Trichoderma longibrachiatum and Aspergillus fischeri Infection as a Cause of Skin Graft Failure in a Patient with Critical Burns after Liver Transplantation
Abstract
1. Introduction
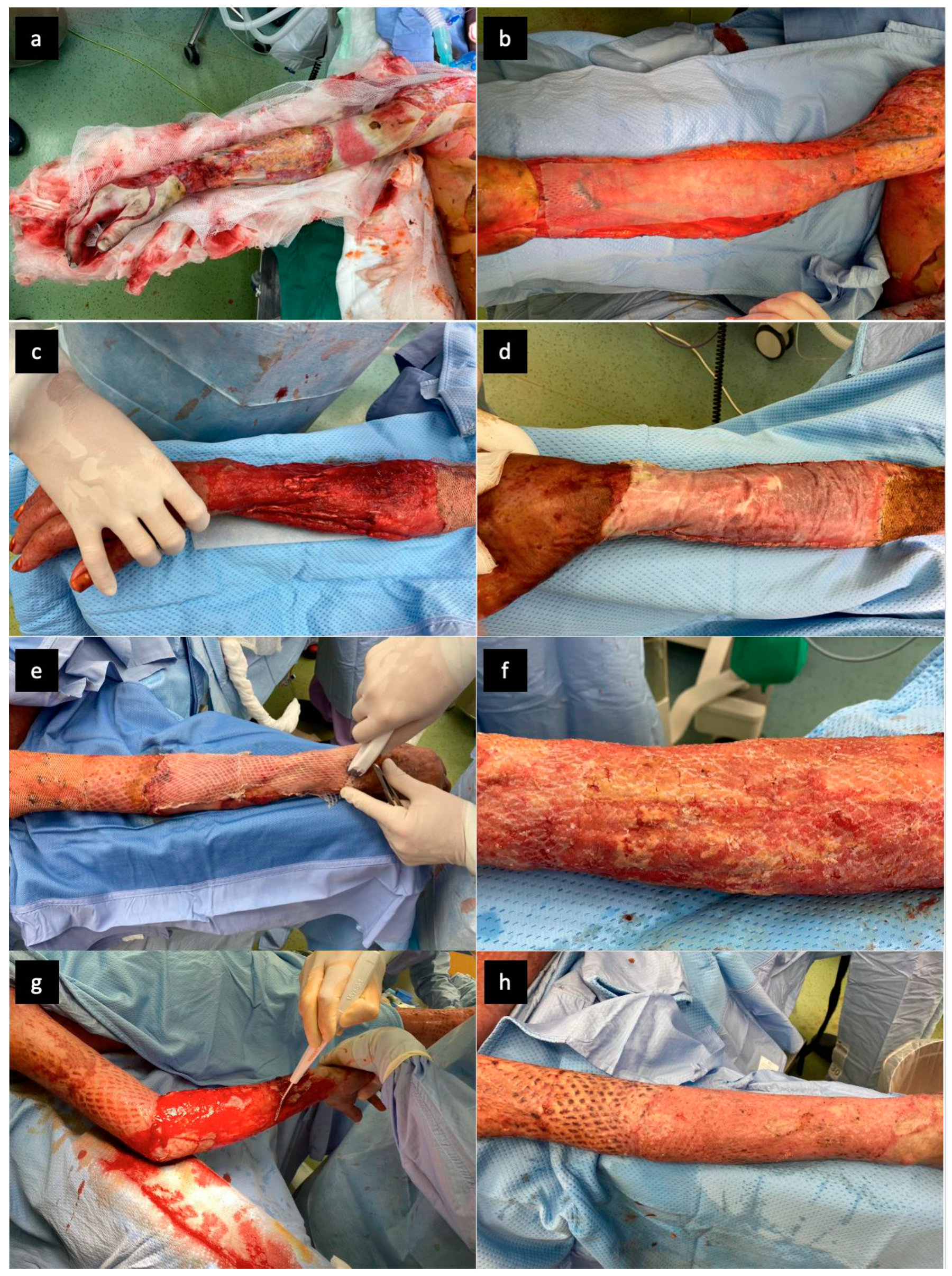
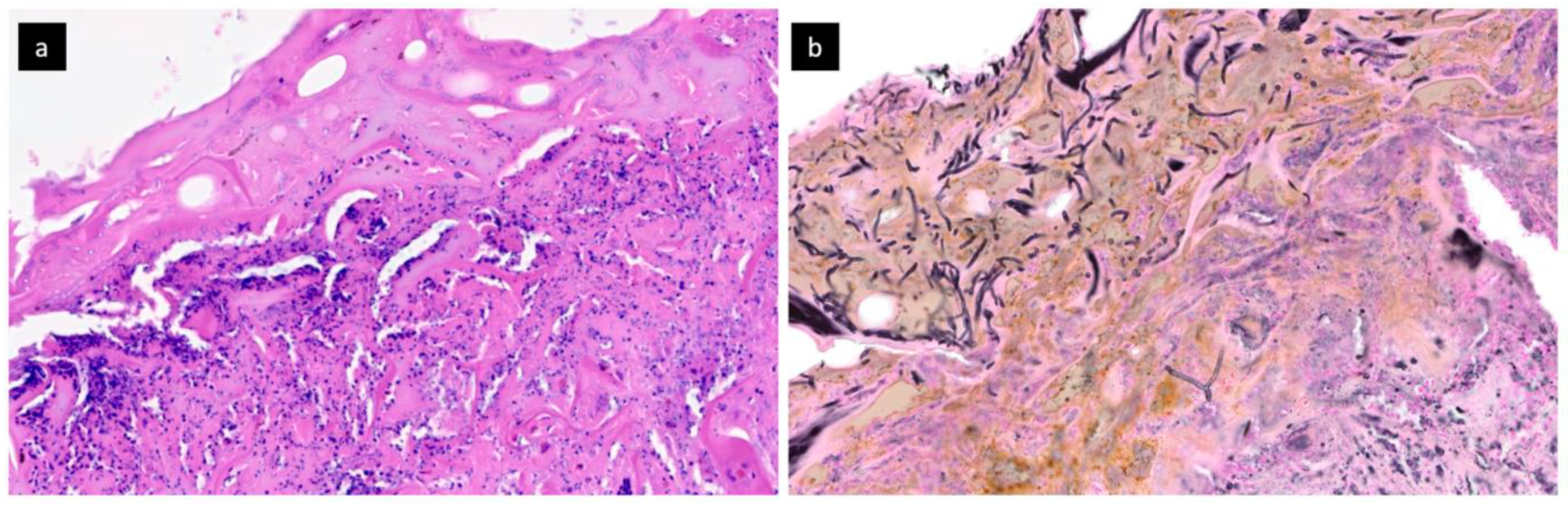
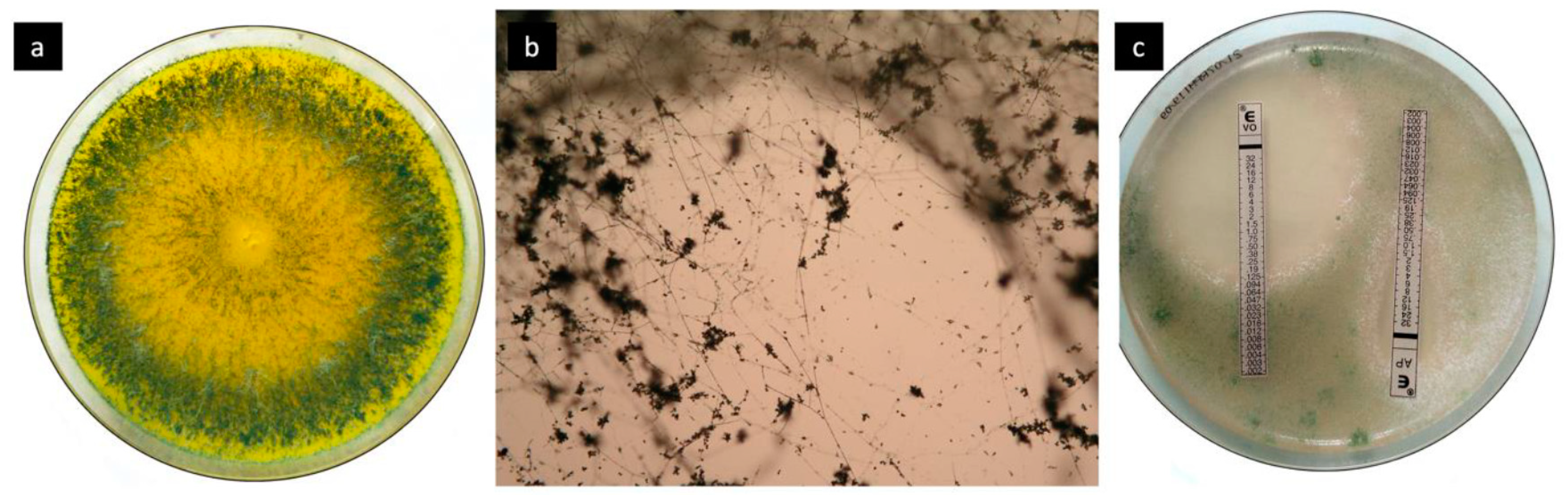
2. Case Report and Results
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kao, Y.; Loh, E.; Hsu, C.; Lin, H.; Huang, C.; Chou, Y.; Lien, C.; Tam, K. Fluid Resuscitation in Patients With Severe Burns: A Meta-analysis of Randomized Controlled Trials. Acad. Emerg. Med. 2018, 25, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Gacto-Sanchez, P. Surgical treatment and management of the severely burn patient: Review and update. Med. Intensiv. 2017, 41, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Vigani, A.; Culler, C.A. Systemic and Local Management of Burn Wounds. Vet. Clin. N. Am. Small Anim. Pract. 2017, 47, 1149–1163. [Google Scholar] [CrossRef]
- Rowley-Conwy, G. Management of burns in intensive and acute care. Nurs. Stand. 2013, 27, 63–64. [Google Scholar] [CrossRef]
- Spronk, I.; Legemate, C.; Oen, I.; Van Loey, N.; Polinder, S.; Van Baar, M. Health related quality of life in adults after burn injuries: A systematic review. PLoS ONE 2018, 13, e0197507. [Google Scholar] [CrossRef]
- Kool, M.B.; Geenen, R.; Egberts, M.R.; Wanders, H.; Van Loey, N.E. Patients’ perspectives on quality of life after burn. Burns 2017, 43, 747–756. [Google Scholar] [CrossRef]
- Shahrokhi, S.; Arno, A.; Jeschke, M.G. The use of dermal substitutes in burn surgery: Acute phase. Wound Repair Regen. 2014, 22, 14–22. [Google Scholar] [CrossRef]
- van der Veen, V.C.; van der Wal, M.B.; van Leeuwen, M.C.; Ulrich, M.M.; Middelkoop, E. Biological background of dermal substitutes. Burns 2010, 36, 305–321. [Google Scholar] [CrossRef]
- Uccioli, L.; Meloni, M.; Izzo, V.; Giurato, L. Use of Nevelia Dermal-Epidermal Regenerative Template in the Management of Ischemic Diabetic Foot Postsurgical Wounds. Int. J. Low. Extrem. Wounds 2020, 19, 282–288. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, B.; Orlandi, F.; D’Autilio, M.F.L.M.; Scioli, M.G.; Orlandi, A.; Cervelli, V.; Gentile, P. Long-term follow-up comparison of two different bi-layer dermal substitutes in tissue regeneration: Clinical outcomes and histological findings. Int. Wound J. 2018, 15, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Yiğitbaş, H. Our experience with dermal substitute Nevelia® in the treatment of severely burned patients. Turk. J. Trauma Emerg. Surg. 2019, 25, 520–526. [Google Scholar] [CrossRef]
- Ferrer, C.; Colom, F.; Fraseés, S.; Mulet, E.; Abad, J.L.; Alioó, J.L. Detection and Identification of Fungal Pathogens by PCR and by ITS2 and 5.8S Ribosomal DNA Typing in Ocular Infections. J. Clin. Microbiol. 2001, 39, 2873–2879. [Google Scholar] [CrossRef] [PubMed]
- Lipový, B.; Brychta, P.; Řihová, H.; Hanslianová, M.; Loskotová, A.; Jarkovský, J.; Kaloudová, Y.; Suchánek, I. Prevalence of infectious complications in burn patients requiring intensive care: Data from a pan-European study. Epidemiol. Mikrobiol. Imunol. 2016, 65, 25–32. [Google Scholar] [PubMed]
- Ceniceros, A.; Pértega, S.; Galeiras, R.; Mourelo, M.; Lopez, E.; Broullon, J.; Sousa, L.; Freire, D.; Pértega-Díaz, S. Predicting mortality in burn patients with bacteraemia. Infection 2015, 44, 215–222. [Google Scholar] [CrossRef]
- Egozi, D.; Hussein, K.; Filson, S.; Mashiach, T.; Ullmann, Y.; Raz-Pasteur, A. Bloodstream infection as a predictor for mortality in severe burn patients: An 11-year study. Epidemiol. Infect. 2013, 142, 2172–2179. [Google Scholar] [CrossRef]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn Wound Infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef]
- Lachiewicz, A.M.; Hauck, C.G.; Weber, D.J.; Cairns, A.B.; Van Duin, D. Bacterial Infections After Burn Injuries: Impact of Multidrug Resistance. Clin. Infect. Dis. 2017, 65, 2130–2136. [Google Scholar] [CrossRef]
- Horvath, E.E.; Murray, C.K.; Vaughan, G.M.; Chung, K.K.; Hospenthal, D.R.; Wade, C.E.; Holcomb, J.B.; Wolf, S.; Mason, A.D.; Cancio, L.C. Fungal Wound Infection (Not Colonization) Is Independently Associated with Mortality in Burn Patients. Ann. Surg. 2007, 245, 978–985. [Google Scholar] [CrossRef]
- Becker, W.K.; Cioffi, W.G.; McManus, A.T.; Kim, S.H.; McManus, W.F.; Mason, A.D.; Pruitt, B.A. Fungal Burn Wound Infection. Arch. Surg. 1991, 126, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.H. Fungal infection of burn wounds in patients with open and occlusive treatment methods. East. Mediterr. Health J. 1999, 5, 333–336. [Google Scholar]
- Murray, C.K.; Loo, F.L.; Hospenthal, D.R.; Cancio, L.C.; Jones, J.A.; Kim, S.H.; Holcomb, J.B.; Wade, C.E.; Wolf, S. Incidence of systemic fungal infection and related mortality following severe burns. Burns 2008, 34, 1108–1112. [Google Scholar] [CrossRef]
- Howard, P.; Cancio, L.; McManus, A.; Goodwin, C.; Kim, S.; Pruitt, B. What’s new in burn-associated infections? Curr. Surg. 1999, 56, 397–405. [Google Scholar] [CrossRef]
- Schofield, C.M.; Murray, C.K.; Horvath, E.E.; Cancio, L.C.; Kim, S.H.; Wolf, S.; Hospenthal, D.R. Correlation of culture with histopathology in fungal burn wound colonization and infection. Burns 2007, 33, 341–346. [Google Scholar] [CrossRef]
- Schaal, J.; Leclerc, T.; Soler, C.; Donat, N.; Cirrode, A.; Jault, P.; Bargues, L. Epidemiology of filamentous fungal infections in burned patients: A French retrospective study. Burns 2015, 41, 853–863. [Google Scholar] [CrossRef]
- Katz, T.; Wasiak, J.; Cleland, H.; Padiglione, A. Incidence of non-candidal fungal infections in severe burn injury: An Australian perspective. Burns 2014, 40, 881–886. [Google Scholar] [CrossRef]
- Capoor, M.R.; Gupta, S.; Sarabahi, S.; Mishra, A.; Tiwari, V.K.; Aggarwal, P. Epidemiological and clinico-mycological profile of fungal wound infection from largest burn centre in Asia. Mycoses 2011, 55, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Rosanova, M.T.; Brizuela, M.; Villasboas, M.; Guarracino, F.; Alvarez, V.; Santos, P.; Finquelievich, J. Fusarium spp infections in a pediatric burn unit: Nine years of experience. Braz. J. Infect. Dis. 2016, 20, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.P.; Chen, S.C.-A.; Slavin, M.A. Emerging infections caused by non- Aspergillus filamentous fungi. Clin. Microbiol. Infect. 2016, 22, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Chouaki, T.; Lavarde, V.; Lachaud, L.; Raccurt, C.P.; Hennequin, C. Invasive Infections Due toTrichodermaSpecies: Report of 2 Cases, Findings of In Vitro Susceptibility Testing, and Review of the Literature. Clin. Infect. Dis. 2002, 35, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Kredics, L.; Antal, Z.; Dóczi, I.; Manczinger, L.; Kevei, F.; Nagy, E. Clinical importance of the genus Trichoderma. Acta Microbiol. Immunol. Hung. 2003, 50, 105–117. [Google Scholar] [CrossRef]
- Trabelsi, S.; Hariga, D.; Khaled, S. First case of Trichoderma longibrachiatum infection in a renal transplant recipient in Tunisia and review of the literature. Tunis Med. 2010, 88, 52–57. [Google Scholar]
- Chen, S.C.-A.; Blyth, C.C.; Sorrell, T.C.; Slavin, M. Pneumonia and Lung Infections due to Emerging and Unusual Fungal Pathogens. Semin. Respir. Crit. Care Med. 2011, 32, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Groll, A.; Hiemenz, J.; Fleming, R.; Roilides, E.; Anaissie, E. Infections due to emerging and uncommon medically important fungal pathogens. Clin. Microbiol. Infect. 2004, 10, 48–66. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Sutton, D.A.; Cano-Lira, J.F.; Gené, J.; Fothergill, A.W.; Wiederhold, N.P.; Guarro, J. Phylogeny of the Clinically Relevant Species of the Emerging Fungus Trichoderma and Their Antifungal Susceptibilities. J. Clin. Microbiol. 2014, 52, 2112–2125. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Kusne, S.; Sutton, D.A.; Manez, R.; Carrau, R.; Nichols, K.A.L.; Skedros, D.; Todo, S.; Rinaldi, M.G.; Nichols, L.; et al. Acute invasive sinusitis due to Trichoderma longibrachiatum in a liver and small bowel transplant recipient. Clin. Infect. Dis. 1998, 26, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Kerr, C.M.; Perfect, J.R.; Craven, P.C.; Jorgensen, J.H.; Drutz, D.J.; Shelburne, J.D.; Gallis, H.A.; Gutman, R.A. Fungal Peritonitis in Patients on Continuous Ambulatory Peritoneal Dialysis. Ann. Intern. Med. 1983, 99, 334–337. [Google Scholar] [CrossRef]
- Piens, M.A.; Celard, M.; de Monbrison, F.; Grando, J.; Vandenesch, F.; Mottolese, C.; Picot, S. Trichoderma infection of cerebro-spinal fluid shunt device in a non immunocompromised patient. J. Mycol. Med. 2004, 14, 49–51. [Google Scholar]
- Ranque, S.; Garcia-Hermoso, D.; Michel-Nguyen, A.; Dumon, H. Isolation of Trichoderma atroviride from a liver transplant. J. Mycol. Méd. 2008, 18, 234–236. [Google Scholar] [CrossRef]
- Seguin, P.; Degeilh, B.; Grulois, I.; Gacouin, A.; Maugendre, S.; Dufour, T.; Dupont, B.; Camus, C. Successful treatment of a brain abscess due toTrichoderma longibrachiatum after surgical resection. Eur. J. Clin. Microbiol. Infect. Dis. 1995, 14, 445–448. [Google Scholar] [CrossRef]
- Tascini, C.; Cardinali, G.; Barletta, V.; Di Paolo, A.; Leonildi, A.; Zucchelli, G.; Corte, L.; Colabella, C.; Roscini, L.; Consorte, A.; et al. First Case of Trichoderma longibrachiatum CIED (Cardiac Implantable Electronic Device)-Associated Endocarditis in a Non-immunocompromised Host: Biofilm Removal and Diagnostic Problems in the Light of the Current Literature. Mycopathologia 2015, 181, 297–303. [Google Scholar] [CrossRef]
- Struck, M.; Gille, J. Fungal infections in burns: A comprehensive review. Ann. Burn. Fire Disasters 2013, 26, 147–153. [Google Scholar]
- Kyriopoulos, E.; Kyriakopoulos, A.; Karonidis, A.; Gravvanis, A.; Gamatsi, I.; Tsironis, C.; Tsoutsos, D. Burn injuries and soft tissue traumas complicated by mucormycosis infection: A report of six cases and review of the literature. Ann. Burn. Fire Disasters 2015, 28, 280–287. [Google Scholar]
- Gonzalez, S.R.; Wolter, K.G.; Yuen, J.C. Infectious Complications Associated with the Use of Integra: A Systematic Review of the Literature. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2869. [Google Scholar] [CrossRef]
- Peck, M.D.; Kessler, M.; Meyer, A.A.; Morris, P.A.B. A Trial of the Effectiveness of Artificial Dermis in the Treatment of Patients with Burns Greater Than 45% Total Body Surface Area. J. Trauma Acute Care Surg. 2002, 52, 971–978. [Google Scholar] [CrossRef]
- Munster, A.M.; Smith-Meek, M.; Shalom, A. Acellular allograft dermal matrix: Immediate or delayed epidermal coverage? Burns 2001, 27, 150–153. [Google Scholar] [CrossRef]
- Branski, L.K.; Herndon, D.N.; Pereira, C.; Mlcak, R.P.; Celis, M.M.; Lee, J.O.; Sanford, A.P.; Norbury, W.B.; Zhang, X.-J.; Jeschke, M.G. Longitudinal assessment of Integra in primary burn management: A randomized pediatric clinical trial. Crit. Care Med. 2007, 35, 2615–2623. [Google Scholar] [CrossRef]
- Bloemen, M.C.T.; Van Der Wal, M.B.A.; Verhaegen, P.D.H.M.; Nieuwenhuis, M.; Van Baar, M.E.; Van Zuijlen, P.P.M.; Middelkoop, E. Clinical effectiveness of dermal substitution in burns by topical negative pressure: A multicenter randomized controlled trial. Wound Repair Regen. 2012, 20, 797–805. [Google Scholar] [CrossRef]
- Lagus, H.; Sarlomo-Rikala, M.; Böhling, T.; Vuola, J. Prospective study on burns treated with Integra®, a cellulose sponge and split thickness skin graft: Comparative clinical and histological study—Randomized controlled trial. Burns 2013, 39, 1577–1587. [Google Scholar] [CrossRef]
- Bootun, R. Effects of immunosuppressive therapy on wound healing. Int. Wound J. 2012, 10, 98–104. [Google Scholar] [CrossRef]
- Zhang, H.; Qu, W.; Nazzal, M.; Ortiz, J. Burn patients with history of kidney transplant experience increased incidence of wound infection. Burns 2020, 46, 609–615. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipový, B.; Raška, F.; Kocmanová, I.; Hanslianová, M.; Hladík, M.; Holoubek, J.; Bezdíček, M.; Macháček, C. Trichoderma longibrachiatum and Aspergillus fischeri Infection as a Cause of Skin Graft Failure in a Patient with Critical Burns after Liver Transplantation. J. Fungi 2021, 7, 487. https://doi.org/10.3390/jof7060487
Lipový B, Raška F, Kocmanová I, Hanslianová M, Hladík M, Holoubek J, Bezdíček M, Macháček C. Trichoderma longibrachiatum and Aspergillus fischeri Infection as a Cause of Skin Graft Failure in a Patient with Critical Burns after Liver Transplantation. Journal of Fungi. 2021; 7(6):487. https://doi.org/10.3390/jof7060487
Chicago/Turabian StyleLipový, Břetislav, Filip Raška, Iva Kocmanová, Markéta Hanslianová, Martin Hladík, Jakub Holoubek, Matěj Bezdíček, and Ctirad Macháček. 2021. "Trichoderma longibrachiatum and Aspergillus fischeri Infection as a Cause of Skin Graft Failure in a Patient with Critical Burns after Liver Transplantation" Journal of Fungi 7, no. 6: 487. https://doi.org/10.3390/jof7060487
APA StyleLipový, B., Raška, F., Kocmanová, I., Hanslianová, M., Hladík, M., Holoubek, J., Bezdíček, M., & Macháček, C. (2021). Trichoderma longibrachiatum and Aspergillus fischeri Infection as a Cause of Skin Graft Failure in a Patient with Critical Burns after Liver Transplantation. Journal of Fungi, 7(6), 487. https://doi.org/10.3390/jof7060487





