Arbuscular Mycorrhization Enhances Nitrogen, Phosphorus and Potassium Accumulation in Vicia faba by Modulating Soil Nutrient Balance under Elevated CO2
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Experimental Design and Treatments
2.3. Harvest and Sampling
2.4. Mycorrhizal Colonization
2.5. Determination of Carbon, Nitrogen, Phosphorus, and Potassium in Plants and Soils
2.6. Statistical Analysis
3. Results
3.1. Mycorrhizal Colonization, Nodule Biomass, and Plant Growth Parameters
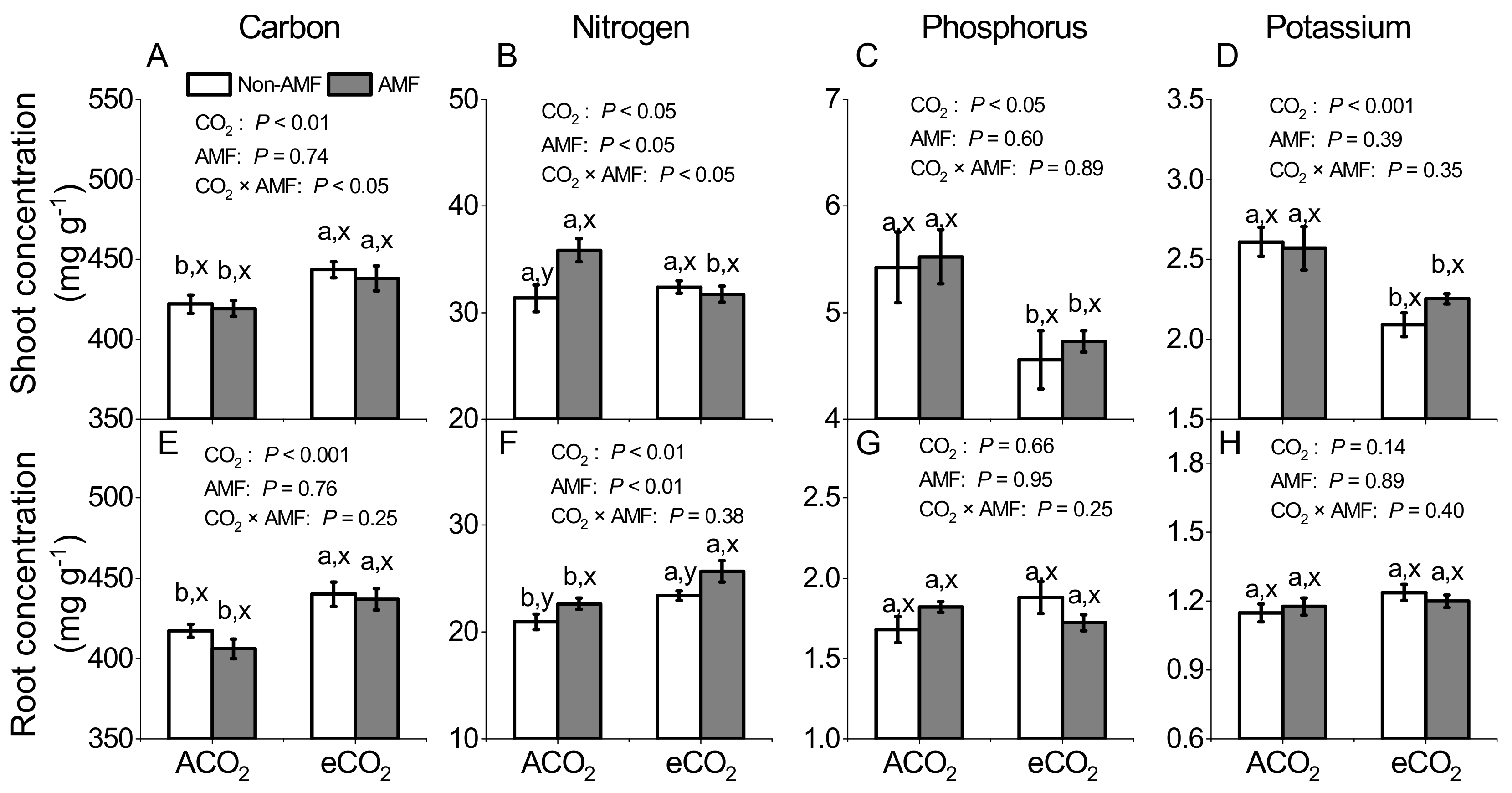
3.2. Effects of AMF and CO2 on Plant Carbon, Nitrogen, Phosphorus and Potassium Concentrations
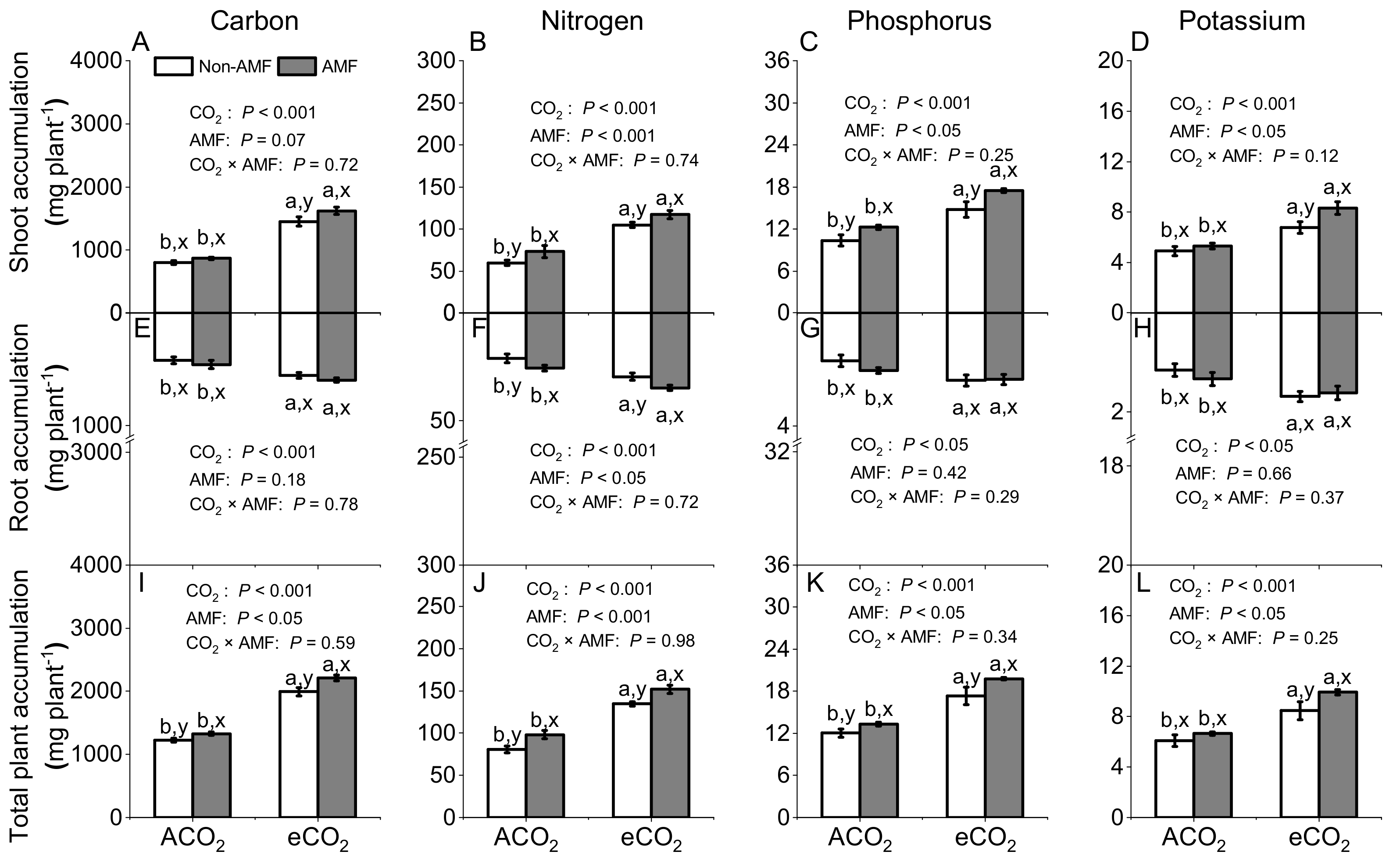
3.3. Effects of AMF and CO2 on Plant Carbon, Nitrogen, Phosphorus, and Potassium Accumulations
3.4. Effects of AMF and CO2 on Nutrient Use Efficiency
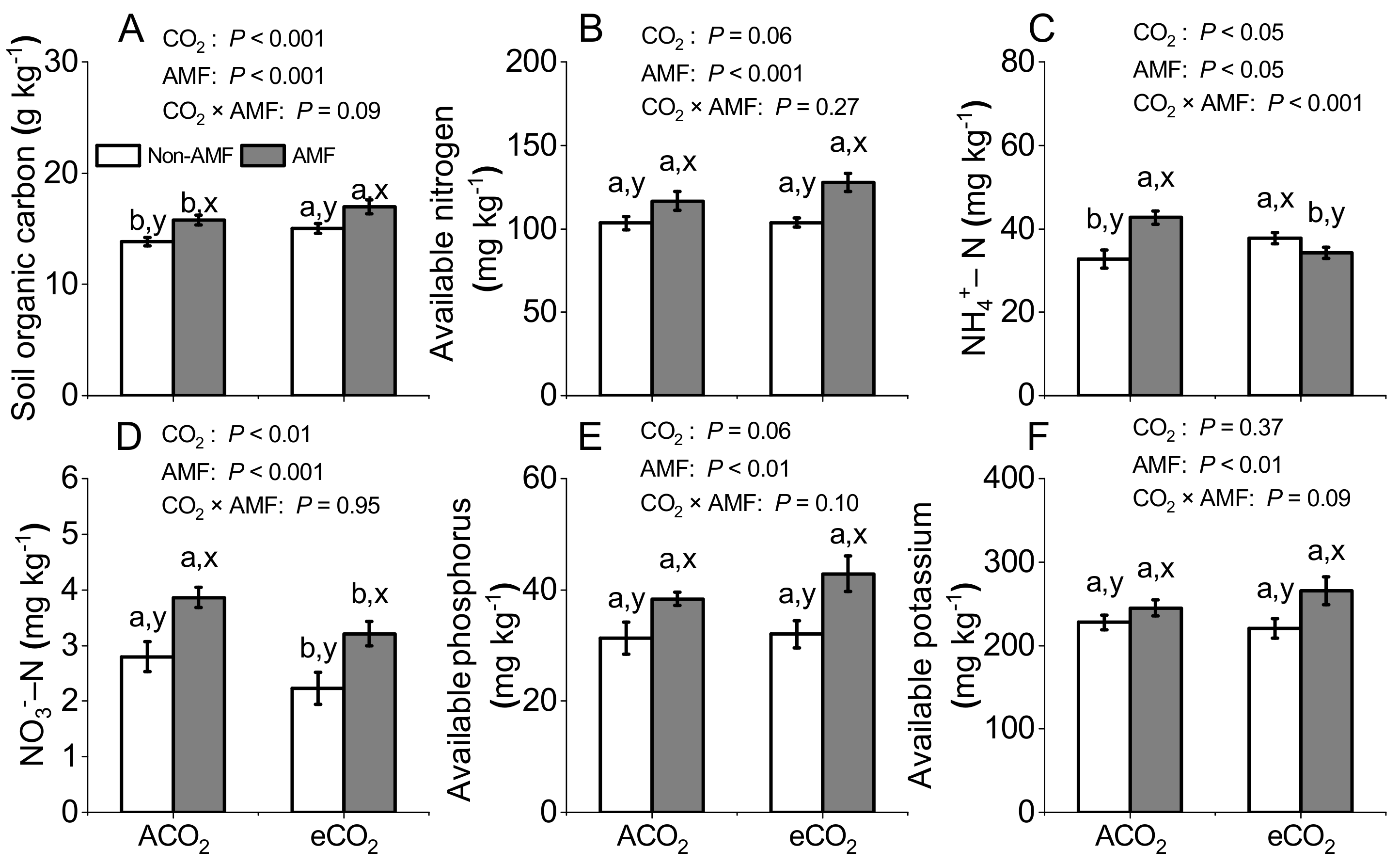
3.5. Effects of AMF and CO2 on Soil Nutrients
3.6. Correlations between Biomass Production and Plant Tissue or Soil Nitrogen, Phosphorus, and Potassiumconcentration
4. Discussion
4.1. Mycorrhizal Colonization and Nodule Biomass Increased under eCO2
4.2. AMF and eCO2 Synergistically Improve Carbon Accumulation and Biomass Production
4.3. AMF and eCO2 Synergistically Improve Nitrogen Accumulation in Plant
4.4. AMF and eCO2 Synergistically Improve Phosphorus and Potassium Accumulation in Plants
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Wang, S.H.; Zhang, Y.G.; Ju, W.M.; Chen, J.M.; Ciais, P.; Cescatti, A.; Sardans, J.; Janssens, I.A.; Wu, M.S.; Berry, J.A.; et al. Recent global decline of CO2 fertilization effects on vegetation photosynthesis. Science 2020, 370, 1295–1300. [Google Scholar] [CrossRef]
- Parvin, S.; Uddin, S.; Tausz-Posch, S.; Armstrong, R.; Tausz, M. Carbon sink strength of nodules but not other organs mod-ulates photosynthesis of faba bean (Vicia faba) grown under elevated [CO2] and different water supply. New Phytol. 2020, 227, 132–145. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, C.; Shao, S.; Li, J.; Xie, H.T.; Zhang, W.; Chen, F.S.; He, H.B.; Zhang, X.D. Interactive effects of elevated CO2 and nitrogen fertilization levels on photosynthesized carbon allocation in a temperate spring wheat and soil system. Pedosphere 2021, 31, 191–203. [Google Scholar] [CrossRef]
- Shi, S.M.; Qiu, Y.L.; Wen, M.; Xu, X.; Dong, X.S.; Xu, C.Y.; He, X.H. Daytime, Not Nighttime, Elevated Atmospheric Carbon Dioxide Exposure Improves Plant Growth and Leaf Quality of Mulberry (Morus alba L.) Seedlings. Front. Plant Sci. 2021, 11, 609031. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, G.J.; Christy, B.; Nuttall, J.; Huth, N.; Cammarano, D.; Stöckle, C.; Basso, B.; Shcherbak, I.; Fitzgerald, G.; Luo, Q.; et al. Response of wheat growth, grain yield and water use to elevated CO2 under a Free-Air CO2 Enrichment (FACE) experiment and modelling in a semi-arid environment. Glob. Chang. Biol. 2015, 21, 2670–2686. [Google Scholar] [CrossRef]
- Yoshida, H.; Horie, T.; Nakazono, K.; Ohno, H.; Nakagawa, H. Simulation of the effects of genotype and N availability on rice growth and yield response to an elevated atmospheric CO2 concentration. Field Crops Res. 2011, 124, 433–440. [Google Scholar] [CrossRef]
- Thomey, M.L.; Slattery, R.A.; Köhler, I.H.; Bernacchi, C.J.; Ort, D.R. Yield response of field-grown soybean exposed to heat waves under current and elevated [CO2]. Glob. Chang. Biol. 2019, 25, 4352–4368. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Lugassi, N.; Egbaria, A.; Granot, D.; Yermiyahu, U. The role of nitrogen in photosynthetic acclimation to elevated [CO2] in tomatoes. Plant Soil 2018, 434, 397–411. [Google Scholar] [CrossRef]
- Aljazairi, S.; Arias, C.; Nogues, S. Carbon and nitrogen allocation and partitioning in traditional and modern wheat genotypes under pre-industrial and future CO2 conditions. Plant Biol. 2014, 17, 647–659. [Google Scholar] [CrossRef]
- Jakobsen, I.; Smith, S.E.; Smith, F.A.; Watts-Williams, S.J.; Clausen, S.S.; Grønlund, M. Plant growth responses to elevated atmospheric CO2 are increased by phosphorus sufficiency but not by arbuscular mycorrhizas. J. Exp. Bot. 2016, 67, 6173–6186. [Google Scholar] [CrossRef][Green Version]
- Dabu, X.; Li, S.; Cai, Z.; Ge, T.; Hai, M. The effect of potassium on photosynthetic acclimation in cucumber during CO2 en-richment. Photosynthetica 2019, 57, 640–645. [Google Scholar] [CrossRef]
- Singh, A.K.; Rai, A.; Kushwaha, M.; Chauhan, P.S.; Pandey, V.; Singh, N. Tree growth rate regulate the influence of elevated CO2 on soil biochemical responses under tropical condition. J. Environ. Manag. 2019, 231, 1211–1221. [Google Scholar] [CrossRef]
- Jin, J.; Armstrong, R.; Tang, C.X. Impact of elevated CO2 on grain nutrient concentration varies with crops and soils–A long-term FACE study. Sci. Total Environ. 2019, 651, 2641–2647. [Google Scholar] [CrossRef]
- Terrer, C.; Jackson, R.B.; Prentice, I.C.; Keenan, T.F.; Kaiser, C.; Vicca, S.; Fisher, J.B.; Reich, P.B.; Stocker, B.D.; Hungate, B.A.; et al. Nitrogen and phosphorus constrain the CO2 fertilization of global plant biomass. Nat. Clim. Chang. 2019, 9, 684–689. [Google Scholar] [CrossRef]
- Du, C.; Wang, X.; Zhang, M.; Jing, J.; Gao, Y. Effects of elevated CO2 on plant C-N-P stoichiometry in terrestrial ecosystems: A meta-analysis. Sci. Total Environ. 2019, 650, 697–708. [Google Scholar] [CrossRef]
- Hao, X.; Li, P.; Han, X.; Norton, R.M.; Lam, S.K.; Zong, Y.; Sun, M.; Lin, E.; Gao, Z. Effects of free-air CO2 enrichment (FACE) on N, P and K uptake of soybean in northern China. Agric. For. Meteorol. 2016, 218–219, 261–266. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef]
- Smith, F.A.; Grace, E.J.; Smith, S.E. More than a carbon economy: Nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytol. 2009, 182, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Habeeb, T.H.; Abdel-Mawgoud, M.; Yehia, R.S.; Khalil, A.M.A.; Saleh, A.M.; AbdElgawad, H. Interactive impact of ar-buscular mycorrhizal fungi and elevated CO2 on growth and functional food value of Thymus vulgare. J. Fungi 2020, 6, 168. [Google Scholar] [CrossRef]
- Becklin, K.M.; Mullinix, G.W.R.; Ward, J.K. Host plant physiology and mycorrhizal functioning shift across a glacial through future CO2 gradient. Plant Physiol. 2016, 172, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Baslam, M.; Erice, G.; Goicoechea, N. Impact of arbuscular mycorrhizal fungi (AMF) and atmospheric CO2 concentration on the biomass production and partitioning in the forage legume alfalfa. Symbiosis 2012, 58, 171–181. [Google Scholar] [CrossRef]
- Saleh, A.M.; Abdel-Mawgoud, M.; Hassan, A.R.; Habeeb, T.H.; Yehia, R.S.; AbdElgawad, H. Global metabolic changes induced by arbuscular mycorrhizal fungi in oregano plants grown under ambient and elevated levels of atmospheric CO2. Plant Physiol. Biochem. 2020, 151, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, N.; Baslam, M.; Erice, G.; Irigoyen, J.J. Increased photosynthetic acclimation in alfalfa associated with arbuscular mycorrhizal fungi (AMF) and cultivated in greenhouse under elevated CO2. J. Plant Physiol. 2014, 171, 1774–1781. [Google Scholar] [CrossRef]
- Zhu, X.C.; Song, F.B.; Liu, S.Q.; Liu, F.L. Arbuscular mycorrhiza improve growth, nitrogen uptake, and nitrogen use efficiency in wheat grown under elevated CO2. Mycorrhiza 2016, 26, 133–140. [Google Scholar] [CrossRef]
- Treseder, K.K. A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol. 2004, 164, 347–355. [Google Scholar] [CrossRef]
- Butterly, C.R.; Armstrong, R.; Chen, D.; Tang, C. Carbon and nitrogen partitioning of wheat and field pea grown with two nitrogen levels under elevated CO2. Plant Soil 2015, 391, 367–382. [Google Scholar] [CrossRef]
- Loladze, I. Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. eLife 2014, 3, e02245. [Google Scholar] [CrossRef]
- Olesniewicz, K.S.; Thomas, R.B. Effects of mycorrhizal colonization on biomass production and nitrogen fixation of black locust (Robinia pseudoacacia) seedlings grown under elevated atmospheric carbon dioxide. New Phytol. 1999, 142, 133–140. [Google Scholar] [CrossRef]
- Baslam, M.; Garmendia, I.; Goicoechea, N. Elevated CO2 may impair the beneficial effect of arbuscular mycorrhizal fungi on the mineral and phytochemical quality of lettuce. Ann. Appl. Biol. 2012, 161, 180–191. [Google Scholar] [CrossRef]
- Chen, X.; Tu, C.; Burton, M.G.; Watson, D.M.; Burkey, K.O.; Hu, S. Plant nitrogen acquisition and interactions under elevated carbon dioxide: Impact of endophytes and mycorrhizae. Glob. Chang. Biol. 2007, 13, 1238–1249. [Google Scholar] [CrossRef]
- Ziska, L.H.; Ghannoum, O.; Baker, J.; Conroy, J.; Bunce, J.A.; Kobayashi, K.; Okada, M. A global perspective of ground level,‘ambient’carbon dioxide for assessing the response of plants to atmospheric CO2. Glob. Chang. Biol. 2001, 7, 789–796. [Google Scholar]
- Brundrett, M.; Bougher, N.; Dell, B.; Grove, T.; Malajczuk, N. Working with Mycorrhizas in Forestry and Agriculture (ACIAR Monograph 32); The Australian Centre for International Agricultural Research: Canberra, Australia, 1996; Volume 34, pp. 173–212. [Google Scholar]
- Yang, J.H.; Wang, C.L.; Dai, H.L. Soil Agrochemical Analysis and Environmental Monitoring Techniques; Chinese Dadi Press: Beijing, China, 2008; pp. 18–64. [Google Scholar]
- Carvalho, J.M.; Barreto, R.F.; Prado, R.d.M.; Habermann, E.; Martinez, C.A.; Branco, R.B.F. Elevated [CO2] and warming increase the macronutrient use efficiency and biomass of Stylosanthes capitata Vogel under field conditions. J. Agron. Crop Sci. 2020, 206, 597–606. [Google Scholar] [CrossRef]
- Staddon, P.L.; Fitter, A.H.; Graves, J.D. Effect of elevated atmospheric CO2 on mycorrhizal colonization, external mycorrhizal hyphal production and phosphorus inflow in Plantago lanceolata and Trifolium repens in association with the arbuscular mycorrhizal fungus Glomus mosseae. Glob. Chang. Biol. 1999, 5, 347–358. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Z.; Sun, H.; Yang, W.; Xu, H. The response patterns of arbuscular mycorrhizal and ectomycorrhizal symbionts under elevated CO2: A meta-analysis. Front. Microbiol. 2018, 9, 1248. [Google Scholar] [CrossRef] [PubMed]
- Sanders, I.; Streitwolf-Engel, R.; Van der Heijden, M.; Boller, T.; Wiemken, A. Increased allocation to external hyphae of arbuscular mycorrhizal fungi under CO2 enrichment. Oecologia 1998, 117, 496–503. [Google Scholar] [CrossRef]
- Rillig, M.C.; Allen, M.F. What is the role of arbuscular mycorrhizal fungi in plant-to-ecosystem responses to elevated atmospheric CO2? Mycorrhiza 1999, 9, 1–8. [Google Scholar] [CrossRef]
- Compant, S.; Van Der Heijden, M.G.; Sessitsch, A. Climate change effects on beneficial plant-microorganism interactions. FEMS Microbiol. Ecol. 2010, 73, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, P.; Pérez-Fernández, M.; Valentine, A. The role of arbuscular mycorrhizal colonization in the carbon and nutrient economy of the tripartite symbiosis with nodulated Phaseolus vulgaris. Soil Biol. Biochem. 2008, 40, 1019–1027. [Google Scholar] [CrossRef]
- Baslam, M.; Antolín, M.; Gogorcena, Y.; Munoz, F.J.; Goicoechea, N. Changes in alfalfa forage quality and stem carbohydrates induced by arbuscular mycorrhizal fungi and elevated atmospheric CO2. Ann. Appl. Biol. 2014, 164, 190–199. [Google Scholar] [CrossRef]
- Antolín, M.C.; Fiasconaro, M.L.; Sánchez-Díaz, M. Relationship between photosynthetic capacity, nitrogen assimilation and nodule metabolism in alfalfa (Medicago sativa) grown with sewage sludge. J. Hazard. Mater. 2010, 182, 210–216. [Google Scholar] [CrossRef]
- Harris, D.; Pacovsky, R.S.; Paul, E.A. Carbon economy of soybean–Rhizobium–Glomus associations. New Phytol. 1985, 101, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Houshmandfar, A.; Fitzgerald, G.J.; O’Leary, G.; Tausz-Posch, S.; Fletcher, A.; Tausz, M. The relationship between transpiration and nutrient uptake in wheat changes under elevated atmospheric CO2. Physiol. Plant. 2018, 163, 516–529. [Google Scholar] [CrossRef]
- Ericsson, T. Growth and shoot: Root ratio of seedlings in relation to nutrient availability. Plant Soil 1995, 168, 205–214. [Google Scholar] [CrossRef]
- Ellsworth, D.S.; Anderson, I.C.; Crous, K.Y.; Cooke, J.; Drake, J.E.; Gherlenda, A.N.; Gimeno, T.E.; Macdonald, C.A.; Medlyn, B.E.; Powell, J.R.; et al. Elevated CO2 does not increase eucalypt forest productivity on a low-phosphorus soil. Nat. Clim. Chang. 2017, 7, 279–282. [Google Scholar] [CrossRef]
- Irigoyen, J.; Goicoechea, N.; Antolín, M.; Pascual, I.; Sánchez-Díaz, M.; Aguirreolea, J.; Morales, F. Growth, photosynthetic acclimation and yield quality in legumes under climate change simulations: An updated survey. Plant Sci. 2014, 226, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Gavito, M.E.; Curtis, P.S.; Mikkelsen, T.N.; Jakobsen, I. Atmospheric CO2 and mycorrhiza effects on biomass allocation and nutrient uptake of nodulated pea (Pisum sativum L.) plants. J. Exp. Bot. 2000, 51, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Staddon, P.L.; Ramsey, C.B.; Ostle, N.; Ineson, P.; Fitter, A.H. Rapid turnover of hyphae of mycorrhizal fungi determined by AMS microanalysis of 14C. Science 2003, 300, 1138–1140. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.N.; Jakobsen, I. Symbiotic exchange of carbon and phosphorus between cucumber and three arbuscular mycorrhizal fungi. New Phytol. 1993, 124, 481–488. [Google Scholar] [CrossRef]
- Sekhar, K.M.; Sreeharsha, R.V.; Reddy, A.R. Differential responses in photosynthesis, growth and biomass yields in two mul-berry genotypes grown under elevated CO2 atmosphere. J. Photochem. Photobiol. B 2015, 151, 172–179. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Asensio, J.S.R.; Cousins, A.B. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and arabidopsis. Science 2010, 328, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Drake, B.G.; Gonzalez-Meler, M.A.; Long, S.P. More efficient plants: A consequence of rising atmospheric CO2? Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 609–639. [Google Scholar] [CrossRef]
- An, Y.; Wan, S.; Zhou, X.; Subedar, A.A.; Wallace, L.L.; Luo, Y. Plant nitrogen concentration, use efficiency, and contents in a tallgrass prairie ecosystem under experimental warming. Glob. Chang. Biol. 2005, 11, 1733–1744. [Google Scholar] [CrossRef]
- Fellbaum, C.R.; Gachomo, E.W.; Beesetty, Y.; Choudhari, S.; Strahan, G.D.; Pfeffer, P.E.; Kiers, E.T.; Bücking, H. Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2012, 109, 2666–2671. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, P.; Sahoo, S.; Senapati, A.; Kumar, U.; Mitra, D.; Parameswaran, C.; Anandan, A.; Kumar, A.; Jahan, A.; Nayak, A.K. Understanding interaction effect of arbuscular mycorrhizal fungi in rice under elevated carbon dioxide conditions. J. Basic Microbiol. 2019, 59, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Liu, B.; Gilna, B.; Zhang, Y.; Zhu, C.; Ma, H.; Pang, J.; Chen, G.; Zhu, J. Elevated CO2 effects on nutrient competition between a C3 crop (Oryza sativa L.) and a C4 weed (Echinochloa crusgalli L.). Nutr. Cycl. Agroecosyst. 2011, 89, 93–104. [Google Scholar] [CrossRef]
- Zhu, C.; Zeng, Q.; Yu, H.; Liu, S.; Dong, G.; Zhu, J. Effect of elevated CO2 on the growth and macronutrient (N, P and K) uptake of annual wormwood (Artemisia annua L.). Pedosphere 2016, 26, 235–242. [Google Scholar] [CrossRef]
- Goicoechea, N.; Bettoni, M.M.; Fuertes-Mendizábal, T.; Gonzalez-Murua, C.; Aranjuelo, I. Durum wheat quality traits affected by mycorrhizal inoculation, water availability and atmospheric CO2 concentration. Crop. Pasture Sci. 2016, 67, 147. [Google Scholar] [CrossRef]
- Li, P.; Han, X.; Zong, Y.; Li, H.; Lin, E.; Han, Y.; Hao, X. Effects of free-air CO2 enrichment (FACE) on the uptake and utilization of N, P and K in Vigna radiata. Agric. Ecosyst. Environ. 2015, 202, 120–125. [Google Scholar] [CrossRef]
- Habermann, E.; De Oliveira, E.A.D.; Contin, D.R.; Martin, J.A.B.S.; Curtarelli, L.; Gonzalez-Meler, M.A.; Martinez, C.A. Stomatal development and conductance of a tropical forage legume are regulated by elevated [CO2] under moderate warming. Front. Plant Sci. 2019, 10, 609. [Google Scholar] [CrossRef]
- McGrath, J.M.; Lobell, D.B. Reduction of transpiration and altered nutrient allocation contribute to nutrient decline of crops grown in elevated CO2concentrations. Plant Cell Environ. 2012, 36, 697–705. [Google Scholar] [CrossRef]
- Jifon, J.L.; Drouillard, D.L.; Graham, J.H.; Syvertsen, J.P. Growth depression of mycorrhizal Citrus seedlings grown at high phosphorus supply is mitigated by elevated CO2. New Phytol. 2002, 153, 133–142. [Google Scholar] [CrossRef]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Tausz, M.; Norton, R.M.; Tausz-Posch, S.; Löw, M.; Seneweera, S.; O’Leary, G.; Armstrong, R.; Fitzgerald, G.J. Can additional N fertiliser ameliorate the elevated CO2 -induced depression in grain and tissue N concentrations of wheat on a high soil N background? J. Agron. Crop Sci. 2017, 203, 574–583. [Google Scholar] [CrossRef]
- Cheng, L.; Booker, F.L.; Tu, C.; Burkey, K.O.; Zhou, L.; Shew, H.D.; Rufty, T.W.; Hu, S. Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 2012, 337, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Hoosbeek, M.R. Elevated CO2 increased phosphorous loss from decomposing litter and soil organic matter at two FACE ex-periments with trees. Biogeochemistry 2016, 127, 89–97. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Horwath, W.R.; Dorodnikov, M.; Blagodatskaya, E. Review and synthesis of the effects of elevated atmospheric CO2 on soil processes: No changes in pools, but increased fluxes and accelerated cycles. Soil Biol. Biochem. 2019, 128, 66–78. [Google Scholar] [CrossRef]
- Hodge, A.; Fitter, A.H. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc. Natl. Acad. Sci. USA 2010, 107, 13754–13759. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nat. Cell Biol. 2005, 435, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Lagomarsino, A.; Moscatelli, M.C.; Hoosbeek, M.R.; De Angelis, P.; Grego, S. Assessment of soil nitrogen and phosphorous availability under elevated CO2 and N-fertilization in a short rotation poplar plantation. Plant Soil 2008, 308, 131–147. [Google Scholar] [CrossRef]
- Lin, X.G.; Hu, J.L.; Chu, H.Y.; Yin, R.; Yuan, X.X.; Zhang, H.Y.; Zhu, J.G. Response of soil ammonia-oxidizing bacteria to enriched atmospheric CO2. Rural Eco-Environ. 2005, 21, 41–46. [Google Scholar]


| Treatment | AM (%) | Nodule Biomass (g plant−1) | Shoot Biomass (g plant−1) | Root Biomass (g plant−1) | Root/Shoot Ratio | Total Biomass (g plant−1) | |
|---|---|---|---|---|---|---|---|
| ACO2 | M− | 0 | 0.04 ± 0.01b,x | 1.91 ± 0.04b,y | 1.01 ± 0.09b,x | 0.53 ± 0.06a,x | 2.91 ± 0.07b,y |
| M+ | 43.01 ± 2.49b | 0.05 ± 0.01b,x | 2.15 ± 0.08b,x | 1.13 ± 0.08b,x | 0.57 ± 0.05a,x | 3.18 ± 0.09b,x | |
| eCO2 | M− | 0 | 0.07 ± 0.00a,y | 3.24 ± 0.09a,y | 1.26 ± 0.05a,x | 0.39 ± 0.01b,x | 4.50 ± 0.08a,y |
| M+ | 59.64 ± 3.04a | 0.13 ± 0.02a,x | 3.69 ± 0.09a,x | 1.39 ± 0.14a,x | 0.37 ± 0.01b,x | 5.05 ± 0.12a,x | |
| ANOVA | |||||||
| CO2 | ** | *** | *** | ** | ** | *** | |
| AMF | * | ** | ns | ns | ** | ||
| CO2 × AMF | ** | ns | ns | ns | ns | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, S.; Luo, X.; Dong, X.; Qiu, Y.; Xu, C.; He, X. Arbuscular Mycorrhization Enhances Nitrogen, Phosphorus and Potassium Accumulation in Vicia faba by Modulating Soil Nutrient Balance under Elevated CO2. J. Fungi 2021, 7, 361. https://doi.org/10.3390/jof7050361
Shi S, Luo X, Dong X, Qiu Y, Xu C, He X. Arbuscular Mycorrhization Enhances Nitrogen, Phosphorus and Potassium Accumulation in Vicia faba by Modulating Soil Nutrient Balance under Elevated CO2. Journal of Fungi. 2021; 7(5):361. https://doi.org/10.3390/jof7050361
Chicago/Turabian StyleShi, Songmei, Xie Luo, Xingshui Dong, Yuling Qiu, Chenyang Xu, and Xinhua He. 2021. "Arbuscular Mycorrhization Enhances Nitrogen, Phosphorus and Potassium Accumulation in Vicia faba by Modulating Soil Nutrient Balance under Elevated CO2" Journal of Fungi 7, no. 5: 361. https://doi.org/10.3390/jof7050361
APA StyleShi, S., Luo, X., Dong, X., Qiu, Y., Xu, C., & He, X. (2021). Arbuscular Mycorrhization Enhances Nitrogen, Phosphorus and Potassium Accumulation in Vicia faba by Modulating Soil Nutrient Balance under Elevated CO2. Journal of Fungi, 7(5), 361. https://doi.org/10.3390/jof7050361







