszewski, T.; Matuszewski, Ł. Anticancer, antioxidant, and antibacterial activities of low molecular weight bioactive subfractions isolated from cultures of wood degrading fungus Cerrena unicolor. PLoS ONE 2018, 13, e0197044. [Google Scholar] [CrossRef] [Green Version]
- Sheeba, H.; Ali, M.S.; Anuradha, V. In-vitro Anti-cancer Activity of Endophytic Fungi Isolated from Ziziphus mauritiana in Cervical Cancer Cell Line. Eur. J. Med. Plants 2020, 31, 38–48. [Google Scholar] [CrossRef]
- Li, T.-H.; Hou, C.-C.; Chang, C.L.-T.; Yang, W.-C. Anti-Hyperglycemic Properties of Crude Extract and Triterpenes from Poria cocos. Evid. Based Complement. Altern. Med. eCAM 2011, 2011, 128402. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.-Y.; Korivi, M.; Chaing, Y.-Y.; Chien, T.-Y.; Tsai, Y.-C. Pleurotus tuber-regium Polysaccharides Attenuate Hyperglycemia and Oxidative Stress in Experimental Diabetic Rats. Evid. Based Complement. Alternat. Med. 2012, 2012, 856381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.-G.; Yi, S.-H.; Lee, J.-S. Production and Characterization of a New α-Glucosidase Inhibitory Peptide from Aspergillus oryzae N159-1. Mycobiology 2013, 41, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Stojkovic, D.; Smiljkovic, M.; Ciric, A.; Glamoclija, J.; Van Griensven, L.; Ferreira, I.C.F.R.; Sokovic, M. An insight into antidiabetic properties of six medicinal and edible mushrooms: Inhibition of α-amylase and α-glucosidase linked to type-2 diabetes. S. Afr. J. Bot. 2019, 120, 100–103. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-J.; Lin, C.-S.; Lu, C.-C.; Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Tseng, S.-F.; Wu, T.-R.; Chen, Y.-Y.M.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nature Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [Green Version]
- Bugeda, A.; Garrigues, S.; Gandía, M.; Manzanares, P.; Marcos, J.F.; Coca, M. The Antifungal Protein AfpB Induces Regulated Cell Death in Its Parental Fungus Penicillium digitatum. mSphere 2020, 5, e00595-20. [Google Scholar] [CrossRef] [PubMed]
- Gashaw, G.; Fassil, A.; Redi, F. Evaluation of the Antibacterial Activity of Pleurotus spp. Cultivated on Different Agricultural Wastes in Chiro, Ethiopia. Int. J. Microbiol. 2020, 2020, 9312489. [Google Scholar] [CrossRef]
- Souza Filho, P.F.; Nair, R.B.; Andersson, D.; Lennartsson, P.R.; Taherzadeh, M.J. Vegan-mycoprotein concentrate from pea-processing industry byproduct using edible filamentous fungi. Fungal Biol. Biotechnol. 2018, 5, 1–10. [Google Scholar]
- Rózsa, S.; Măniuțiu, D.-N.; Poșta, G.; Gocan, T.-M.; Andreica, I.; Bogdan, I.; Rózsa, M.; Laza, V. Influence of the Culture Substrate on the Agaricus blazei Murrill Mushrooms Vitamins Content. Plants 2019, 8, 316. [Google Scholar] [CrossRef] [Green Version]
- Uzma, F.; Mohan, C.D.; Hashem, A.; Konappa, N.M.; Rangappa, S.; Kamath, P.V.; Singh, B.P.; Mudili, V.; Gupta, V.K.; Siddaiah, C.N.; et al. Endophytic Fungi—Alternative Sources of Cytotoxic Compounds: A Review. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A Review of Mushrooms as a Potential Source of Dietary Vitamin, D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [Green Version]
- Kimatu, B.M.; Zhao, L.; Biao, Y.; Ma, G.; Yang, W.; Pei, F.; Hu, Q. Antioxidant potential of edible mushroom (Agaricus bisporus) protein hydrolysates and their ultrafiltration fractions. Food Chem. 2017, 230, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E. Determination of antioxidant and antimicrobial properties of Agaricus bisporus from Romanian markets. Analele Univ. Ovid. Const. Seria Chimie 2012, 23, 47–52. [Google Scholar]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C.F.R. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food. Chem.Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef]
- Muna, G.A.; John, M.; Benson, M.; Ogoyi, D. Antioxidant properties of cultivated edible mushroom (Agaricus bisporus) in Kenya. Afr. J. Biotechnol. 2015, 14, 1401–1408. [Google Scholar]
- Patinho, I.; Saldaña, E.; Selani, M.M.; de Camargo, A.C.; Merlo, T.C.; Menegali, B.S.; de Souza Silva, A.P.; Contreras-Castillo, C.J. Use of Agaricus bisporus mushroom in beef burgers: Antioxidant, flavor enhancer and fat replacing potential. Food. Prod. Process. Nutr. 2019, 1, 7. [Google Scholar] [CrossRef]
- Gąsecka, M.; Magdziak, Z.; Siwulski, M.; Mleczek, M. Profile of phenolic and organic acids, antioxidant properties and ergosterol content in cultivated and wild growing species of Agaricus. Eur. Food. Res. Technol. 2018, 244, 259–268. [Google Scholar] [CrossRef]
- Hetland, G.; Johnson, E.; Lyberg, T.; Kvalheim, G. The mushroom Agaricus blazei Murill elicits medicinal effects on tumor, infection, allergy, and inflammation through its modulation of innate immunity and amelioration of Th1/Th2 imbalance and inflammation. Adv. Pharmacol. Sci. 2011, 2011, 1–10. [Google Scholar]
- Shimizu, T.; Kawai, J.; Ouchi, K.; Kikuchi, H.; Osima, Y.; Hidemi, R. Agarol, an ergosterol derivative from Agaricus blazei, induces caspase-independent apoptosis in human cancer cells. Int. J. Oncol. 2016, 48, 1670–1678. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, Y.; Furutani, Y.; Matsuoka, R.; Furukawa, T. Hot water extract of Agaricus blazei Murrill specifically inhibits growth and induces apoptosis in human pancreatic cancer cells. BMC Complement. Altern. Med. 2018, 18, 319. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Sumiyoshi, Y.; Hashine, K.; Shirato, A.; Kyo, S.; Inoue, M. Phase I clinical study of the dietary supplement, Agaricus blazei Murill, in cancer patients in remission. Evid. Based. Complement. Alternat. Med. 2011, 2011, 192381. [Google Scholar] [CrossRef] [Green Version]
- Taofiq, O.; Rodrigues, F.; Barros, L.; Peralta, R.M.; Barreiro, M.F.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Agaricus blazei Murrill from Brazil: An ingredient for nutraceutical and cosmeceutical applications. Food. Funct. 2019, 10, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.-C.; Deng, J.-S.; Huang, S.-S.; Wu, S.-H.; Chen, C.-C.; Lin, W.-R.; Lin, H.-Y.; Huang, G.-J. Anti-inflammatory activity of Sanghuangporus sanghuang mycelium. Int. J. Mol. Sci 2017, 18, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, C.; Ma, J.; Han, C.; Jin, Y.; Zhao, G.; He, X. Extraction and antioxidant activity of total triterpenoids in the mycelium of a medicinal fungus, Sanghuangporus sanghuang. Sci. Rep. 2019, 9, 7418. [Google Scholar] [CrossRef]
- Bakir, T.; Karadeniz, M.; Unal, S. Investigation of antioxidant activities of Pleurotus ostreatus stored at different temperatures. Food Sci. Nutr. 2018, 6, 1040–1044. [Google Scholar] [CrossRef] [Green Version]
- Gąsecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P. Phenolic composition and antioxidant properties of Pleurotus ostreatus and Pleurotus eryngii enriched with selenium and zinc. Eur. Food Res. Technol. 2016, 242, 723–732. [Google Scholar] [CrossRef] [Green Version]
- Kumari, M.; Taritla, S.; Sharma, A.; Jayabaskaran, C. Antiproliferative and Antioxidative Bioactive Compounds in Extracts of Marine-Derived Endophytic Fungus Talaromyces purpureogenus. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Caicedo, N.H.; Davalos, A.F.; Puente, P.A.; Rodríguez, A.Y.; Caicedo, P.A. Antioxidant activity of exo-metabolites produced by Fusarium oxysporum: An endophytic fungus isolated from leaves of Otoba gracilipes. Microbiol. Open 2019, 8, e903. [Google Scholar] [CrossRef] [Green Version]
- Pan, F.; Su, T.-J.; Cai, S.-M.; Wu, W. Fungal endophyte-derived Fritillaria unibracteata var. wabuensis: Diversity, antioxidant capacities in vitro and relations to phenolic, flavonoid or saponin compounds. Sci. Rep. 2017, 7, 42008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledo, C.; Barroetaveña, C.; Fernandes, Â.; Barros, L.; Ferreira, I. Chemical and antioxidant properties of wild edible mushrooms from native Nothofagus spp. forest, Argentina. Molecules 2016, 21, 1201. [Google Scholar] [CrossRef] [PubMed]
- Raman, J.; Jang, K.-Y.; Oh, Y.-L.; Oh, M.; Im, J.-H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and Nutritional Value of Prominent Pleurotus spp.: An Overview. Mycobiology 2021, 49, 1–14. [Google Scholar] [CrossRef] [PubMed]
- González-Palma, I.; Escalona-Buendía, H.B.; Ponce-Alquicira, E.; Téllez-Téllez, M.; Gupta, V.K.; Díaz-Godínez, G.; Soriano-Santos, J. Evaluation of the Antioxidant Activity of Aqueous and Methanol Extracts of Pleurotus ostreatus in Different Growth Stages. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.-B.; Cheung, P.C. Use of stimulatory agents to enhance the production of bioactive exopolysaccharide from Pleurotus tuber-regium by submerged fermentation. J. Agric. Food Chem. 2011, 59, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-B.; Chen, L.; Cheung, P.C.K. Proteomic insights into the stimulatory effect of Tween 80 on mycelial growth and exopolysaccharide production of an edible mushroom Pleurotus tuber-regium. Biotechnol. Lett. 2012, 34, 1863–1867. [Google Scholar] [CrossRef]
- Yap, H.-Y.Y.; Fung, S.-Y.; Ng, S.-T.; Tan, C.-S.; Tan, N.-H. Shotgun proteomic analysis of tiger milk mushroom (Lignosus rhinocerotis) and the isolation of a cytotoxic fungal serine protease from its sclerotium. J. Ethnopharmacol. 2015, 174, 437–451. [Google Scholar] [CrossRef]
- Nallathamby, N.; Phan, C.-W.; Seow, S.L.-S.; Baskaran, A.; Lakshmanan, H.; Abd Malek, S.N.; Sabaratnam, V. A Status Review of the Bioactive Activities of Tiger Milk Mushroom Lignosus rhinocerotis (Cooke) Ryvarden. Front. Pharmacol. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Chai, Y.; Wang, G.; Fan, L.; Zhao, M. A proteomic analysis of mushroom polysaccharide-treated HepG2 cells. Sci. Rep. 2016, 6, 23565. [Google Scholar] [CrossRef] [PubMed]
- Matuszewska, A.; Stefaniuk, D.; Jaszek, M.; Pięt, M.; Zając, A.; Matuszewski, Ł.; Cios, I.; Grąz, M.; Paduch, R.; Bancerz, R. Antitumor potential of new low molecular weight antioxidative preparations from the white rot fungus Cerrena unicolor against human colon cancer cells. Sci. Rep. 2019, 9, 1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sezer, Y.Ç.; Süfer, Ö.; Sezer, G. Extraction of phenolic compounds from oven and microwave dried mushrooms (Agaricus bisporus and Pleurotus ostreatus) by using methanol, ethanol and aceton as solvents. Indian J. Pharm. Educ. Res. 2017, 51, 393–397. [Google Scholar] [CrossRef] [Green Version]
- Boonsong, S.; Klaypradit, W.; Wilaipun, P. Antioxidant activities of extracts from five edible mushrooms using different extractants. Agric. Nat. Resour. 2016, 50, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Investigation of antioxidant and antimicrobial activities of different extracts of auricularia and Termitomyces species of mushrooms. Sci. World J. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Abdullah, N.; Ismail, S.M.; Aminudin, N.; Shuib, A.S.; Lau, B.F. Evaluation of selected culinary-medicinal mushrooms for antioxidant and ACE inhibitory activities. Evid. Based. Complement. Alternat. Med. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Diao, X.; Wang, T.; Chen, G.; Lin, Q.; Yang, X.; Xu, J. Phylogenetic diversity and antioxidant activities of culturable fungal endophytes associated with the mangrove species Rhizophora stylosa and R. mucronata in the South China Sea. PLoS ONE 2018, 13, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.-L.; Guo, T.-T.; Ren, Z.-X.; Zhang, N.-S.; Wang, M.-L. Diversity and Antioxidant Activity of Culturable Endophytic Fungi from Alpine Plants of Rhodiola crenulata, R. angusta, and R. sachalinensis. PLoS ONE 2015, 10, e0118204. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Zhou, C.; Zhou, D.; Ou, S.; Zhang, X.; Huang, H. Structure characterization of a novel polysaccharide from Hericium erinaceus fruiting bodies and its immunomodulatory activities. Food Funct. 2018, 9, 294–306. [Google Scholar] [CrossRef]
- Zeng, X.; Ling, H.; Yang, J.; Chen, J.; Guo, S. Proteome analysis provides insight into the regulation of bioactive metabolites in Hericium erinaceus. Gene 2018, 666, 108–115. [Google Scholar] [CrossRef]
- Vidović, S.S.; Mujić, I.O.; Zeković, Z.P.; Lepojević, Ž.D.; Tumbas, V.T.; Mujić, A.I. Antioxidant properties of selected Boletus mushrooms. Food Biophys. 2010, 5, 49–58. [Google Scholar] [CrossRef]
- Yuswan, M.; Al-Obaidi, J.R.; Rahayu, A.; Sahidan, S.; Shazrul, F.; Fauzi, D. New bioactive molecules with potential antioxidant activity from various extracts of wild edible Gelam mushroom (Boletus spp.). Adv. Biosci. Biotechnol. 2015, 6, 320. [Google Scholar] [CrossRef] [Green Version]
- Jaworska, G.; Pogoń, K.; Skrzypczak, A.; Bernaś, E. Composition and antioxidant properties of wild mushrooms Boletus edulis and Xerocomus badius prepared for consumption. J. Food Sci. Technol. 2015, 52, 7944–7953. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Hu, Y.; Duan, X.; Tang, T.; Shen, Y.; Hu, B.; Liu, A.; Chen, H.; Li, C.; Liu, Y. Characterization and antioxidant activities of polysaccharides from thirteen boletus mushrooms. Int. J. Biol. Macromol. 2018, 113, 1–7. [Google Scholar] [CrossRef]
- Song, H.-Y.; Kim, D.-H.; Kim, J.-M. Comparative transcriptome analysis of dikaryotic mycelia and mature fruiting bodies in the edible mushroom Lentinula edodes. Sci. Rep. 2018, 8, 8983. [Google Scholar] [CrossRef]
- Jamil, N.A.M.; Rashid, N.M.N.; Hamid, M.H.A.; Rahmad, N.; Al-Obaidi, J.R. Comparative nutritional and mycochemical contents, biological activities and LC/MS screening of tuber from new recipe cultivation technique with wild type tuber of tiger’s milk mushroom of species Lignosus rhinocerus. World. J. Microbiol. Biotechnol. 2018, 34, 1. [Google Scholar] [CrossRef]
- Pinto, E.; Monteiro, C.; Maia, M.; Faria, M.A.; Lopes, V.; Lameiras, C.; Pinheiro, D. Aspergillus Species and Antifungals Susceptibility in Clinical Setting in the North of Portugal: Cryptic Species and Emerging Azoles Resistance in A. fumigatus. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Hamed, A.A.; Abdel-Aziz, M.S.; El Hady, F.K.A. Antimicrobial and antioxidant activities of different extracts from Aspergillus unguis SPMD-EGY grown on different media. Bull. Nat. Res. Cent. 2018, 42, 29. [Google Scholar] [CrossRef]
- Finnigan, T.J.A.; Wall, B.T.; Wilde, P.J.; Stephens, F.B.; Taylor, S.L.; Freedman, M.R. Mycoprotein: The Future of Nutritious Nonmeat Protein, a Symposium Review. Curr. Dev. Nutr. 2019, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza Filho, P.F.; Andersson, D.; Ferreira, J.A.; Taherzadeh, M.J. Mycoprotein: Environmental impact and health aspects. World J. Microbiol. Biotechnol. 2019, 35, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Zeng, H.; Wang, K.; Lin, H.; Li, P.; Huang, Y.; Zhou, S.; Zhang, W.; Chen, C.; Fan, H. Microwave-assisted aqueous two-phase extraction of diverse polysaccharides from Lentinus edodes: Process optimization, structure characterization and antioxidant activity. Int. J. Biol. Macromol. 2019, 136, 305–315. [Google Scholar] [CrossRef]
- Chen, X.; Ji, H.; Xu, X.; Liu, A. Optimization of polysaccharide extraction process from grifola frondosa and its antioxidant and anti-tumor research. J. Food Meas. Charact. 2019, 13, 144–153. [Google Scholar] [CrossRef]
- Niu, L.-L.; Wu, Y.-R.; Liu, H.-P.; Wang, Q.; Li, M.-Y.; Jia, Q. Optimization of extraction process, characterization and antioxidant activities of polysaccharide from Leucopaxillus giganteus. J. Food Meas. Charact. 2021, 15, 2842–2853. [Google Scholar] [CrossRef]
- González, A.; Cruz, M.; Losoya, C.; Nobre, C.; Loredo, A.; Rodríguez, R.; Contreras, J.; Belmares, R. Edible mushrooms as a novel protein source for functional foods. Food Funct. 2020, 11, 7400–7414. [Google Scholar]
- Rahmad, N.; Al-Obaidi, J.R.; Rashid, N.M.N.; Zean, N.B.; Yusoff, M.H.Y.M.; Shaharuddin, N.S.; Jamil, N.A.M.; Saleh, N.M. Comparative proteomic analysis of different developmental stages of the edible mushroom Termitomyces heimii. Biol. Res. 2014, 47, 30. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Gu, B.; Huang, J.; Jiang, S.; Chen, Y.; Yin, Y.; Pan, Y.; Yu, G.; Li, Y.; Wong, B.H.C.; et al. Transcriptome and Proteome Exploration to Provide a Resource for the Study of Agrocybe aegerita. PLoS ONE 2013, 8, e56686. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Bau, T. De novo assembly and characterization of the transcriptome of a wild edible mushroom Leucocalocybe mongolica and identification of SSR markers. Biotechnol. Biotechnol. Equip. 2017, 31, 1148–1159. [Google Scholar]
- Yoo, S.-i.; Lee, H.-Y.; Markkandan, K.; Moon, S.; Ahn, Y.J.; Ji, S.; Ko, J.; Kim, S.-J.; Ryu, H.; Hong, C.P. Comparative transcriptome analysis identified candidate genes involved in mycelium browning in Lentinula edodes. BMC Genom. 2019, 20, 121. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Miyagi, A.; Yoneyama, S.; Gisusi, S.; Tokuji, Y.; Kawai-Yamada, M. CE–MS-based metabolomics reveals the metabolic profile of maitake mushroom (Grifola frondosa) strains with different cultivation characteristics. Biosci. Biotechnol. Biochem. 2017, 81, 2314–2322. [Google Scholar] [CrossRef] [Green Version]
- Souza Filho, P.F.; Zamani, A.; Taherzadeh, M.J. Edible protein production by filamentous fungi using starch plant wastewater. Waste Biomass. Valor. 2019, 10, 2487–2496. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Hoo, P.C.-X.; Tan, L.T.-H.; Pusparajah, P.; Khan, T.M.; Lee, L.-H.; Goh, B.-H.; Chan, K.-G. Golden needle mushroom: A culinary medicine with evidenced-based biological activities and health promoting properties. Front. Pharmacol. 2016, 7, 474. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Hu, T.; Li, H.; Jin, G.; Zhang, Y. Metabonomic profiling in study hepatoprotective effect of polysaccharides from Flammulina velutipes on carbon tetrachloride-induced acute liver injury rats using GC–MS. Int. J. Biol. Macromol. 2018, 110, 285–293. [Google Scholar] [CrossRef]
- Jacinto-Azevedo, B.; Valderrama, N.; Henríquez, K.; Aranda, M.; Aqueveque, P. Nutritional value and biological properties of Chilean wild and commercial edible mushrooms. Food Chem. 2021, 356, 129651. [Google Scholar] [CrossRef]
- Punitha, S.C.; Rajasekaran, M. Proximate, elemental and GC-MS study of the edible mushroom Volvariella volvacea (Bull Ex Fr) singer. J. Chem. Pharm. Res. 2015, 7, 511–518. [Google Scholar]
- Chittaragi, A.; Naika, R.; Vinayaka, K. Nutritive value of few wild mushrooms from the Western Ghats of Shivamogga District, Karnataka, India. Asian J. Pharm. Clin. Res. 2014, 7, 50–53. [Google Scholar]
- Vega, A.; Franco, H. Productividad y calidad de los cuerpos fructíferos de los hongos comestibles Pleurotus pulmonarius RN2 y P. djamor RN81 y RN82 cultivados sobre sustratos lignocelulósicos. Inf. Tecnol. 2013, 24, 69–78. [Google Scholar] [CrossRef]
- Singdevsachan, S.K.; Patra, J.K.; Thatoi, H. Nutritional and bioactive potential of two wild edible mushrooms (Lentinus sajor-caju and Lentinus torulosus) from Similipal Biosphere Reserve, India. Food Sci. Biotechnol. 2013, 22, 137–145. [Google Scholar] [CrossRef]
- Leal, A.R.; Barros, L.; Barreira, J.C.M.; Sousa, M.J.; Martins, A.; Santos-Buelga, C.; Ferreira, I.C.F.R. Portuguese wild mushrooms at the “pharma–nutrition” interface: Nutritional characterization and antioxidant properties. Food Res. Int. 2013, 50, 1–9. [Google Scholar] [CrossRef]
- Reis, F.S.; Stojković, D.; Soković, M.; Glamočlija, J.; Ćirić, A.; Barros, L.; Ferreira, I.C.F.R. Chemical characterization of Agaricus bohusii, antioxidant potential and antifungal preserving properties when incorporated in cream cheese. Food Res. Int. 2012, 48, 620–626. [Google Scholar] [CrossRef]
- Palazzolo, E.; Letizia Gargano, M.; Venturella, G. The nutritional composition of selected wild edible mushrooms from Sicily (southern Italy). Int. J. Food Sci. Nutr. 2012, 63, 79–83. [Google Scholar] [CrossRef]
- Kumari, D.; Reddy, M.; Upadhyay, R. Nutritional composition and antioxidant activities of 18 different wild Cantharellus mushrooms of Northwestern Himalayas. Food Sci. Technol. Int. 2011, 17, 557–567. [Google Scholar] [CrossRef]
- Vaz, J.A.; Barros, L.; Martins, A.; Santos-Buelga, C.; Vasconcelos, M.H.; Ferreira, I.C.F.R. Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem. 2011, 126, 610–616. [Google Scholar] [CrossRef] [Green Version]
- Michael, H.W.; Bultosa, G.; Pant, L.M. Nutritional contents of three edible oyster mushrooms grown on two substrates at Haramaya, Ethiopia, and sensory properties of boiled mushroom and mushroom sauce. Int. J. Food Sci. Technol. 2011, 46, 732–738. [Google Scholar] [CrossRef]
- Beluhan, S.; Ranogajec, A. Chemical composition and non-volatile components of Croatian wild edible mushrooms. Food Chem. 2011, 124, 1076–1082. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Santos-Buelga, C.; Ferreira, I.C.F.R. Targeted metabolites analysis in wild Boletus species. LWT Food Sci. Technol. 2011, 44, 1343–1348. [Google Scholar] [CrossRef]
- Kuforiji, O.; Aboaba, O. Application of Candida valida as a protein supplement. J. Food Safety 2010, 30, 969–981. [Google Scholar] [CrossRef]
- Omarini, A.; Henning, C.; Ringuelet, J.; Zygadlo, J.A.; Albertó, E. Volatile composition and nutritional quality of the edible mushroom Polyporus tenuiculus grown on different agro-industrial waste. Int. J. Food Sci. Technol. 2010, 45, 1603–1609. [Google Scholar] [CrossRef]
- Slama, A.; Neffati, M.; Boudabous, A. Biochemical composition of desert truffle Terfezia boudieri Chatin. In Proceedings of the International Symposium on Medicinal and Aromatic Plants-SIPAM2009 853, Djerba, Tunisia, 26–28 March 2009; pp. 285–290. [Google Scholar]
- Dundar, A.; Yesil, O.F.; Acay, H.; Okumus, V.; Ozdemir, S.; Yildiz, A. Antioxidant properties, chemical composition and nutritional value of Terfezia boudieri (Chatin) from Turkey. Food Sci. Technol. Int. 2012, 18, 317–328. [Google Scholar] [CrossRef]
- Pavithra, M.; Sridhar, K.R.; Greeshma, A.A.; Tomita-Yokotani, K. Bioactive potential of the wild mushroom Astraeus hygrometricus in South-west India. Mycology 2016, 7, 191–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, Â.; Barros, L.; Martins, A.; Herbert, P.; Ferreira, I.C.F.R. Nutritional characterisation of Pleurotus ostreatus (Jacq. ex Fr.) P. Kumm. produced using paper scraps as substrate. Food Chem. 2015, 169, 396–400. [Google Scholar] [CrossRef] [Green Version]
- Sivagurunathan, P.; Sivasankari, S. Influence of Chicken Manure on Biological Efficiency of Pleurotus spp. Waste Biomass Valor. 2015, 6, 23–28. [Google Scholar] [CrossRef]
- Rasalanavho, M.; Moodley, R.; Jonnalagadda, S.B. Elemental bioaccumulation and nutritional value of five species of wild growing mushrooms from South Africa. Food Chem. 2020, 319, 126596. [Google Scholar] [CrossRef] [PubMed]
- Contato, A.G.; Inácio, F.D.; de Araújo, C.A.V.; Brugnari, T.; Maciel, G.M.; Haminiuk, C.W.I.; Bracht, A.; Peralta, R.M.; de Souza, C.G.M. Comparison between the aqueous extracts of mycelium and basidioma of the edible mushroom Pleurotus pulmonarius: Chemical composition and antioxidant analysis. J. Food Meas. Charact. 2020, 14, 830–837. [Google Scholar] [CrossRef]
- Yu, Q.; Guo, M.; Zhang, B.; Wu, H.; Zhang, Y.; Zhang, L. Analysis of Nutritional Composition in 23 Kinds of Edible Fungi. J. Food Qual. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Anti-obesity effects of medicinal and edible mushrooms. Molecules 2018, 23, 2880. [Google Scholar]
- Helal, N.A.; Eassa, H.A.; Amer, A.M.; Eltokhy, M.A.; Edafiogho, I.; Nounou, M.I. Nutraceuticals’ Novel Formulations: The Good, the Bad, the Unknown and Patents Involved. Recent Pat. Drug. Deliv. Formul. 2019, 13, 105–156. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhao, C.; Zheng, S.; Mei, X.; Huang, K.; Wang, G.; He, X. Anti-obesity and hypolipidemic effect of water extract from Pleurotus citrinopileatus in C57BL/6J mice. Food Sci. Nutr. 2019, 7, 1295–1301. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.R.; Kim, J.E.; Choi, J.Y.; Park, J.J.; Kim, H.R.; Song, B.R.; Choi, Y.W.; Kim, K.M.; Song, H.; Hwang, D.Y. Anti-obesity effect in high-fat-diet-induced obese C57BL/6 mice: Study of a novel extract from mulberry (Morus alba) leaves fermented with Cordyceps militaris. Exp. Ther. Med. 2019, 17, 2185–2193. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.; Su, M.; Duan, Y.; Huang, Y. Eurotium cristatum, a potential probiotic fungus from Fuzhuan brick tea, alleviated obesity in mice by modulating gut microbiota. Food Funct. 2019, 10, 5032–5045. [Google Scholar] [CrossRef]
- Uzor, P.F.; Osadebe, P.O.; Nwodo, N.J. Antidiabetic activity of extract and compounds from an endophytic fungus Nigrospora oryzae. Pharm. Res. 2017, 67, 308–311. [Google Scholar] [CrossRef]
- Yap, H.-Y.Y.; Tan, N.-H.; Ng, S.-T.; Tan, C.-S.; Fung, S.-Y. Inhibition of protein glycation by tiger milk mushroom [Lignosus rhinocerus (Cooke) Ryvarden] and search for potential anti-diabetic activity-related metabolic pathways by genomic and transcriptomic data mining. Front. Pharmacol. 2018, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Kaur, A. Antidiabetic potential of a peptide isolated from an endophytic Aspergillus awamori. J. Appl. Microbiol. 2016, 120, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Ogbole, O.O.; Nkumah, A.O.; Linus, A.U.; Falade, M.O. Molecular identification, in vivo and in vitro activities of Calvatia gigantea (macro-fungus) as an antidiabetic agent. Mycology 2019, 10, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Kaur, A. Evaluation of antidiabetic and antioxidant potential of endophytic fungi isolated from medicinal plants. Int. J. Green. Pharm. 2018, 12, 6–14. [Google Scholar]
- Wu, T.; Xu, B. Antidiabetic and antioxidant activities of eight medicinal mushroom species from China. Int. J. Med. Mushrooms. 2015, 17, 129–140. [Google Scholar] [CrossRef]
- Zutz, C.; Bandian, D.; Neumayer, B.; Speringer, F.; Gorfer, M.; Wagner, M.; Strauss, J.; Rychli, K. Fungi treated with small chemicals exhibit increased antimicrobial activity against facultative bacterial and yeast pathogens. Biomed Res. Int. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wiese, J.; Imhoff, J.F. Marine bacteria and fungi as promising source for new antibiotics. Drug Develop. Res. 2019, 80, 24–27. [Google Scholar] [CrossRef] [Green Version]
- Aly, A.H.; Debbab, A.; Proksch, P. Fifty years of drug discovery from fungi. Fungal Divers 2011, 50, 3. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar]
- Deshmukh, S.K.; Verekar, S.A.; Bhave, S.V. Endophytic fungi: A reservoir of antibacterials. Front. Microbiol. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Meng, W.; Cao, C.; Wang, J.; Shan, W.; Wang, Q. Antibacterial and antifungal compounds from marine fungi. Mar. Drugs 2015, 13, 3479–3513. [Google Scholar] [CrossRef]
- Svahn, K.S.; Göransson, U.; El-Seedi, H.; Bohlin, L.; Larsson, D.J.; Olsen, B.; Chryssanthou, E. Antimicrobial activity of filamentous fungi isolated from highly antibiotic-contaminated river sediment. Infec. Ecol. Epidemiol. 2012, 2, 11591. [Google Scholar] [CrossRef]
- Malhadas, C.; Malheiro, R.; Pereira, J.A.; de Pinho, P.G.; Baptista, P. Antimicrobial activity of endophytic fungi from olive tree leaves. World J. Microbiol. Biotechnol. 2017, 33, 46. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, A.; Gupta, P.; Kumar, N.; Mishra, J.; Kumar, A.; Rakhee; Misra, K. Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes), Inhibits Candida Biofilms: A Metabolomic Approach. Int. J. Med. Mush. 2017, 19, 685–696. [Google Scholar] [CrossRef]
- Gargano, M.L.; Zervakis, G.I.; Isikhuemhen, O.S.; Venturella, G.; Calvo, R.; Giammanco, A.; Fasciana, T.; Ferraro, V. Ecology, Phylogeny, and Potential Nutritional and Medicinal Value of a Rare White “Maitake” Collected in a Mediterranean Forest. Diversity 2020, 12, 230. [Google Scholar] [CrossRef]
- Yuan, B.; Zhao, L.; Rakariyatham, K.; Han, Y.; Gao, Z.; Kimatu, B.M.; Hu, Q.; Xiao, H. Isolation of a novel bioactive protein from an edible mushroom Pleurotus eryngii and its anti-inflammatory potential. Food Funct. 2017, 8, 2175–2183. [Google Scholar] [CrossRef]
- Yu, G.-J.; Yin, Y.-L.; Yu, W.-H.; Liu, W.; Jin, Y.-X.; Shrestha, A.; Yang, Q.; Ye, X.-D.; Sun, H. Proteome Exploration to Provide a Resource for the Investigation of Ganoderma lucidum. PLoS ONE 2015, 10, e0119439. [Google Scholar] [CrossRef] [Green Version]
- Younis, A.M.; Wu, F.-S.; El Shikh, H.H. Antimicrobial activity of extracts of the oyster culinary medicinal mushroom Pleurotus ostreatus (higher basidiomycetes) and identification of a new antimicrobial compound. Int. J. Med. Mushrooms 2015, 17, 579–590. [Google Scholar] [CrossRef]
- Hosoe, T.; Fukushima, K.; Takizawa, K.; Itabashi, T.; Kawahara, N.; Vidotto, V.; Kawai, K.-I. A New Antifungal Macrolide, Eushearilide, Isolated from Eupenicillium shearii. J. Antibiot. 2006, 59, 597–600. [Google Scholar] [CrossRef]
- Tonoi, T.; Inohana, T.; Sato, T.; Noda, Y.; Ikeda, M.; Akutsu, M.; Murata, T.; Maekawa, Y.; Tanaka, A.; Seki, R. Total Synthesis and Antimicrobial Evaluation of 23-Demethyleushearilide and Extensive Antimicrobial Evaluation of All Synthetic Stereoisomers of (16Z, 20E)-Eushearilide and (16E, 20E)-Eushearilide. Molecules 2019, 24, 3437. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.J.; Ferreira, I.C.F.R.; Lourenço, I.; Costa, E.; Martins, A.; Pintado, M. Wild Mushroom Extracts as Inhibitors of Bacterial Biofilm Formation. Pathogens 2014, 3, 667–679. [Google Scholar] [CrossRef] [Green Version]
- Bin, L.; Wei, L.; Xiaohong, C.; Mei, J.; Mingsheng, D. In vitro antibiofilm activity of the melanin from Auricularia auricula, an edible jelly mushroom. Ann. Microbiol. 2012, 62, 1523–1530. [Google Scholar] [CrossRef]
- Papetti, A.; Signoretto, C.; Spratt, D.A.; Pratten, J.; Lingström, P.; Zaura, E.; Ofek, I.; Wilson, M.; Pruzzo, C.; Gazzani, G. Components in Lentinus edodes mushroom with anti-biofilm activity directed against bacteria involved in caries and gingivitis. Food Funct. 2018, 9, 3489–3499. [Google Scholar] [CrossRef]
- Kaur, N.; Arora, D.S. Prospecting the antimicrobial and antibiofilm potential of Chaetomium globosum an endophytic fungus from Moringa oleifera. AMB Express 2020, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Meenambiga, S.; Rajagopal, K. Antibiofilm activity and molecular docking studies of bioactive secondary metabolites from endophytic fungus Aspergillus nidulans on oral Candida albicans. J. Appl. Pharm. Sci. 2018, 8, 37–45. [Google Scholar]
- Shomali, N.; Onar, O.; Karaca, B.; Demirtas, N.; Cihan, A.C.; Akata, I.; Yildirim, O. Antioxidant, anticancer, antimicrobial, and antibiofilm properties of the culinary-medicinal fairy ring mushroom, Marasmius oreades (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 571–582. [Google Scholar] [CrossRef]
- Kaur, N.; Arora, D.S.; Kalia, N.; Kaur, M. Antibiofilm, antiproliferative, antioxidant and antimutagenic activities of an endophytic fungus Aspergillus fumigatus from Moringa oleifera. Mol. Biol. Rep. 2020, 47, 2901–2911. [Google Scholar] [CrossRef]
- Qader, M.M.; Hamed, A.A.; Soldatou, S.; Abdelraof, M.; Elawady, M.E.; Hassane, A.S.I.; Belbahri, L.; Ebel, R.; Rateb, M.E. Antimicrobial and Antibiofilm Activities of the Fungal Metabolites Isolated from the Marine Endophytes Epicoccum nigrum M13 and Alternaria alternata 13A. Mar. Drugs 2021, 19, 232. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Kaushal, M.; Kumar, P.; Mukhopadhyay, K.; Kumar, A. Fungal-derived xenobiotic exhibits antibacterial and antibiofilm activity against Staphylococcus aureus. Drug Discov. Ther. 2018, 12, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Karaca, B.; Cihan, A.Ç.; Akata, I.; Altuner, E.M. Anti-Biofilm and Antimicrobial Activities of Five Edible and Medicinal Macrofungi Samples on Some Biofilm Producing Multi Drug Resistant Enterococcus Strains. Turkish J. Agric. Food Sci. Technol. 2020, 8, 69–80. [Google Scholar] [CrossRef] [Green Version]

| Fungal Species | Active Molecules | Effect | Reference |
|---|---|---|---|
| Colletotrichum capsici | Taxol | Anticancer (mitotic inhibitor) | [42] |
| Ganoderma lucidum | C-19 fatty acids | antitumour activity against HL-60 | [43] |
| Penicillium buchwaldii and Penicillium spathulatum | asperphenamate | Anticancer | [44] |
| Schizophyllum commune | hydrophobin SC3 | Anticancer (sarcoma S180 cell line) | [45] |
| Fusarium solani | Taxol, baccatin III | Anticancer (HeLa) | [46] |
| Flammulina velutipe | FIP-fve | Anticancer (A549) | [47] |
| Gomphus clavatus | GCG-1 | Antioxidant (against activity (apoptosis of HepG-2)) | [48] |
| Ganoderma atrum | FIP-gat | Antioxidant (against MDA-MB-231) | [49] |
| Lignosus rhinocerotis | FIP-Lrh | Anticancer (HeLa, A549, MCF-7) | [50] |
| Aspergillus candidus | 3-Hydroxyterphenyllin (3-HT) | Anticancer (ovarian carcinoma cell lines, A2780/) | [51] |
| Ramaria botrytis | ubiquitin-like | Anticancer (293T, HeLa A549, KB and MCF-7) | [52] |
| Fusarium solani | (FIP-nha) | A549 apoptosis | [53] |
| Cerrena unicolor | ex-LMSI, ex-LMSII, and ex-LMSIII | Anticancer (MDA-MB-231, PC3, and MCF7) | [54] |
| Trichoderma viride | 3-beta-hydroxy urs-12-en-28-oic acid | Anticancer (HeLa) | [55] |
| Poria cocos | Triterpenes | Anti-Hyperglycemic | [56] |
| Pleurotus tuber-regium | polysaccharides (1P, 2P, and 3P) | Anti-Hyperglycemia | [57] |
| Aspergillus oryzae | P-1 and P2 peptide | α-Glucosidase Inhibitory | [58] |
| Agaricus blazei, Coprinus comatus, Cordyceps militaris, Inonotus obliquus, Phellinus linteus | p-coumaric acid, p-hydroxybenzoic acid and cinnamic acid | Inhibition of α-amylase | [59] |
| Ganoderma lucidum | (WEGL) mycelium | reduces obesity | [60] |
| Penicillium digitatum | AfpB protein | Antifungal activity | [61] |
| Pleurotus ostreatus and Pleurotus florida | Methanolic extracts | Antimicrobial activity | [62] |
| Monascus purpureus, Aspergillus oryzae, Neurospora intermedia, Fusarium venenatum | Fungal biomass | Vegan protein | [63] |
| Agaricus blazei | Multi-vitamins | Immune sustem stimulators, antimicrobial | [64] |
| Fungal Species | Target | Reference |
|---|---|---|
| Russula delica, Fistulina hepatica, Mycena rosea, Leucopaxilus giganteus, and Lepista nuda | Pseudomonas aeruginosa | [173] |
| Auricularia auricula | Pseudomonas aeruginosa and Pseudomonas fluorescens | [174] |
| Lentinus edodes | Streptococcus mutans | [175] |
| Chaetomium globosum | Staphylococcus aureus, Klebsiella pneumoniae and Candida albicans | [176] |
| Aspergillus nidulans | Candida albicans | [177] |
| Marasmius oreades | Staphylococcus epidermidis and Pseudomonas aeruginosa | [178] |
| Aspergillus fumigatus | Staphylococcus aureus, Klebsiella pneumoniae and Candida albicans | [179] |
| Epicoccum nigrum and Alternaria alternata | Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli and Bacillus subtilis | [180] |
| Aspergillus nidulans | Staphylococcus aureus | [181] |
| Morchella angusticeps, Ganoderma lucidum, Cerioporus squamosus, Trametes versicolor and Lentinula edodes | Enterococcus faecalis | [182] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Obaidi, J.R.; Jambari, N.N.; Ahmad-Kamil, E.I. Mycopharmaceuticals and Nutraceuticals: Promising Agents to Improve Human Well-Being and Life Quality. J. Fungi 2021, 7, 503. https://doi.org/10.3390/jof7070503
Al-Obaidi JR, Jambari NN, Ahmad-Kamil EI. Mycopharmaceuticals and Nutraceuticals: Promising Agents to Improve Human Well-Being and Life Quality. Journal of Fungi. 2021; 7(7):503. https://doi.org/10.3390/jof7070503
Chicago/Turabian StyleAl-Obaidi, Jameel R., Nuzul Noorahya Jambari, and E. I. Ahmad-Kamil. 2021. "Mycopharmaceuticals and Nutraceuticals: Promising Agents to Improve Human Well-Being and Life Quality" Journal of Fungi 7, no. 7: 503. https://doi.org/10.3390/jof7070503
APA StyleAl-Obaidi, J. R., Jambari, N. N., & Ahmad-Kamil, E. I. (2021). Mycopharmaceuticals and Nutraceuticals: Promising Agents to Improve Human Well-Being and Life Quality. Journal of Fungi, 7(7), 503. https://doi.org/10.3390/jof7070503






