Application of Disinfectants for Environmental Control of a Lethal Amphibian Pathogen
Abstract
1. Introduction
2. Materials and Methods
2.1. Bd Culture Maintenance
2.2. Growth Inhibition of Bd by Six Commercial Antifungals
2.3. Killing Ability Concentration Determination of Wofasteril40 against Bd
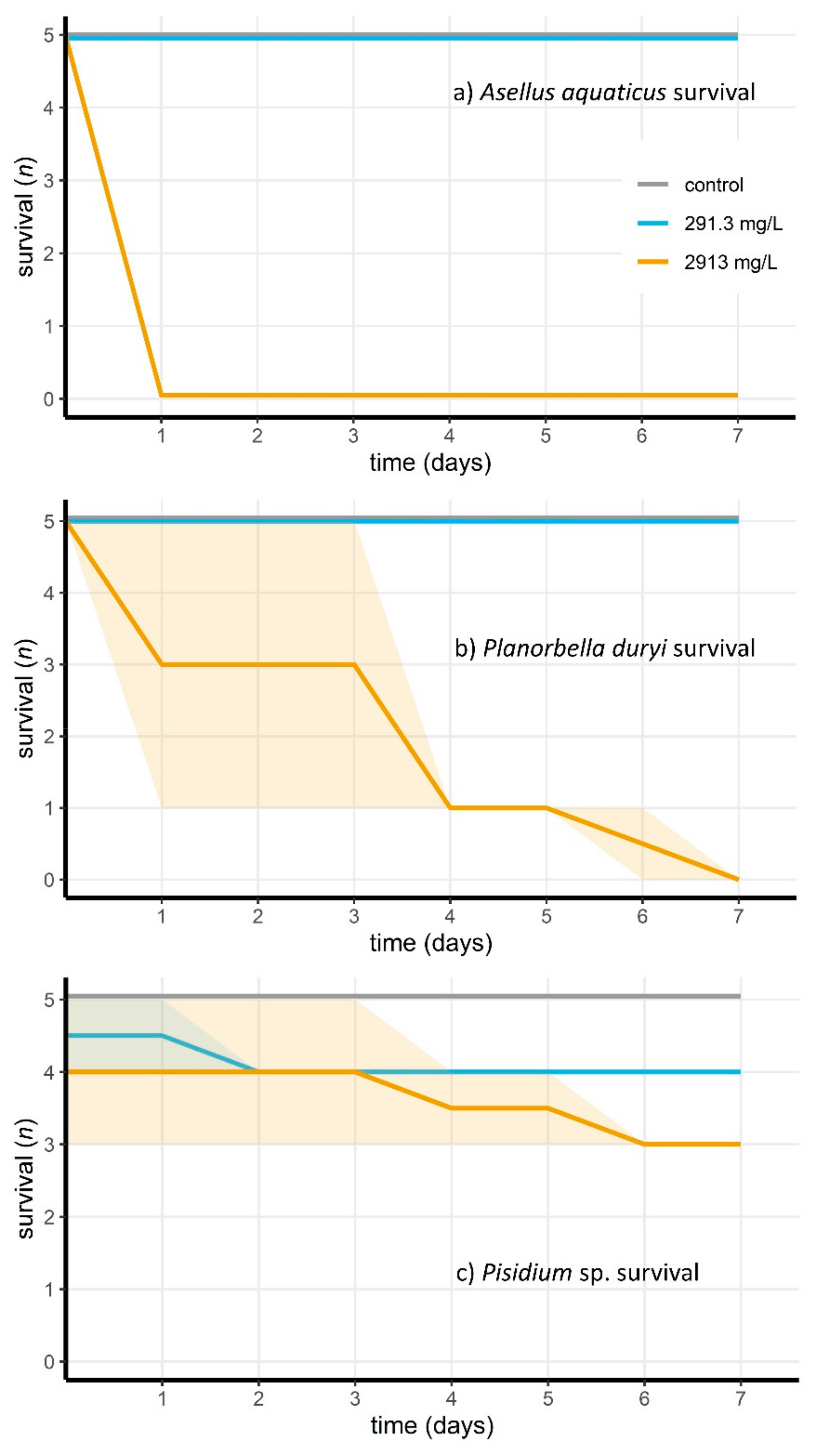
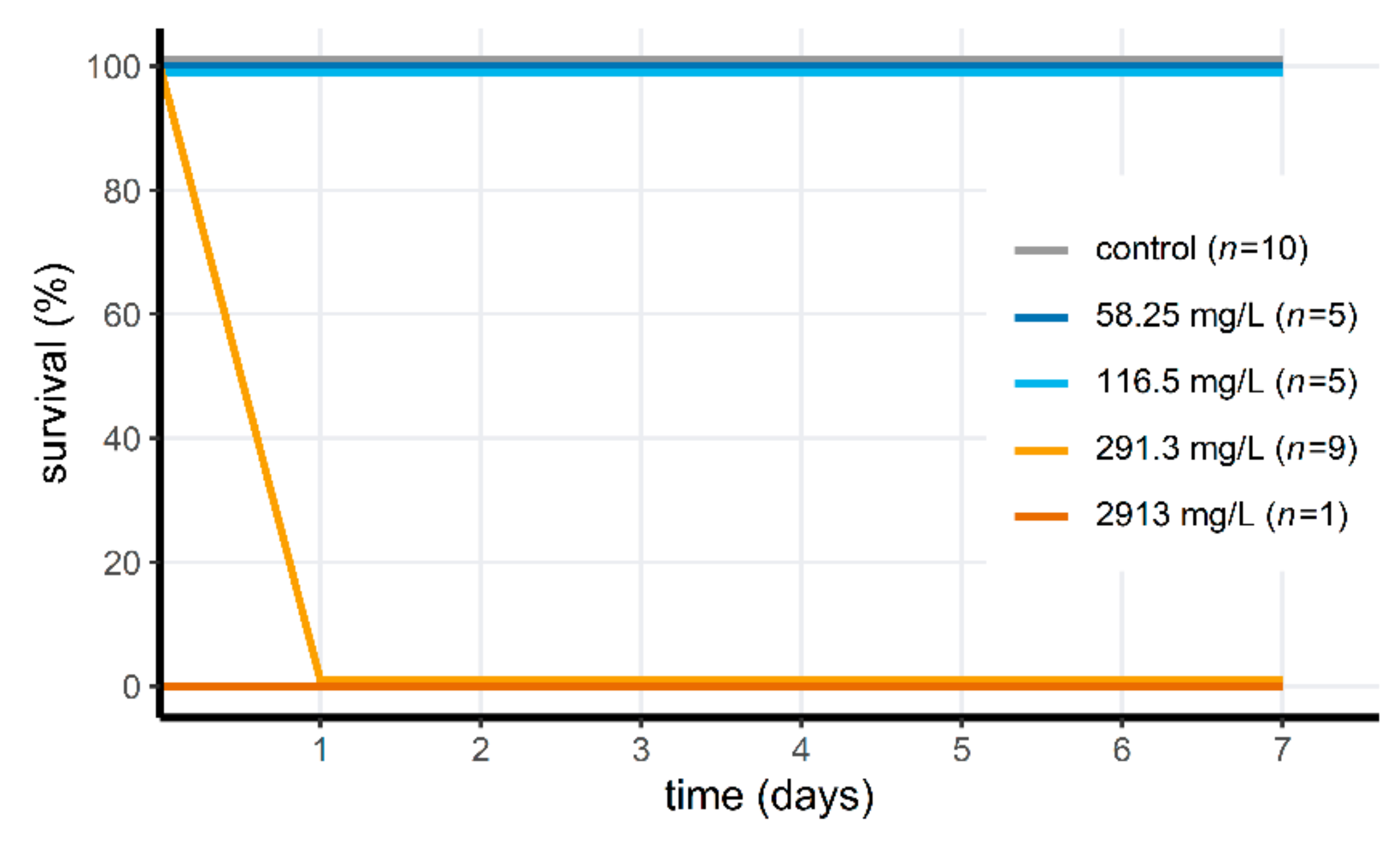
2.4. Acute Toxicity of Wofasteril40 against Tadpoles and Aquatic Invertebrates
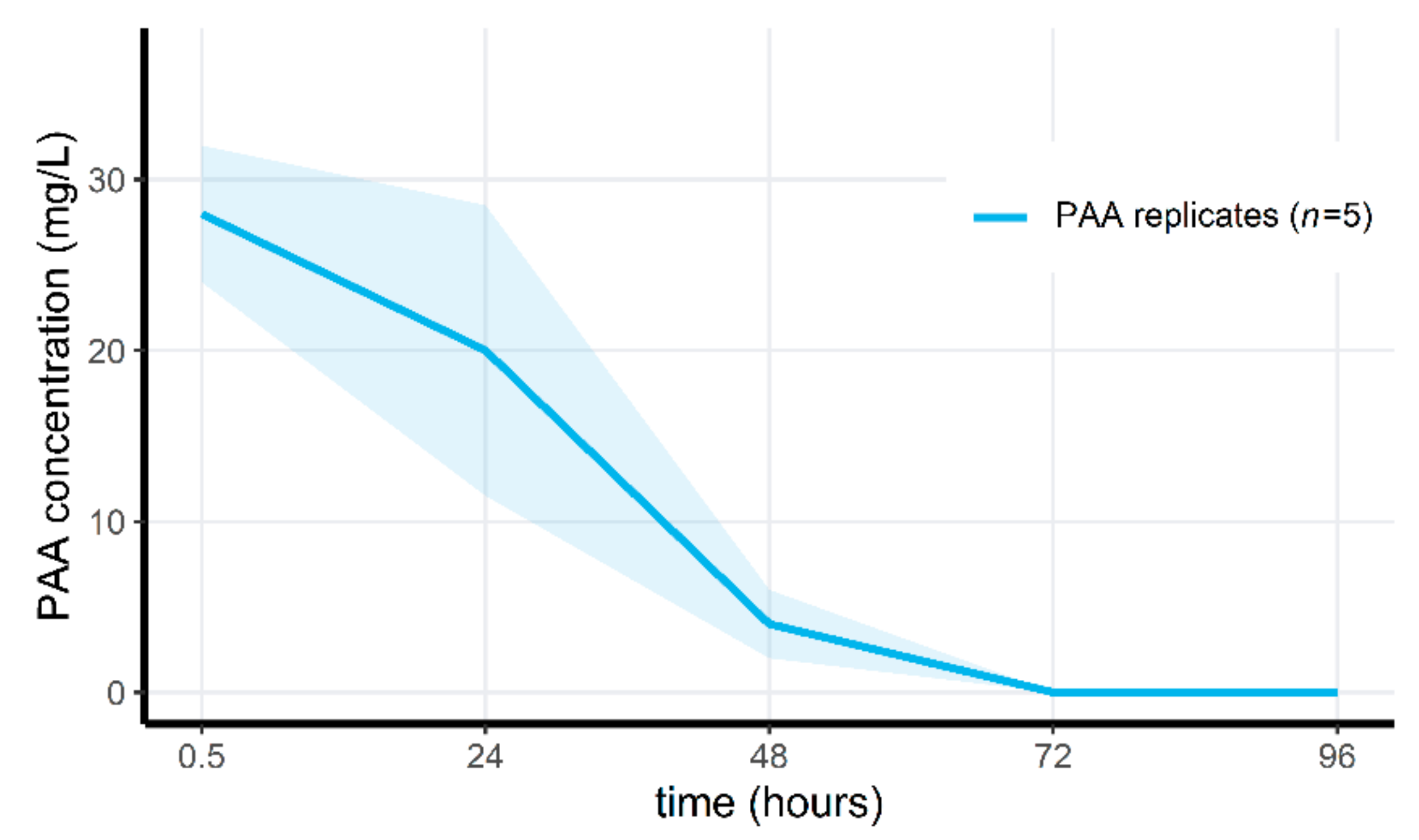
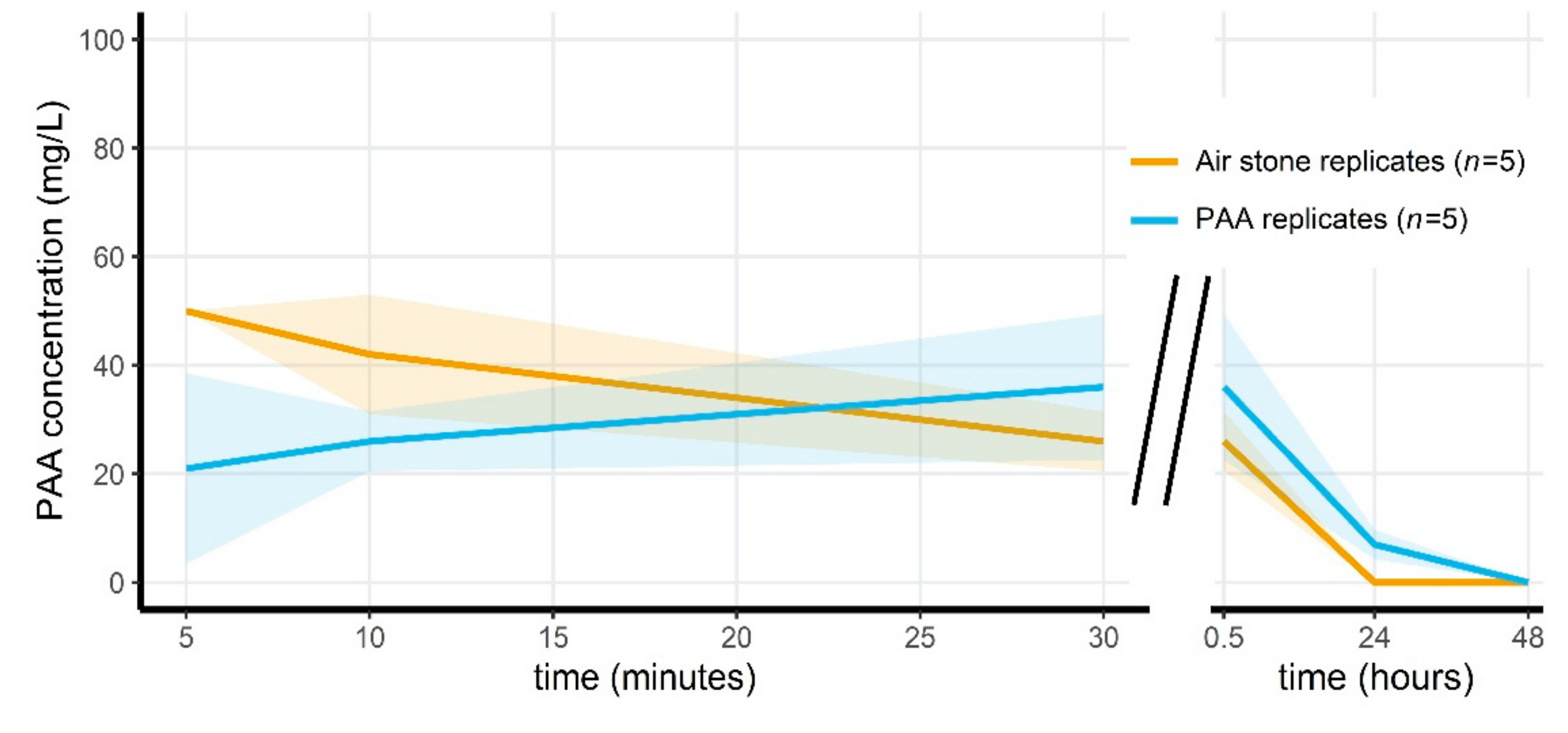
2.5. Exposure of Microcosms to Peracetic Acid
2.6. Statistical Analysis and Graphical Visualisation
3. Results
3.1. Effectiveness of Commercial Products against Bd
3.2. Killing Ability of Peracetic Acid against Bd
3.3. Acute Toxicity of Peracetic Acid for Aquatic Animals
3.4. Peracetic Acid Concentrations Rapidly Decrease in the Microcosms
3.5. Adding PAA to Microcosms Results in a Temporary pH Drop
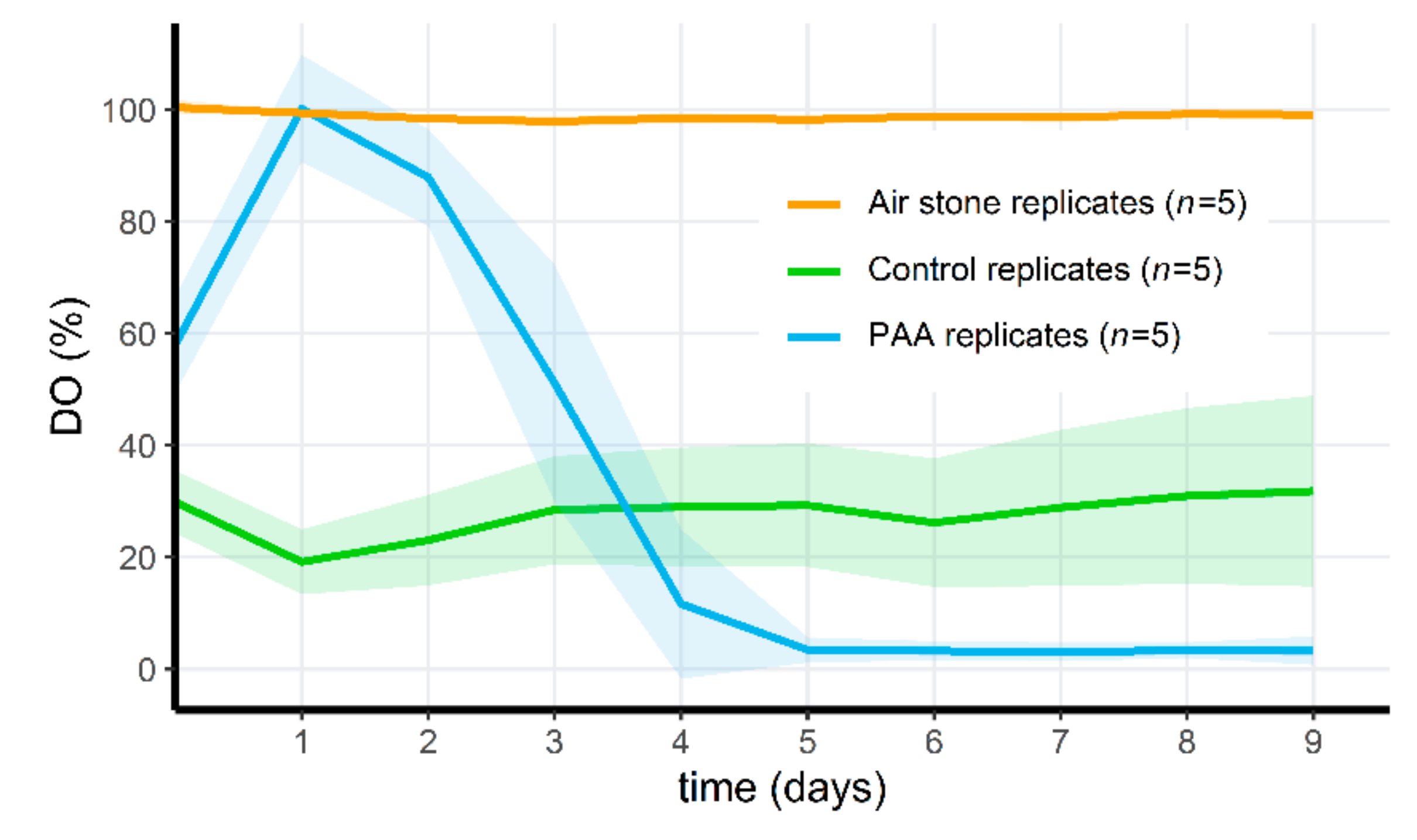
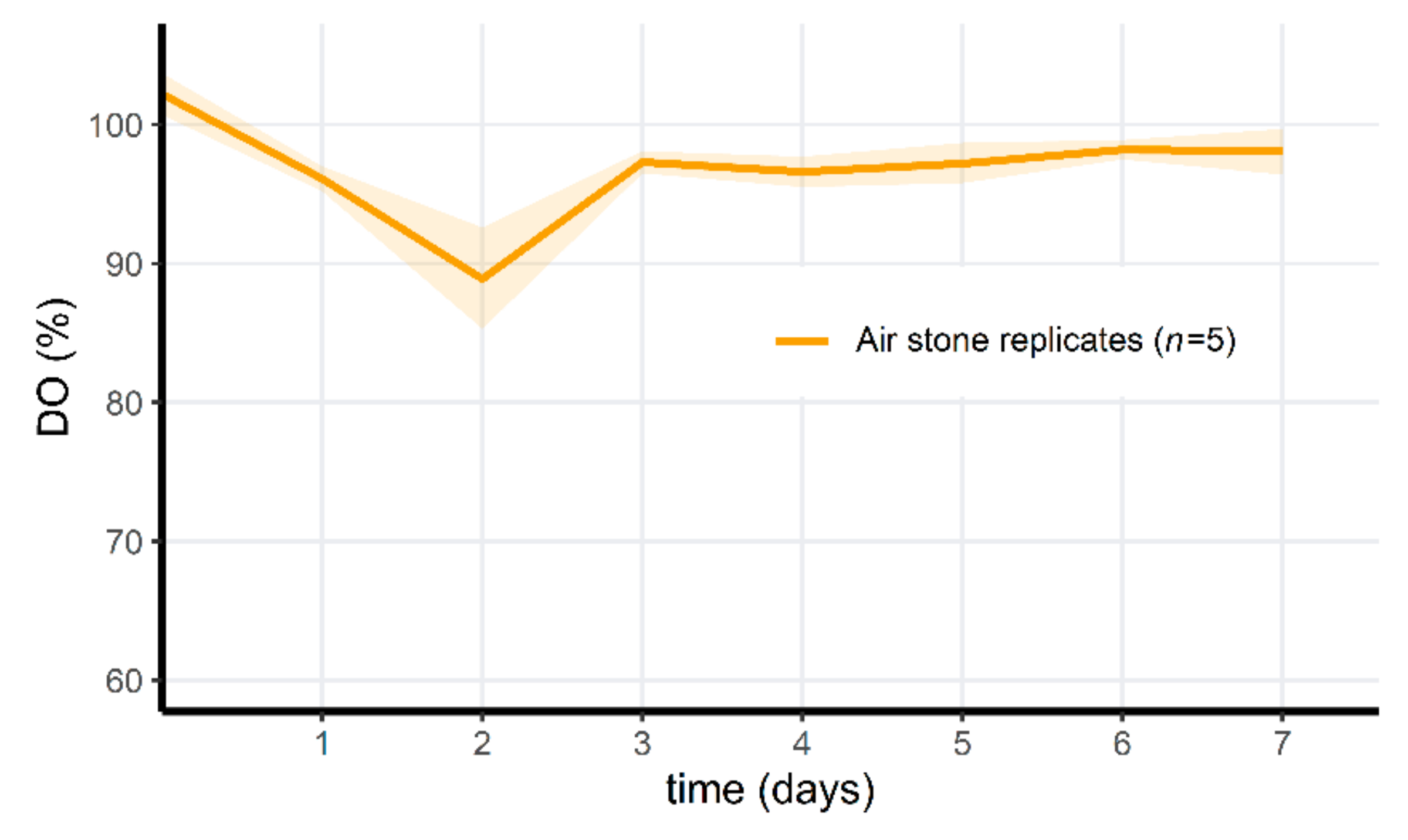
3.6. PAA Treatment of Microcosms Results in a Drop of Oxygen Levels
4. Discussion
4.1. Effectiveness of Commercial Products against Bd
4.2. Killing Ability of Peracetic Acid against Bd
4.3. Peracetic Acid Concentrations That Kill Bd Are Acutely Toxic for Tadpoles
4.4. Disinfecting Aquatic Environments May Result in Drastic Ecosystem Changes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butchart, S.H.M.; Walpole, M.; Collen, B.; van Strien, A.; Scharlemann, J.P.W.; Almond, R.E.A.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global Biodiversity: Indicators of Recent Declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef]
- Hoffmann, M.; Hilton-Taylor, C.; Angulo, A.; Böhm, M.; Brooks, T.M.; Butchart, S.H.M.; Carpenter, K.E.; Chancon, J.; Collen, B.; Cox, N.A.; et al. The Impact of Conservation on the Status of the World’s Vertebrates. Science 2010, 330, 1503–1509. [Google Scholar] [CrossRef]
- Smale, D.A.; Wernberg, T.; Oliver, E.C.J.; Thomsen, M.; Harvey, B.P.; Straub, S.C.; Burrows, M.T.; Alexander, L.V.; Benthuysen, J.A.; Donat, M.G.; et al. Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Chang. 2019, 9, 306–312. [Google Scholar] [CrossRef]
- Johnson, C.N.; Balmford, A.; Brook, B.W.; Buettel, J.C.; Galetti, M.; Guangchun, L.; Wilmshurst, J.M. Biodiversity losses and conservation responses in the Anthropocene. Science 2017, 356, 270–275. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA 2017, 114, e6089–e6096. [Google Scholar] [CrossRef] [PubMed]
- Sánches-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- IUCN. International Union for Conservation of Nature Red List. Available online: http://www.iucn.org/about/work/programmes/species/red_list/ (accessed on 4 May 2021).
- Scheele, B.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Gerber, B.D.; Converse, S.J.; Muths, E.; Crockett, H.J.; Mosjer, B.A.; Bailey, L.L. Identifying species conservation strategies to reduce disease-associated declines. Conserv. Lett. 2017, 11, 1–10. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Briggs, C.J.; Daszak, P. The ecology and impact of chytridiomycosis: An emerging disease of amphibians. Trends Ecol. Evol. 2009, 25, 109–118. [Google Scholar] [CrossRef]
- Frick, W.F.; Pollock, J.F.; Hicks, A.C.; Langwig, K.E.; Reynolds, E.S.; Turner, G.G.; Butchkoski, C.M.; Kunz, T.H. An emerging disease causes regional population collapse of a common North American bat species. Science 2010, 329, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging Infectious Diseases of Wildlife—Threats to Biodiversity and Human Health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef]
- McCallum, H.; Dobson, A. Detecting disease and parasite threats to endangered species and ecosystems. Trends Ecol. Evol. 1995, 10, 190–194. [Google Scholar] [CrossRef]
- Longcore, J.E.; Pessier, A.P.; Nichols, D.K. Batrachochytrium dendrobatidis gen. et sp. nov., a Chytrid Pathogenic to Amphibians. Mycologia 1999, 91, 219–227. [Google Scholar] [CrossRef]
- Martel, A.; Spitzen-van der Sluijs, A.; Blooi, M.; Bert, W.; Ducatelle, R.; Fisher, M.C.; Woeltjes, A.; Bosman, W.; Chiers, K.; Bossuyt, F.; et al. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc. Natl. Acad. Sci. USA 2013, 110, 15325–15329. [Google Scholar] [CrossRef]
- Courtois, E.A.; Loyau, A.; Bourgoin, M.; Schmeller, D.S. Initiation of Batrachochytrium dendrobatidis infection in the absence of physical contact with infected hosts—A field study in a high altitude lake. Oikos 2017, 126, 843–851. [Google Scholar] [CrossRef]
- Hagman, M.; Alford, R.A. Patterns of Batrachochytrium dendrobatidis transmission between tadpoles in a high-elevation rainforest stream in tropical Australia. Dis. Aquat. Organ. 2015, 115, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; Sanchez-Tomé, E.; Fernández-Loras, A.; Oliver, J.A.; Fisher, M.C.; Garner, T.W.J. Successful elimination of a lethal wildlife infectious disease in nature. Biol. Lett. 2015, 11, 20150874. [Google Scholar] [CrossRef]
- Martel, A.; Vila-Escale, M.; Fernández-Giberteau, D.; Martinez-Silvestre, A.; Canessa, S.; Van Praet, S.; Pannon, P.; Chiers, K.; Ferran, A.; Kelly, M.; et al. Integral chain management of wildlife diseases. Conserv. Lett. 2020, e12707. [Google Scholar] [CrossRef]
- Culp, S.J.; Beland, F.A. Malachite Green: A Toxicological Review. Int. J. Toxicol. 1996, 15, 219–238. [Google Scholar] [CrossRef]
- Leal, J.F.; Neves, M.G.P.M.S.; Santos, E.B.H.; Esteves, V.I. Use of formalin in intensive aquaculture: Properties, application and effects on fish and water quality. Rev. Aquacult. 2018, 10, 281–295. [Google Scholar] [CrossRef]
- Srivastava, S.; Sinha, R.; Roy, D. Toxicological effects of malachite green. Aquat. Toxicol. 2004, 66, 319–329. [Google Scholar] [CrossRef]
- Nelson, C.R.; Hites, R.A. Aromatic amines in and near the Buffalo River. Environ. Sci. Technol. 1980, 14, 1147–1149. [Google Scholar] [CrossRef]
- Swenberg, J.A.; Moeller, B.C.; Lu, K.; Rager, J.E.; Fry, R.; Starr, T.B. Formaldehyde carcinogenicity research: 30 years and counting for mode of action, epidemiology, and cancer risk assessment. Toxicol. Pathol. 2013, 41, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Monarca, S.; Richardson, S.D.; Feretti, D.; Grottolo, M.; Thruston, A.D., Jr.; Zani, C.; Navazio, G.; Ragazzo, P.; Zerbini, I.; Alberti, A. Mutagenicity and disinfection by-products in surface drinking water disinfected with peracetic acid. Environ. Toxicol. Chem. 2002, 21, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.; King, L.; Skonberg, D. Demonstration of Sustainable Cod Farming from Egg to Grow-Out in Maine; NOAA Final Report; University of Maine: Orono, ME, USA, 2007. [Google Scholar]
- Brown, N. Halibut aquaculture in North America. In Practical Flatfish Culture and Stock Enhancement; Daniels, H.V., Watanabe, W., Eds.; Blackwell Publishing: Ames, IA, USA, 2010; pp. 1–29. [Google Scholar]
- Straus, D.L.; Meinelt, T.; Farmer, B.D.; Beck, B.H. Acute toxicity and histopathology of channel catfish fry exposed to peracetic acid. Aquaculture 2012, 342, 134–138. [Google Scholar] [CrossRef]
- Patrick, G.; Tarnecki, A.M.; Rhody, N.; Schloesser, R.; Main, K.; Yanong, R.; Francis-Floyd, R. Disinfection of almaco jack (Seriola rivoliana Valenciennes) eggs: Evaluation of three chemicals. Aquacult. Res. 2019, 50, 3793–3801. [Google Scholar] [CrossRef]
- Lubello, C.; Gori, R.; Nicese, F.P.; Ferrini, F. Municipal-treated wastewater reuse for plant nurseries irrigation. Water Res. 2004, 38, 2939–2947. [Google Scholar] [CrossRef] [PubMed]
- Baldry, M.G.C. The bactericidal, fungicidal and sporicidal properties of hydrogen peroxide and peracetic acid. J. Appl. Bacteriol. 1983, 54, 417–423. [Google Scholar] [CrossRef]
- Montori, A.; San Sebastian, O.; Franch, M.; Pujol-Buxó, E.; Llorente, G.A.; Fernández-Loras, A.; Richter-Boix, Á.; Bosch, J. Observations on the intensity and prevalence of Batrachochytridium dendrobatidis in sympatric and allopatric Epidalea calamita (native) and Discoglossus pictus (invasive) populations. Basic Appl. Herpetol. 2019, 33, 5–17. [Google Scholar] [CrossRef]
- Frankel, T.E.; Bohannon, M.E.; Frankel, J.S. Assessing the impacts of methoxychlor exposure on the viability, reproduction, and locomotor behavior of the Seminole ramshorn snail (Planorbella duryi). Environ. Toxicol. Chem. 2019, 39, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, J.; Kukkonen, J.V.K.; Holopainen, I.J. Toxicokinetics of 2,4,5-Trichlorophenol and Benzo(a)pyrene in the Clam Pisidium amnicum: Effects of Seasonal Temperatures and Trematode Parasites. Arch. Environ. Contam. Toxicol. 2000, 39, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, J.; Honkanen, J.; Kukkonen, J.V.K.; Holopainen, I.J. Bisphenol A Accumulation in the Freshwater Clam Pisidium amnicum at Low Temperatures. Arch. Environ. Contam. Toxicol. 2002, 43, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Ali, H.; Alarifi, S.; Masih, A.P.; Manohardas, S.; Hussain, S.A. Eco-toxic efficacy of nano-sized magnesium oxide in freshwater snail Radix leuteola L. Fresenius Environ. Bull. 2016, 25, 1234–1242. [Google Scholar]
- Factor, C.J.B.; de Chaves, E.R.C. Toxicity of arsenic, aluminum, chromium and nickel to the embryos of the freshwater snail, Radix quadrasi von Möellendorf 1898. Philipp. J. Sci. 2012, 141, 207–216. [Google Scholar]
- Van Ginneken, M.; De Jonge, M.; Bervoets, L.; Blust, R. Uptake and toxicity of Cd, Cu and Pb mixtures in the isopod Asellus aquaticus from waterborne exposure. Sci. Total Environ. 2015, 537, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Green, D.W.J.; Williams, K.A.; Hughes, D.R.L.; Shaik, G.A.R.; Pascoe, D. Toxicity of phenol to Asellus aquaticus (L.)—effects of temperature and episodic exposure. Water Res. 1988, 22, 225–231. [Google Scholar] [CrossRef]
- Berger, L.; Speare, R.; Marantelli, G.; Skerrat, L.F. A zoospore inhibition technique to evaluate the activity of antifungal compounds against Batrachochytrium dendrobatidis and unsuccessful treatment of experimentally infected green tree frogs (Litoria caerulea) by fluconazole and benzalkonium chloride. Res. Vet. Sci. 2009, 87, 106–110. [Google Scholar] [CrossRef]
- De Jong, M.S.; Van Dyk, R.; Weldon, C. Antifungal efficacy of F10SC veterinary disinfectant against Batrachochytrium dendrobatidis. Med. Mycol. 2017, 56, 60–68. [Google Scholar] [CrossRef]
- Van Rooij, P.; Pasmans, F.; Coen, Y.; Martel, A. Efficacy of chemical disinfectants for the containment of the salamander chytrid fungus Batrachochytrium salamandrivorans. PLoS ONE 2017, 12, e0186269. [Google Scholar] [CrossRef]
- Thabah, C.M.; Devi, L.M.; Hooroo, R.N.K.; Dey, S. Morphological alterations in the external gills of some tadpoles in response to pH. J. Morphol. Sci. 2018. [Google Scholar] [CrossRef]
- Wijethunga, U.; Greenlees, M.; Shine, R. Effect of Pond Acidity on Southern Expansion of Cane Toads. Physiol. Biochem. Zool. 2015, 88. [Google Scholar] [CrossRef]
- Pierce, B.A.; Montgomery, J. Effects of Short-Term Acidification on Growth Rates of Tadpoles. J. Herpetol. 1989, 23, 97–102. [Google Scholar] [CrossRef]
- Garner, T.W.J.; Schmidt, B.R.; Martel, A.; Pasmans, F.; Muths, E.; Cunningham, A.A.; Weldon, C.; Fisher, M.C.; Bosch, J. Mitigating amphibian chytridiomycoses in nature. Philos. Trans. R. Soc. B 2016, 371, 20160207. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, D.; Bosch, J.; Briggs, C.J.; Cashins, S.; Davis, L.R.; Lauer, A.; Muths, E.; Puschendorf, R.; Schmidt, B.R.; Sheafor, B.; et al. Mitigating amphibian disease: Strategies to maintain wild populations and control chytridiomycosis. Front. Zool. 2011, 8, 8. [Google Scholar] [CrossRef]

| Product | |
|---|---|
| Active Component | MIC (mg/L) |
| Blagdon® pond anti-fungus and bacterial | 100 |
| Malachite green oxalate | 0.213 |
| Acriflavine hydrochloride | 0.437 |
| F10 ® SC | 15.625 |
| Alkyl (50% C14, 40% C12, 10% C16) dimethyl benzyl ammonium chloride | 0.843 |
| Poly (hexamethylene biguanide) hydrochloride | 0.063 |
| Iso-bethadine ® | 12,875 |
| Povidone-iodine | 1250 |
| Morenicol® FMC-50 | 160 |
| Malachite green | 0.400 |
| Methylene blue | 0.080 |
| Formalin | 32.204 |
| Sera® pond omnisan | 100 |
| Malachite green oxalate | 0.180 |
| Formaldehyde | 5.890 |
| Wofasteril40 ® | 466 |
| Peracetic acid | 116.5–186.4 |
| Hydrogen peroxide | 37.3–163.1 |
| Acetic acid | 116.5–302.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lammens, L.; Martel, A.; Pasmans, F. Application of Disinfectants for Environmental Control of a Lethal Amphibian Pathogen. J. Fungi 2021, 7, 406. https://doi.org/10.3390/jof7060406
Lammens L, Martel A, Pasmans F. Application of Disinfectants for Environmental Control of a Lethal Amphibian Pathogen. Journal of Fungi. 2021; 7(6):406. https://doi.org/10.3390/jof7060406
Chicago/Turabian StyleLammens, Leni, An Martel, and Frank Pasmans. 2021. "Application of Disinfectants for Environmental Control of a Lethal Amphibian Pathogen" Journal of Fungi 7, no. 6: 406. https://doi.org/10.3390/jof7060406
APA StyleLammens, L., Martel, A., & Pasmans, F. (2021). Application of Disinfectants for Environmental Control of a Lethal Amphibian Pathogen. Journal of Fungi, 7(6), 406. https://doi.org/10.3390/jof7060406







