Periprosthetic Fungal Infections in Severe Endoprosthetic Infections of the Hip and Knee Joint—A Retrospective Analysis of a Certified Arthroplasty Centre of Excellence
Abstract
1. Introduction
2. Materials and Methods
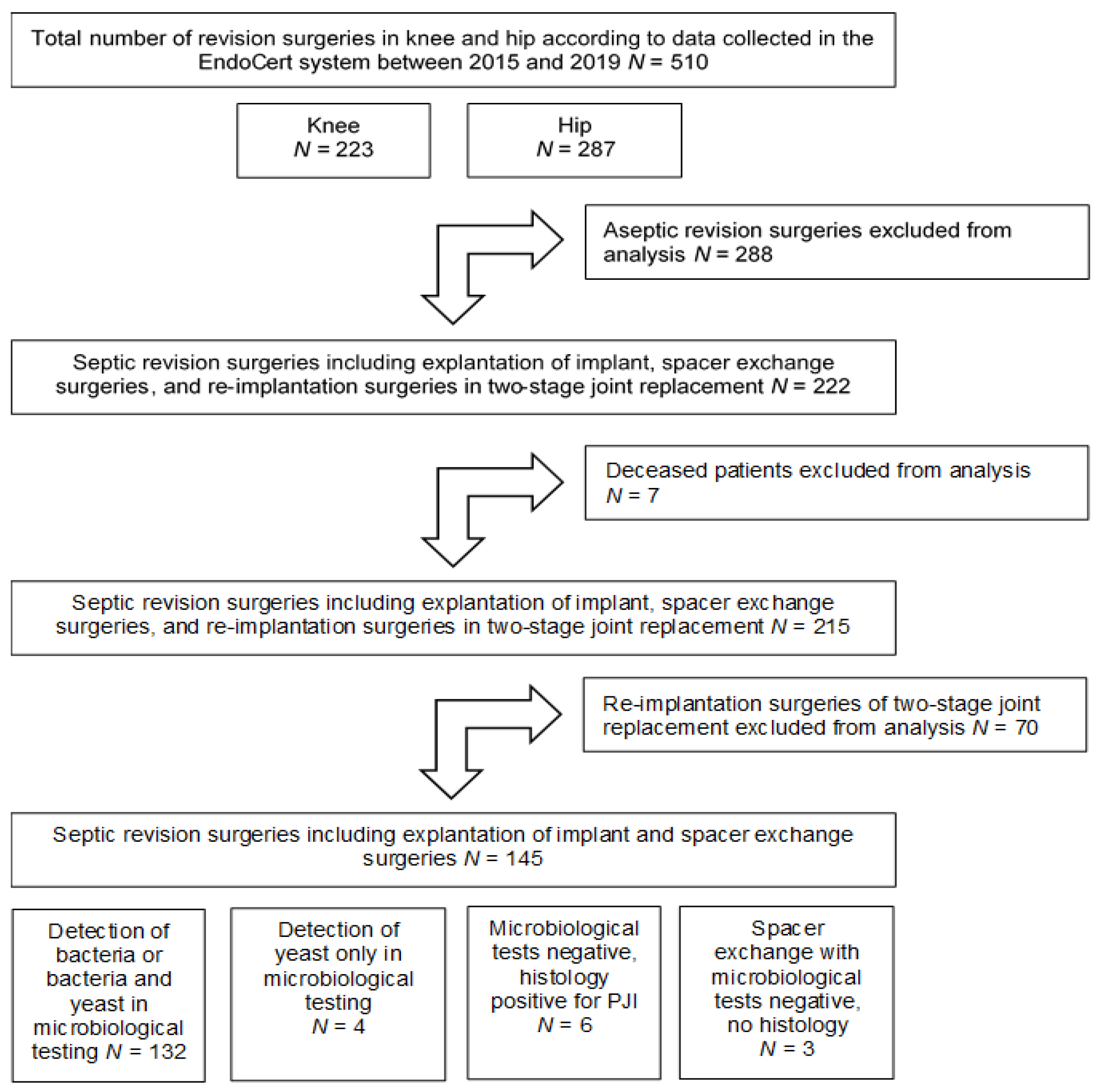
2.1. Patients
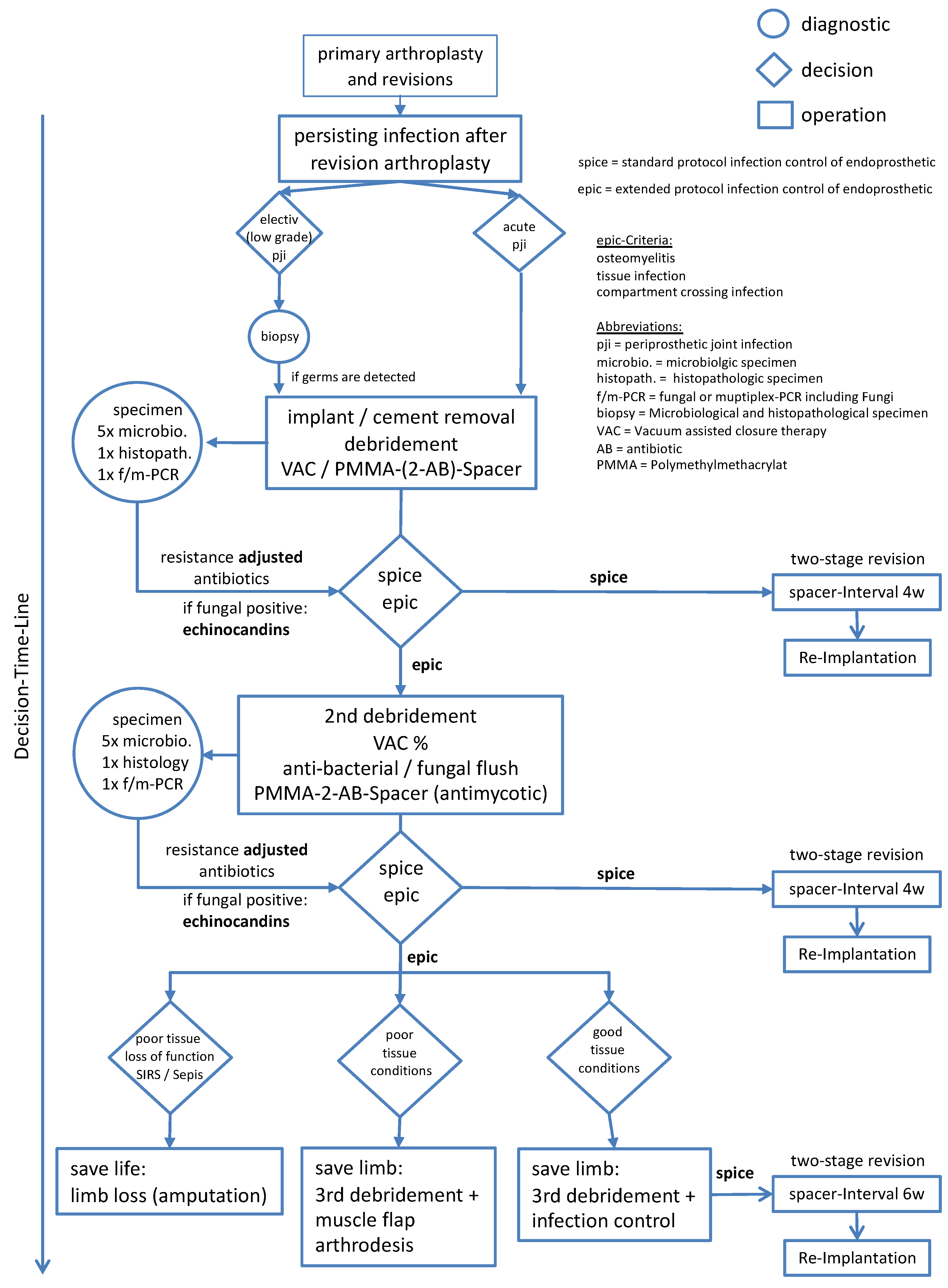
2.2. Treatment
2.3. Microbiological Testings
2.4. Statistics
3. Results
3.1. Patient Data
3.2. Bacterial and Fungal Spectrum of the PJIs
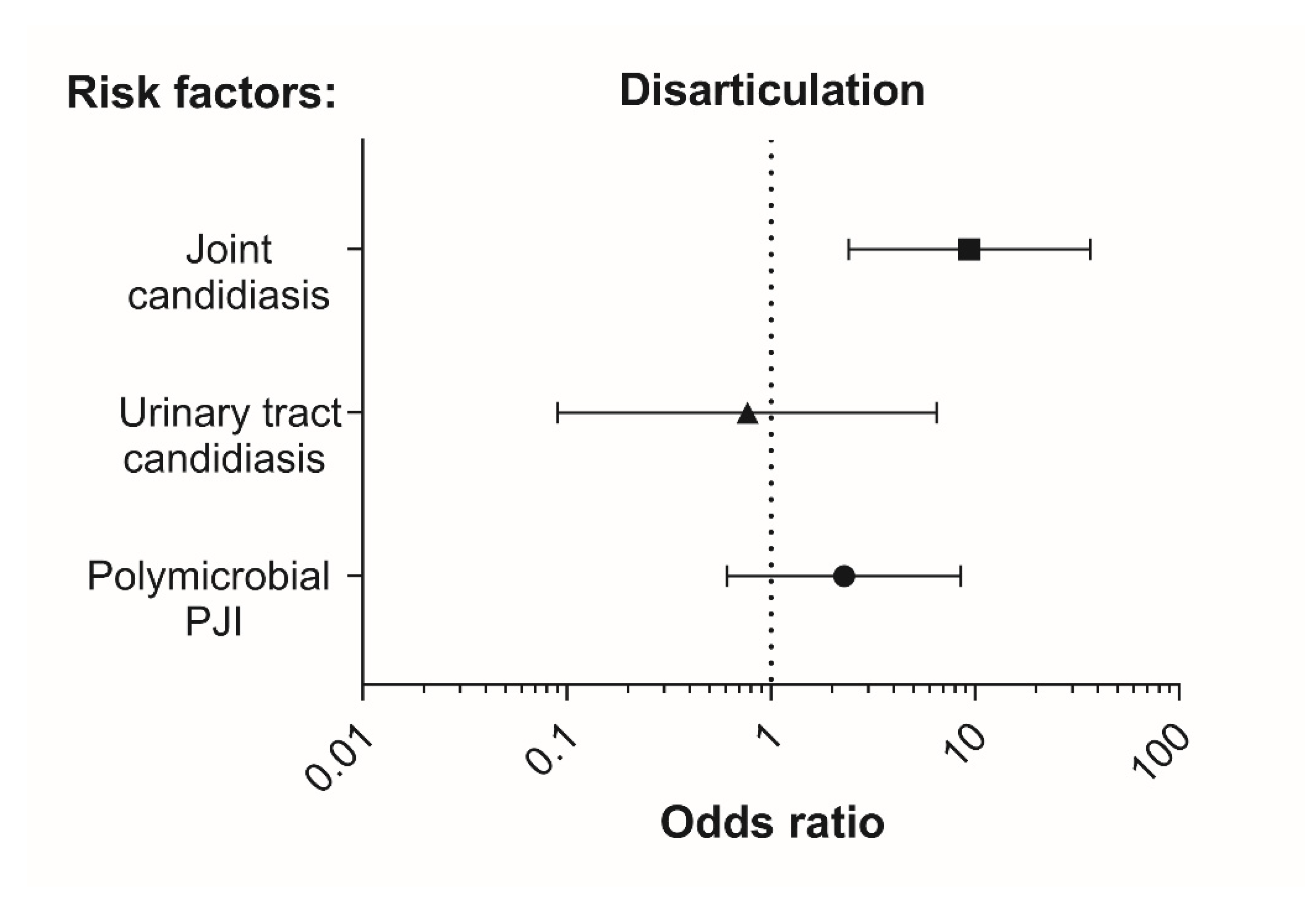
3.3. Association of Candidiasis of the Joint during PJI with Selected Patient Factors
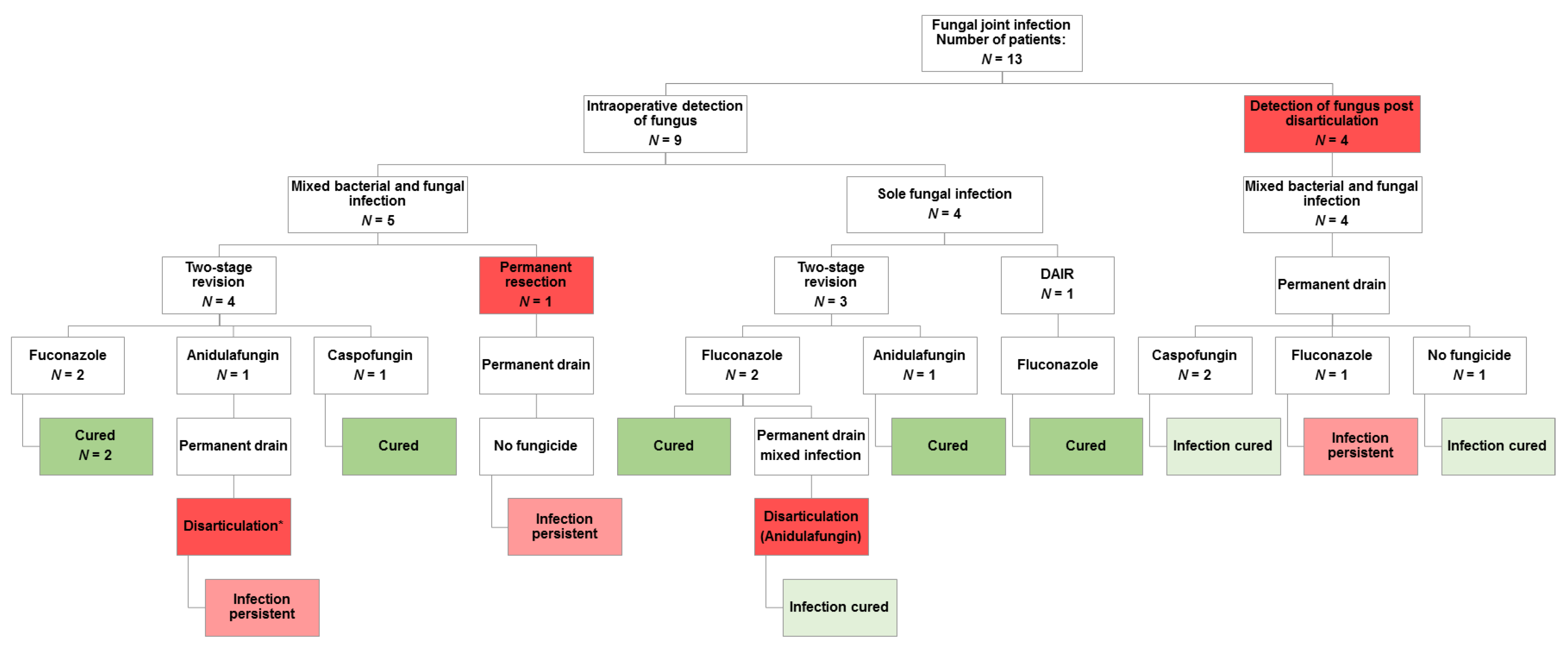
3.4. Fungal Infection and Disarticulation
3.5. Clinical Outcome after Fungal Infection
4. Discussion
4.1. Role and Occurrence of Fungal Infections in PJI
4.2. Biofilm Matrix Formation and Bacterial Symbiosis
4.3. Antifungal Therapy and Choice of Medication
4.4. Surgical Treatment Algorithm and Limits of Therapy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellenrieder, M.; Lenz, R.; Haenle, M.; Bader, R.; Mittelmeier, W. Two-Stage Revision of Implant-Associated Infections after Total Hip and Knee Arthroplasty. GMS Krankenhhyg Interdiszip 2011, 6, Doc17. [Google Scholar] [CrossRef] [PubMed]
- Natsuhara, K.M.; Shelton, T.J.; Meehan, J.P.; Lum, Z.C. Mortality During Total Hip Periprosthetic Joint Infection. J. Arthroplast. 2018. [Google Scholar] [CrossRef] [PubMed]
- Heller, K.-D. Diagnosis of Periprosthetic Infection—What’s Obligation, What’s Optional? Z. Orthop. Unfallchir. 2016, 154, 398–405. [Google Scholar] [CrossRef]
- Ghazavi, M.; Mortazavi, J.; Patzakis, M.; Sheehan, E.; Tan, T.L.; Yazdi, H. Hip and Knee Section, Treatment, Salvage: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplasty 2019, 34, S459–S462. [Google Scholar] [CrossRef]
- Moura, D.L.; Garruço, A. Hip Disarticulation—Case Series Analysis and Literature Review. Rev. Bras. Ortop. 2017, 52, 154–158. [Google Scholar] [CrossRef]
- Preobrazhensky, P.M.; Bozhkova, S.A.; Kazemirsky, A.V.; Tikhilov, R.M.; Kulaba, T.A.; Kornilov, N.N. Functional Outcome of Two-Stage Reimplantation in Patients with Periprosthetic Joint Infection after Primary Total Knee Arthroplasty. Int. Orthop. 2019. [Google Scholar] [CrossRef]
- Kim, J.-K.; Lee, D.-Y.; Kang, D.-W.; Ro, D.-H.; Lee, M.C.; Han, H.-S. Efficacy of Antifungal-Impregnated Cement Spacer against Chronic Fungal Periprosthetic Joint Infections after Total Knee Arthroplasty. Knee 2018, 25, 631–637. [Google Scholar] [CrossRef]
- Xu, S.; Feliu, M.; Lord, A.K.; Lukason, D.P.; Negoro, P.E.; Khan, N.S.; Dagher, Z.; Feldman, M.B.; Reedy, J.L.; Steiger, S.N.; et al. Biguanides Enhance Antifungal Activity against Candida Glabrata. Virulence 2018, 9, 1150–1162. [Google Scholar] [CrossRef]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S. Candida Species: Current Epidemiology, Pathogenicity, Biofilm Formation, Natural Antifungal Products and New Therapeutic Options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef]
- De Luca, C.; Guglielminetti, M.; Ferrario, A.; Calabr, M.; Casari, E. Candidemia: Species Involved, Virulence Factors and Antimycotic Susceptibility. New Microbiol. 2012, 35, 459–468. [Google Scholar]
- Fagotti, L.; Tatka, J.; Salles, M.J.C.; Queiroz, M.C. Risk Factors and Treatment Options for Failure of a Two-Stage Exchange. Curr. Rev. Musculoskelet. Med. 2018, 11, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.S.; Petis, S.M.; Osmon, D.R.; Mabry, T.M.; Berry, D.J.; Hanssen, A.D.; Abdel, M.P. Periprosthetic Joint Infection with Fungal Pathogens. J. Arthroplast. 2018, 33, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Kim, H.J.; Lee, E.J.; Sohn, J.W.; Kim, M.J.; Yoon, Y.K. Prosthetic Joint Infections Caused by Candida Species: A Systematic Review and a Case Series. Mycopathologia 2018. [Google Scholar] [CrossRef]
- Cobo, F.; Rodríguez-Granger, J.; Sampedro, A.; Aliaga-Martínez, L.; Navarro-Marí, J.M. Candida Prosthetic Joint Infection. A Review of Treatment Methods. J. Bone Jt. Infect. 2017, 2, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Groll, A.H.; Buchheidt, D.; Heinz, W.; Bellmann, R.; Cornely, O.; Höhl, R.; Hönigl, M.; Kluge, S.; Kurzai, O.; Lass-Flörl, C.; et al. S1 Leitlinie Diagnose und Therapie von Candida Infektionen: Gemeinsame Empfehlungen der Deutschsprachigen Mykologischen Gesellschaft (DMykG) und der Paul-Ehrlich-Gesellschaft für Chemotherapie (PEG) ICD 10: B37; Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V: Frankfurt, Germany, 2020. [Google Scholar]
- Podbielski, A.; Abele-Horn, M.; Herrmann, M.; Kniehl, E.; Mauch, H.; Rüssmann, H. MIQ 19: Mikrobiologische Diagnostik Der Arthritis Und Osteomyelitis; Urban & Fischer Verlag/Elsevier: Munich, Germany, 2014; Volume II. [Google Scholar]
- Becker, K.; Berner, R.; Eckmann, C.; Eiff, C.; Hartinger, A.; Kempf, V.A.J.; Kühn, J.; Podbielski, A.; Sunderkötter, C.H.; Vogel, U. MIQ 18: Mikrobiologische Diagnostik Der Arthritis Und Osteomyelitis—Ernster, 2nd ed.; Urban & Fischer Verlag/Elsevier: Munich, Germany, 2014. [Google Scholar]
- Azzam, K.; Parvizi, J.; Jungkind, D.; Hanssen, A.; Fehring, T.; Springer, B.; Bozic, K.; Della Valle, C.; Pulido, L.; Barrack, R. Microbiological, Clinical, and Surgical Features of Fungal Prosthetic Joint Infections: A Multi-Institutional Experience. J. Bone Jt. Surg. 2009, 91, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.H.; Yoon, J.Y.; Nam, C.H.; Jung, K.A.; Lee, S.C.; Han, C.D.; Moon, S.H. Fungal Peri-Prosthetic Joint Infection after Primary Total Knee Replacement. J. Bone Jt. Surg. 2012, 94, 656–659. [Google Scholar] [CrossRef]
- Benito, N.; Franco, M.; Ribera, A.; Soriano, A.; Rodriguez-Pardo, D.; Sorlí, L.; Fresco, G.; Fernández-Sampedro, M.; Dolores Del Toro, M.; Guío, L.; et al. Time Trends in the Aetiology of Prosthetic Joint Infections: A Multicentre Cohort Study. Clin. Microbiol. Infect. 2016, 22, 732.e1–732.e8. [Google Scholar] [CrossRef]
- Faschingbauer, M.; Bieger, R.; Kappe, T.; Weiner, C.; Freitag, T.; Reichel, H. Difficult to Treat: Are There Organism-Dependent Differences and Overall Risk Factors in Success Rates for Two-Stage Knee Revision? Arch. Orthop. Trauma Surg. 2020, 140, 1595–1602. [Google Scholar] [CrossRef]
- Fernandes, A. The Microbiological Profiles of Infected Prosthetic Implants with an Emphasis on the Organisms Which Form Biofilms. JCDR 2013. [Google Scholar] [CrossRef] [PubMed]
- Bori, G.; Soriano, A.; García, S.; Gallart, X.; Mallofre, C.; Mensa, J. Neutrophils in Frozen Section and Type of Microorganism Isolated at the Time of Resection Arthroplasty for the Treatment of Infection. Arch. Orthop. Trauma Surg. 2009, 129, 591–595. [Google Scholar] [CrossRef]
- Garvin, K.L.; Konigsberg, B.S. Infection Following Total Knee Arthroplasty: Prevention and Management. Instr. Course Lect. 2012, 61, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Enz, A.; Becker, J.; Warnke, P.; Prall, F.; Lutter, C.; Mittelmeier, W.; Klinder, A. Increased Diagnostic Certainty of Periprosthetic Joint Infections by Combining Microbiological Results with Histopathological Samples Gained via a Minimally Invasive Punching Technique. J. Clin. Med. 2020, 9, 3364. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-D.; Wang, Y.-P.; Chen, C.-F.; Chen, H.-P. The Incidence Rate, Trend and Microbiological Aetiology of Prosthetic Joint Infection after Total Knee Arthroplasty: A 13 Years’ Experience from a Tertiary Medical Center in Taiwan. J. Microbiol. Immunol. Infect. 2018, 51, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Dutronc, H.; Dauchy, F.A.; Cazanave, C.; Rougie, C.; Lafarie-Castet, S.; Couprie, B.; Fabre, T.; Dupon, M. Candida Prosthetic Infections: Case Series and Literature Review. Scand. J. Infect. Dis. 2010, 42, 890–895. [Google Scholar] [CrossRef]
- Kuiper, J.W.; van den Bekerom, M.P.; van der Stappen, J.; Nolte, P.A.; Colen, S. 2-Stage Revision Recommended for Treatment of Fungal Hip and Knee Prosthetic Joint Infections: An Analysis of 164 Patients, 156 from the Literature and 8 Own Cases. Acta Orthop. 2013, 84, 517–523. [Google Scholar] [CrossRef]
- Fortún, J.; Meije, Y.; Buitrago, M.J.; Gago, S.; Bernal-Martinez, L.; Pemán, J.; Pérez, M.; Gómez-G Pedrosa, E.; Madrid, N.; Pintado, V.; et al. Clinical Validation of a Multiplex Real-Time PCR Assay for Detection of Invasive Candidiasis in Intensive Care Unit Patients. J. Antimicrob. Chemother. 2014, 69, 3134–3141. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Castanheira, M. Nosocomial Candidiasis: Antifungal Stewardship and the Importance of Rapid Diagnosis. Med. Myco. 2015, 54, 1–22. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. Non-Culture Diagnostics for Invasive Candidiasis: Promise and Unintended Consequences. J. Fungi 2018, 4, 27. [Google Scholar] [CrossRef]
- Van Merode, A.E.J.; Pothoven, D.C.; van der Mei, H.C.; Busscher, H.J.; Krom, B.P. Surface Charge Influences Enterococcal Prevalence in Mixed-Species Biofilms. J. Appl. Microbiol. 2007, 102, 1254–1260. [Google Scholar] [CrossRef]
- Shirtliff, M.E.; Peters, B.M.; Jabra-Rizk, M.A. Cross-Kingdom Interactions: Candida Albicans and Bacteria. FEMS Microbiol. Lett. 2009, 299, 1–8. [Google Scholar] [CrossRef]
- Morales, D.K.; Hogan, D.A. Candida Albicans Interactions with Bacteria in the Context of Human Health and Disease. PLoS Pathog. 2010, 6, e1000886. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.M.; Ovchinnikova, E.S.; Krom, B.P.; Schlecht, L.M.; Zhou, H.; Hoyer, L.L.; Busscher, H.J.; van der Mei, H.C.; Jabra-Rizk, M.A.; Shirtliff, M.E. Staphylococcus Aureus Adherence to Candida Albicans Hyphae Is Mediated by the Hyphal Adhesin Als3p. Microbiology 2012, 158, 2975–2986. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.P.; Cowley, E.S.; Nobile, C.J.; Hartooni, N.; Newman, D.K.; Johnson, A.D. Anaerobic Bacteria Grow within Candida Albicans Biofilms and Induce Biofilm Formation in Suspension Cultures. Curr. Biol. 2014, 24, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.F.; Tsui, C.; Kucharíková, S.; Andes, D.; Van Dijck, P.; Jabra-Rizk, M.A. Commensal Protection of Staphylococcus Aureus against Antimicrobials by Candida Albicans Biofilm Matrix. mBio 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Andes, D.R.; Krysan, D.J. Candida-Streptococcal Interactions in Biofilm-Associated Oral Diseases. PLoS Pathog. 2018, 14, e1007342. [Google Scholar] [CrossRef]
- De Brucker, K.; Tan, Y.; Vints, K.; De Cremer, K.; Braem, A.; Verstraeten, N.; Michiels, J.; Vleugels, J.; Cammue, B.P.A.; Thevissen, K. Fungal β-1,3-Glucan Increases Ofloxacin Tolerance of Escherichia Coli in a Polymicrobial E. Coli/Candida Albicans Biofilm. Antimicrob. Agents Chemother. 2015, 59, 3052–3058. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Kuhn, D.M.; George, T.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Antifungal Susceptibility of Candida Biofilms: Unique Efficacy of Amphotericin B Lipid Formulations and Echinocandins. Antimicrob. Agents Chemother. 2002, 46, 1773–1780. [Google Scholar] [CrossRef]
- Persyn, A.; Rogiers, O.; Brock, M.; Vande Velde, G.; Lamkanfi, M.; Jacobsen, I.D.; Himmelreich, U.; Lagrou, K.; Van Dijck, P.; Kucharíková, S. Monitoring of Fluconazole and Caspofungin Activity against In Vivo Candida Glabrata Biofilms by Bioluminescence Imaging. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Chandra, J.; Kuhn, D.M.; Ghannoum, M.A. Mechanism of Fluconazole Resistance in Candida Albicans Biofilms: Phase-Specific Role of Efflux Pumps and Membrane Sterols. Infect. Immun. 2003, 71, 4333–4340. [Google Scholar] [CrossRef]
- Enz, A.; Mueller, S.; Mittelmeier, W.; Klinder, A. Severe polymicrobial and fungal periprosthetic osteomyelitis persisting after hip disarticulations treated with caspofungin in risk patients. In Review.
- Rimke, C.; Enz, A.; Bail, H.J.; Heppt, P.; Kladny, B.; von Lewinski, G.; Lohmann, C.H.; Osmanski-Zenk, K.; Haas, H.; Mittelmeier, W. Evaluation of the Standard Procedure for the Treatment of Periprosthetic Joint Infections (PJI) in Germany—Results of a Survey within the EndoCert Initiative. BMC Musculoskelet. Disord. 2020, 21, 694. [Google Scholar] [CrossRef] [PubMed]
- Jakobs, O.; Schoof, B.; Klatte, T.O.; Schmidl, S.; Fensky, F.; Guenther, D.; Frommelt, L.; Gehrke, T.; Gebauer, M. Fungal Periprosthetic Joint Infection in Total Knee Arthroplasty: A Systematic Review. Orthop. Rev. 2015, 7, 5623. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Wissel, M.C.; Shields, R.K.; Salomoni, M.A.; Hao, B.; Press, E.G.; Shields, R.M.; Cheng, S.; Mitsani, D.; Vadnerkar, A.; et al. Performance of Candida Real-Time Polymerase Chain Reaction, β-D-Glucan Assay, and Blood Cultures in the Diagnosis of Invasive Candidiasis. Clin. Infect. Dis. 2012, 54, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- McKeating, C.; White, P.L.; Posso, R.; Palmer, M.; Johnson, E.; McMullan, R. Diagnostic Accuracy of Fungal PCR and β-d-Glucan for Detection of Candidaemia: A Preliminary Evaluation. J. Clin. Pathol. 2018, 71, 420–424. [Google Scholar] [CrossRef] [PubMed]
| Pathogen | Bacterial Spectrum | |
|---|---|---|
| Total Number | Percentage (%) | |
| Staphylococcus epidermidis | 56 | 25,81 |
| Staphylococcus aureus MSSA+ | 23 | 10.60 |
| Enterococcus faecalis | 22 | 10.14 |
| Cutibacterium acnes | 15 | 6.91 |
| Staphylococcus aureus MRSA* | 11 | 5.07 |
| Escherichia coli | 11 | 5.07 |
| Staphylococcus capitis | 9 | 4.15 |
| Staphylococcus hominis | 9 | 4.15 |
| Staphylococcus haemolyticu | 8 | 3.69 |
| Streptococcus agalactiae | 6 | 2.76 |
| Streptococcus dysgalactiae | 6 | 2.76 |
| Proteus mirabilis | 6 | 2.76 |
| Klebsiella pneumoniae | 4 | 1.84 |
| Pseudomonas aeruginosa | 4 | 1.84 |
| Staphylococcus lugdunensis | 3 | 1.38 |
| Enterobacter cloacae | 3 | 1.38 |
| Streptococcus mitis | 3 | 1.38 |
| Staphylococcus caprae | 2 | 0.92 |
| Enterococcus faecium | 2 | 0.92 |
| Corynebacterium sp. | 2 | 0.92 |
| Corynebacterium tuberulostearicum | 2 | 0.92 |
| Micrococcus luteus | 2 | 0.92 |
| Streptococcus anginosus | 1 | 0.46 |
| Corynebacterium striatum | 1 | 0.46 |
| Listeria monocytogenes | 1 | 0.46 |
| Cutibacterium avidum | 1 | 0.46 |
| Parvimonas micra | 1 | 0.46 |
| Finegoldia magna | 1 | 0.46 |
| Klebsiella oxytoca | 1 | 0.46 |
| Morganella morganii | 1 | 0.46 |
| Candida species | Joint | 1. Bacterial Pathogen | 2. Bacterial Pathogen | 3. Bacterial Pathogen | 4. Bacterial Pathogen | 5. Bacterial Pathogen | Duration of Hospital Stay | Disarticulation | Age |
|---|---|---|---|---|---|---|---|---|---|
| C. parapsilosis | hip | none | 19 | no | 81 | ||||
| C. albicans | hip | none | 19 | no | 79 | ||||
| C. glabrata | knee | none | 13 | no | 83 | ||||
| C. albicans | knee | none | 46 | no | 67 | ||||
| C. albicans | hip | S. epidermidis | 20 | yes | 76 | ||||
| C. albicans | hip | S. epidermidis | 175 * | no | 80 | ||||
| C. albicans | knee | S. epidermidis | 175 * | no | 80 | ||||
| C. albicans | hip | MRSA | 28 | no | 66 | ||||
| C. albicans | hip | E. coli | 74 | no | 59 | ||||
| C. albicans | hip | K. pneumoniae | 59 | yes | 81 | ||||
| C. albicans | hip | S. epidermidis | E. faecalis | 175 * | no | 80 | |||
| C. albicans | hip | P. mirabilis | E. faecalis | 63 | yes | 52 | |||
| C. albicans | knee | MRSA | E. faecalis | 139 | yes | 54 | |||
| C. albicans | hip | S. epidermidis | S. capitis | E. coli | 175 * | no | 80 | ||
| C. albicans | hip | S. hominis | S. capitis | P. aeruginosa | 33 | no | 43 | ||
| C. albicans | hip | S. epidermidis | E. faecalis | MRSA | S. agalactiae | 58 | yes | 77 | |
| C. albicans | hip | S. epidermidis | E. faecalis | E. coli | S. haemolyticus | 39 | no | 63 | |
| C. albicans | hip | S. epidermidis | E. faecalis | E. coli | S. aureus | M. morganii | 37 | no | 63 |
| Factor | PJI (Surgical Procedures) | p-Value | ||
|---|---|---|---|---|
| w/o Candida n = 127 | with Candida n = 18 | |||
| Joint | Hip | 66 (52.0%) | 14 (77.8%) | 0.045 # |
| Knee | 61 (48.0%) | 4 (22.2%) | ||
| Age(Years) | mean ± SD | 70.2 ± 9.4 | 70.2 ± 12.2 | 0.473 § |
| median; Min–Max | 72.0; 43–88 | 76.5; 43–83 | ||
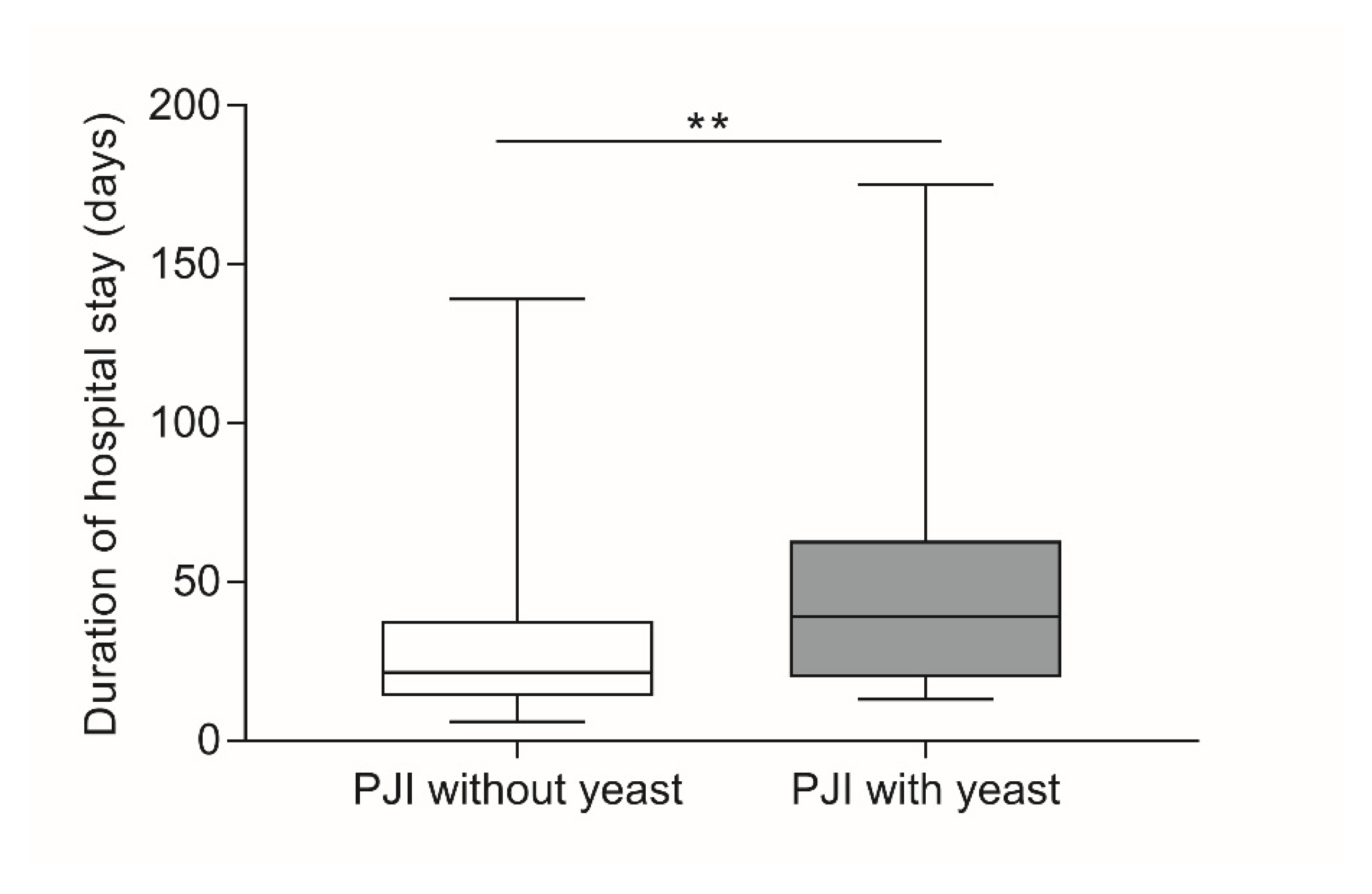
| Duration of hospital stay (days) | mean ± SD | 27.9 ± 19.7 | 54.8 ± 45.8 | 0.003 § |
| median; Min–Max | 21.5; 6–139 | 39; 13–175 | ||
| Previous revisions | Yes | 54 (42.5%) | 15 (83.3%) | 0.002 # |
| Polymicrobial infection (≥2 microbial species including fungi) | Yes | 42 (35.6%) | 14 (77.8%) | 0.001 # |
| Disarticulation | Yes | 5 (3.9%) | 5 (27.8%) | 0.003 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enz, A.; Mueller, S.C.; Warnke, P.; Ellenrieder, M.; Mittelmeier, W.; Klinder, A. Periprosthetic Fungal Infections in Severe Endoprosthetic Infections of the Hip and Knee Joint—A Retrospective Analysis of a Certified Arthroplasty Centre of Excellence. J. Fungi 2021, 7, 404. https://doi.org/10.3390/jof7060404
Enz A, Mueller SC, Warnke P, Ellenrieder M, Mittelmeier W, Klinder A. Periprosthetic Fungal Infections in Severe Endoprosthetic Infections of the Hip and Knee Joint—A Retrospective Analysis of a Certified Arthroplasty Centre of Excellence. Journal of Fungi. 2021; 7(6):404. https://doi.org/10.3390/jof7060404
Chicago/Turabian StyleEnz, Andreas, Silke C. Mueller, Philipp Warnke, Martin Ellenrieder, Wolfram Mittelmeier, and Annett Klinder. 2021. "Periprosthetic Fungal Infections in Severe Endoprosthetic Infections of the Hip and Knee Joint—A Retrospective Analysis of a Certified Arthroplasty Centre of Excellence" Journal of Fungi 7, no. 6: 404. https://doi.org/10.3390/jof7060404
APA StyleEnz, A., Mueller, S. C., Warnke, P., Ellenrieder, M., Mittelmeier, W., & Klinder, A. (2021). Periprosthetic Fungal Infections in Severe Endoprosthetic Infections of the Hip and Knee Joint—A Retrospective Analysis of a Certified Arthroplasty Centre of Excellence. Journal of Fungi, 7(6), 404. https://doi.org/10.3390/jof7060404






