Empirical Support for the Pattern of Competitive Exclusion between Insect Parasitic Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Growth Assays
2.2. Insect Bioassays
2.3. Penetration Test
2.4. Germination and Appressorium Formation Assays
2.5. Liquid Incubation and Biomass Quantification
3. Results
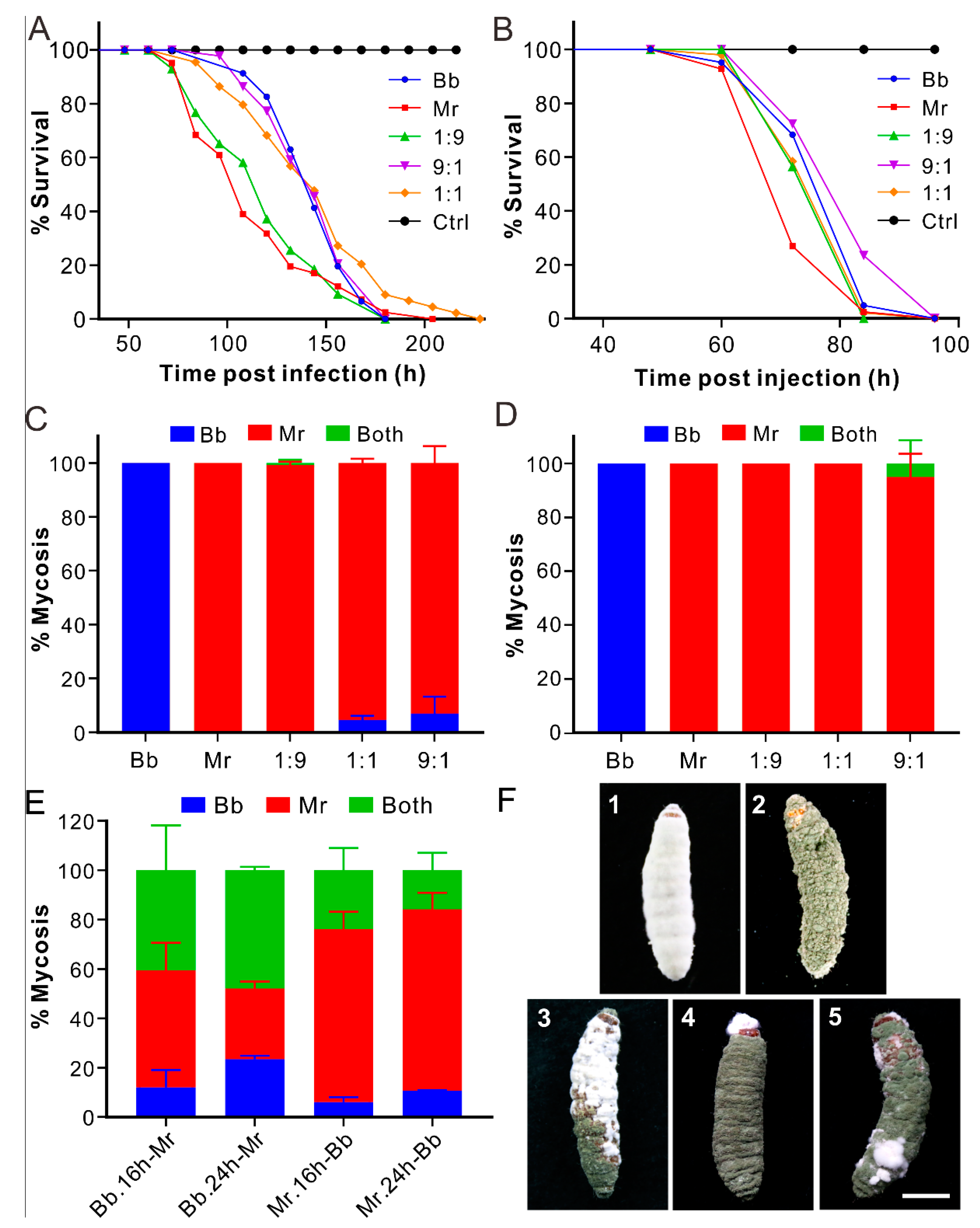
3.1. Non-Synergistic Effect of Coinfection on Fungal Virulence
3.2. Dominant Mycosis of Insect Cadavers by Metarhizium
3.3. Biased Mycosis of Insect Cadavers after Sequential Infections
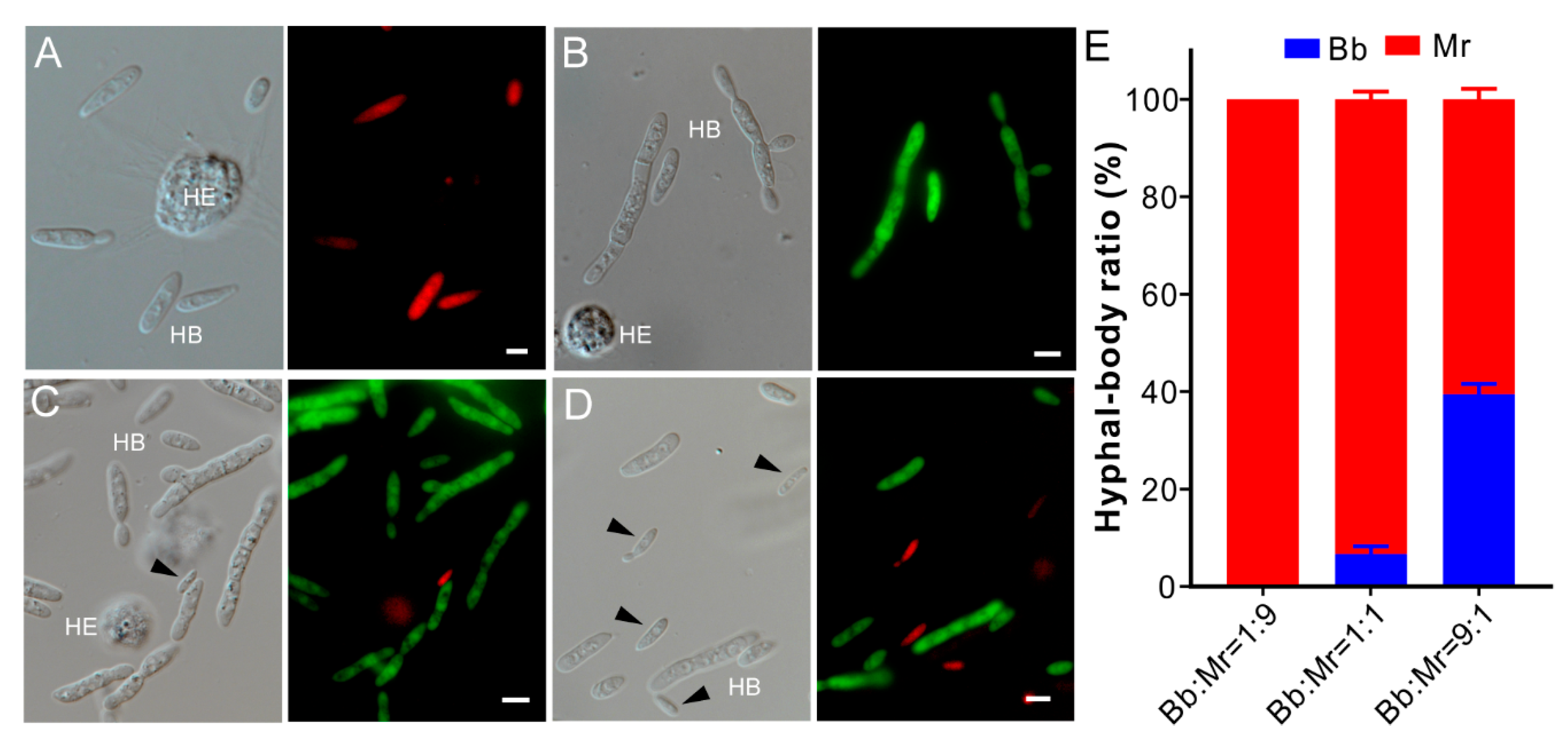
3.4. Dual Inhibition of Spore Germination or Appressorium Formation
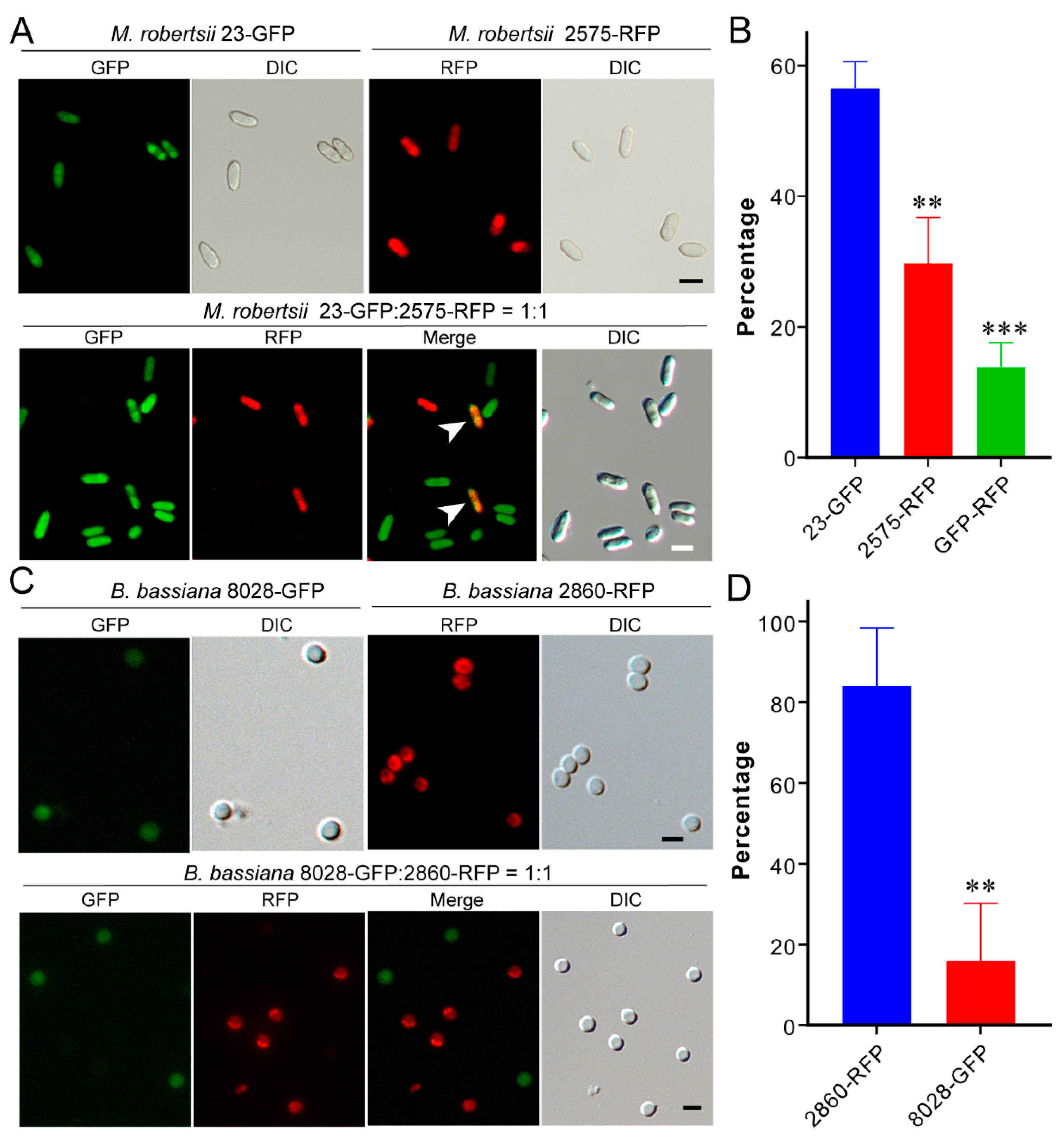
3.5. One-Sided Dominance of M. robertsii within Insect Hemocoels
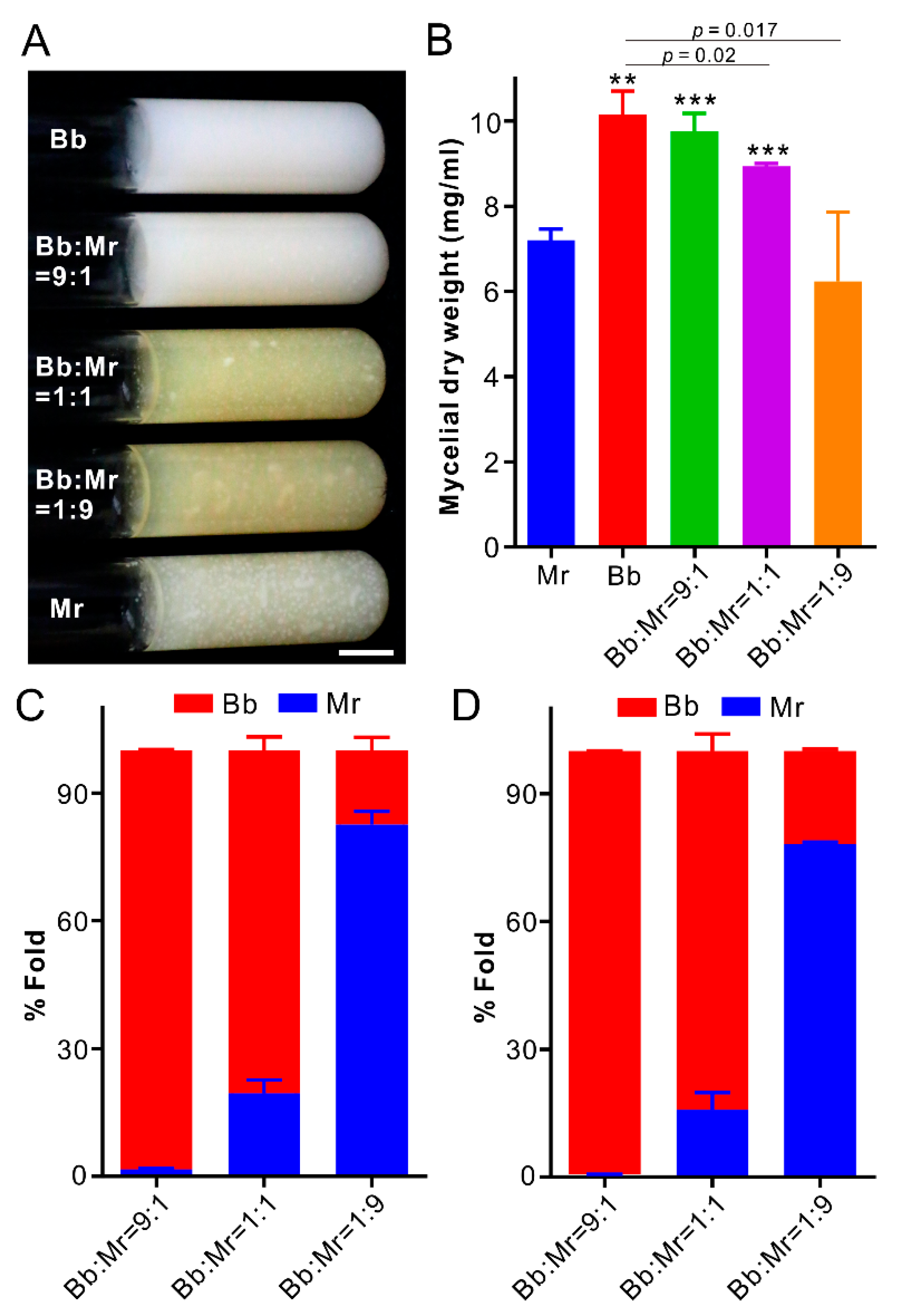
3.6. Dominance of B. bassiana during Co-Culturing in Artificial Medium
3.7. Biased Mycosis of Insects after Infection with Different Strains
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stork, N.E. How many species of insects and other terrestrial arthropods are there on earth? Annu. Rev. Entomol. 2018, 63, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; Tittensor, D.P.; Adl, S.; Simpson, A.G.; Worm, B. How many species are there on Earth and in the ocean? PLoS Biol. 2011, 9, e1001127. [Google Scholar] [CrossRef] [PubMed]
- Lovett, B.; St Leger, R.J. The insect pathogens. Microbiol. Spectr. 2017, 5, FUNK-0001-2016. [Google Scholar]
- St Leger, R.J.; Wang, J.B. Metarhizium: Jack of all trades, master of many. Open Biol. 2020, 10, 200307. [Google Scholar] [CrossRef] [PubMed]
- Steinwender, B.M.; Enkerli, J.; Widmer, F.; Eilenberg, J.; Kristensen, H.L.; Bidochka, M.J.; Meyling, N.V. Root isolations of Metarhizium spp. from crops reflect diversity in the soil and indicate no plant specificity. J. Invertebr. Pathol. 2015, 132, 142–148. [Google Scholar] [CrossRef]
- Meyling, N.V.; Eilenberg, J. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: Potential for conservation biological control. Biol. Control 2007, 43, 145–155. [Google Scholar] [CrossRef]
- Mei, L.J.; Chen, M.; Shang, Y.; Tang, G.; Tao, Y.; Zeng, L.; Huang, B.; Li, Z.; Zhan, S.; Wang, C.S. Population genomics and evolution of a fungal pathogen after releasing exotic strains to control insect pests for 20 years. ISME J. 2020, 14, 1422–1434. [Google Scholar] [CrossRef]
- Hajek, A.E.; St Leger, R.J. Interactions between Fungal Pathogens and Insect Hosts. Annu. Rev. Entomol. 1994, 39, 293–322. [Google Scholar] [CrossRef]
- Wang, C.; Shah, F.A.; Patel, N.; Li, Z.Z.; Butt, T.M. Molecular investigation on strain genetic relatedness and population structure of Beauveria bassiana. Environ. Microbiol. 2003, 5, 908–915. [Google Scholar] [CrossRef]
- Clifton, E.H.; Castrillo, L.A.; Gryganskyi, A.; Hajek, A.E. A pair of native fungal pathogens drives decline of a new invasive herbivore. Proc. Natl. Acad. Sci. USA 2019, 116, 9178–9180. [Google Scholar] [CrossRef]
- Bremermann, H.J.; Thieme, H.R. A competitive exclusion principle for pathogen virulence. J. Math. Biol. 1989, 27, 179–190. [Google Scholar] [CrossRef]
- Zheng, X.D.; Deng, L.L.; Qiang, W.Y.; Cressman, R.; Tao, Y. Limiting similarity of competitive species and demographic stochasticity. Phys. Rev. E 2017, 95, 042404. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.R.; Steidinger, B.S.; Bruns, T.D.; Peay, K.G. Competition-colonization tradeoffs structure fungal diversity. ISME J. 2018, 12, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Harbison, C.W.; Bush, S.E.; Malenke, J.R.; Clayton, D.H. Comparative transmission dynamics of competing parasite species. Ecology 2008, 89, 3186–3194. [Google Scholar] [CrossRef] [PubMed]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Wang, C.S.; Feng, M.G. Advances in fundamental and applied studies in China of fungal biocontrol agents for use against arthropod pests. Biol. Control 2014, 68, 129–135. [Google Scholar] [CrossRef]
- de Faria, M.R.; Wraight, S.P. Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Uma Maheswara Rao, C.; Uma Devi, K.; Akbar Ali Khan, P. Effect of combination treatment with entomopathogenic fungi Beauveria bassiana and Nomuraea rileyi (Hypocreales) on Spodoptera litura (Lepidoptera: Noctuidaeae). Biocontrol Sci. Technol. 2007, 16, 221–232. [Google Scholar] [CrossRef]
- Seid, A.M.; Fredensborg, B.L.; Steinwender, B.M.; Meyling, N.V. Temperature-dependent germination, growth and co-infection of Beauveria spp. isolates from different climatic regions. Biocontrol Sci Techn. 2019, 29, 411–426. [Google Scholar] [CrossRef]
- Inglis, G.D.; Johnson, D.L.; Cheng, K.J.; Goettel, M.S. Use of pathogen combinations to overcome the constraints of temperature on entomopathogenic hyphomycetes against grasshoppers. Biol. Control 1997, 8, 143–152. [Google Scholar] [CrossRef]
- Wang, C.; Fan, M.; Li, Z.Z.; Butt, T.M. Molecular monitoring and evaluation of the application of the insect-pathogenic fungus Beauveria bassiana in southeast China. J. Appl. Microbiol. 2004, 96, 861–870. [Google Scholar] [CrossRef]
- Wang, C.S.; Wang, S.B. Insect pathogenic fungi: Genomics, molecular interactions, and genetic improvements. Annu. Rev. Entomol. 2017, 62, 73–90. [Google Scholar] [CrossRef]
- Valero-Jimenez, C.A.; Faino, L.; Spring In’t Veld, D.; Smit, S.; Zwaan, B.J.; van Kan, J.A. Comparative genomics of Beauveria bassiana: Uncovering signatures of virulence against mosquitoes. BMC Genom. 2016, 17, 986. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xiao, G.; Zheng, P.; Shang, Y.; Su, Y.; Zhang, X.; Liu, X.; Zhan, S.; St Leger, R.J.; Wang, C. Trajectory and genomic determinants of fungal-pathogen speciation and host adaptation. Proc. Natl. Acad. Sci. USA 2014, 111, 16796–16801. [Google Scholar] [CrossRef] [PubMed]
- Cen, K.; Li, B.; Lu, Y.Z.; Zhang, S.W.; Wang, C.S. Divergent LysM effectors contribute to the virulence of Beauveria bassiana by evasion of insect immune defenses. PLoS Pathog. 2017, 13, e1006604. [Google Scholar] [CrossRef]
- Fang, W.; Pei, Y.; Bidochka, M.J. Transformation of Metarhizium anisopliae mediated by Agrobacterium tumefaciens. Can. J. Microbiol. 2006, 52, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Gottar, M.; Gobert, V.; Matskevich, A.; Reichhart, J.; Wang, C.; Butt, T.; BeIvin, M.; Hoffmann, J.; Ferrandon, D. Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell 2006, 127, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.R.; Shang, Y.F.; Li, S.Q.; Wang, C.S. MrHex1 is required for Woronin body formation, fungal development and virulence in Metarhizium robertsii. J. Fungi 2020, 6, 172. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Lu, M.; Ling, E.; Li, P.; Wang, C.S. A M35 family metalloprotease is required for fungal virulence against insects by inactivating host prophenoloxidases and beyond. Virulence 2020, 11, 222–237. [Google Scholar] [CrossRef]
- Shang, J.M.; Shang, Y.F.; Tang, G.R.; Wang, C.S. Identification of a key G-protein coupled receptor in mediating appressorium formation and fungal virulence against insects. Sci. China Life Sci. 2021, 64, 466–477. [Google Scholar]
- Chen, Y.X.; Li, B.; Cen, K.; Lu, Y.Z.; Zhang, S.W.; Wang, C.S. Diverse effect of phosphatidylcholine biosynthetic genes on phospholipid homeostasis, cell autophagy and fungal developments in Metarhizium robertsii. Environ. Microbiol. 2018, 20, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; St Leger, R.J. The Metarhizium anisopliae perilipin homolog MPL1 regulates lipid metabolism, appressorial turgor pressure, and virulence. J. Biol. Chem. 2007, 282, 21110–21115. [Google Scholar] [CrossRef] [PubMed]
- Alizon, S.; de Roode, J.C.; Michalakis, Y. Multiple infections and the evolution of virulence. Ecol. Lett. 2013, 16, 556–567. [Google Scholar] [CrossRef] [PubMed]
- López-Villavicencio, M.; Jonot, O.; Coantic, A.; Hood, M.E.; Enjalbert, J.; Giraud, T. Multiple infections by the anther smut pathogen are frequent and involve related strains. PLoS Pathog. 2007, 3, e176. [Google Scholar] [CrossRef] [PubMed]
- Susi, H.; Barres, B.; Vale, P.F.; Laine, A.L. Co-infection alters population dynamics of infectious disease. Nat. Commun. 2015, 6, 5975. [Google Scholar] [CrossRef] [PubMed]
- Abbate, J.L.; Gladieux, P.; Hood, M.E.; de Vienne, D.M.; Antonovics, J.; Snirc, A.; Giraud, T. Co-occurrence among three divergent plant-castrating fungi in the same Silene host species. Mol. Ecol. 2018, 27, 3357–3370. [Google Scholar] [CrossRef] [PubMed]
- Pauli, G.; Moura Mascarin, G.; Eilenberg, J.; Delalibera Junior, I. Within-host competition between two entomopathogenic fungi and a granulovirus in Diatraea saccharalis (Lepidoptera: Crambidae). Insects 2018, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Z.; Butt, T. Molecular studies of co-formulated strains of the entomopathogenic fungus, Beauveria bassiana. J. Invertebr. Pathol. 2002, 80, 29–34. [Google Scholar] [CrossRef]
- Adler, P.B.; Fajardo, A.; Kleinhesselink, A.R.; Kraft, N.J. Trait-based tests of coexistence mechanisms. Ecol. Lett. 2013, 16, 1294–1306. [Google Scholar] [CrossRef]
- Meyling, N.V.; Lubeck, M.; Buckley, E.P.; Eilenberg, J.; Rehner, S.A. Community composition, host range and genetic structure of the fungal entomopathogen Beauveria in adjoining agricultural and seminatural habitats. Mol. Ecol. 2009, 18, 1282–1293. [Google Scholar] [CrossRef]
- Ramos, Y.; Portal, O.; Lysoe, E.; Meyling, N.V.; Klingen, I. Diversity and abundance of Beauveria bassiana in soils, stink bugs and plant tissues of common bean from organic and conventional fields. J. Invertebr. Pathol. 2017, 150, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.F.; Xiao, G.H.; Zheng, P.; Cen, K.; Zhan, S.; Wang, C.S. Divergent and convergent evolution of fungal pathogenicity. Genome Biol. Evol. 2016, 8, 1374–1387. [Google Scholar] [CrossRef]
- Lee, M.R.; Powell, J.R.; Oberle, B.; Cornwell, W.K.; Lyons, M.; Rigg, J.L.; Zanne, A.E. Good neighbors aplenty: Fungal endophytes rarely exhibit competitive exclusion patterns across a span of woody habitats. Ecology 2019, 100, e02790. [Google Scholar] [CrossRef]
- Kennedy, P. Ectomycorrhizal fungi and interspecific competition: Species interactions, community structure, coexistence mechanisms, and future research directions. New Phytol. 2010, 187, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.E.; Bever, J.D. Trade-offs between arbuscular mycorrhizal fungal competitive ability and host growth promotion in Plantago lanceolata. Oecologia 2009, 160, 807–816. [Google Scholar] [CrossRef]
- Wang, S.; O’Brien, T.R.; Pava-Ripoll, M.; St Leger, R.J. Local adaptation of an introduced transgenic insect fungal pathogen due to new beneficial mutations. Proc. Natl. Acad. Sci. USA 2011, 108, 20449–20454. [Google Scholar] [CrossRef] [PubMed]
- Grainger, T.N.; Letten, A.D.; Gilbert, B.; Fukami, T. Applying modern coexistence theory to priority effects. Proc. Natl. Acad. Sci. USA 2019, 116, 6205–6210. [Google Scholar] [CrossRef]
- Levine, J.M.; HilleRisLambers, J. The importance of niches for the maintenance of species diversity. Nature 2009, 461, 254–257. [Google Scholar] [CrossRef]
- Araujo, J.P.M.; Evans, H.C.; Kepler, R.; Hughes, D.P. Zombie-ant fungi across continents: 15 new species and new combinations within Ophiocordyceps. I. Myrmecophilous hirsutelloid species. Stud. Mycol. 2018, 90, 119–160. [Google Scholar] [CrossRef]
- Thomas, M.B.; Watson, E.L.; Valverde-Garcia, P. Mixed infections and insect-pathogen interactions. Ecol. Lett. 2003, 6, 183–188. [Google Scholar] [CrossRef]
- Ulrich, Y.; Schmid-Hempel, P. Host modulation of parasite competition in multiple infections. Proc. Biol. Sci. 2012, 279, 2982–2989. [Google Scholar] [CrossRef] [PubMed]
- de Roode, J.C.; Culleton, R.; Cheesman, S.J.; Carter, R.; Read, A.F. Host heterogeneity is a determinant of competitive exclusion or coexistence in genetically diverse malaria infections. Proc. Biol. Sci. 2004, 271, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Duwadi, D.; Shrestha, A.; Yilma, B.; Kozlovski, I.; Sa-Eed, M.; Dahal, N.; Jukosky, J. Identification and screening of potent antimicrobial peptides in arthropod genomes. Peptides 2018, 103, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.O.; Boomsma, J.J. Let your enemy do the work: Within-host interactions between two fungal parasites of leaf-cutting ants. Proc. Biol. Sci. 2004, 271, S104–S106. [Google Scholar] [CrossRef]
- Chouvenc, T.; Efstathion, C.A.; Elliott, M.L.; Su, N.-Y. Resource competition between two fungal parasites in subterranean termites. Naturwissenschaften 2012, 99, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Milutinovic, B.; Stock, M.; Grasse, A.V.; Naderlinger, E.; Hilbe, C.; Cremer, S. Social immunity modulates competition between coinfecting pathogens. Ecol. Lett. 2020, 23, 565–574. [Google Scholar] [CrossRef]
- Maistrou, S.; Natsopoulou, M.E.; Jensen, A.B.; Meyling, N.V. Virulence traits within a community of the fungal entomopathogen Beauveria: Associations with abundance and distribution. Fungal Ecol. 2020, 48, 100992. [Google Scholar] [CrossRef]
- Klinger, E.G.; Vojvodic, S.; DeGrandi-Hoffman, G.; Welker, D.L.; James, R.R. Mixed infections reveal virulence differences between host-specific bee pathogens. J. Invertebr. Pathol. 2015, 129, 28–35. [Google Scholar] [CrossRef]
- Behie, S.W.; Zelisko, P.M.; Bidochka, M.J. Endophytic insect-parasitic fungi translocate nitrogen directly from insects to plants. Science 2012, 336, 1576–1577. [Google Scholar] [CrossRef]
- Behie, S.W.; Moreira, C.C.; Sementchoukova, I.; Barelli, L.; Zelisko, P.M.; Bidochka, M.J. Carbon translocation from a plant to an insect-pathogenic endophytic fungus. Nat. Commun. 2017, 8, 14245. [Google Scholar] [CrossRef]
- Mayfield, M.M.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Potts, J.R.; Petrovskii, S.V. Fortune favours the brave: Movement responses shape demographic dynamics in strongly competing populations. J. Theor. Biol. 2017, 420, 190–199. [Google Scholar] [CrossRef]
- Zheng, P.; Xia, Y.; Zhang, S.; Wang, C. Genetics of Cordyceps and related fungi. Appl. Microbiol. Biotechnol. 2013, 97, 2797–2804. [Google Scholar] [CrossRef] [PubMed]
- Saupe, S.J. Molecular genetics of heterokaryon incompatibility in filamentous ascomycetes. Microbiol. Mol. Biol. Rev. 2000, 64, 489–502. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Yi, W.; Chen, S.; Wang, C. Empirical Support for the Pattern of Competitive Exclusion between Insect Parasitic Fungi. J. Fungi 2021, 7, 385. https://doi.org/10.3390/jof7050385
Li S, Yi W, Chen S, Wang C. Empirical Support for the Pattern of Competitive Exclusion between Insect Parasitic Fungi. Journal of Fungi. 2021; 7(5):385. https://doi.org/10.3390/jof7050385
Chicago/Turabian StyleLi, Shiqin, Wenjuan Yi, Siyi Chen, and Chengshu Wang. 2021. "Empirical Support for the Pattern of Competitive Exclusion between Insect Parasitic Fungi" Journal of Fungi 7, no. 5: 385. https://doi.org/10.3390/jof7050385
APA StyleLi, S., Yi, W., Chen, S., & Wang, C. (2021). Empirical Support for the Pattern of Competitive Exclusion between Insect Parasitic Fungi. Journal of Fungi, 7(5), 385. https://doi.org/10.3390/jof7050385






