Breakthrough Invasive Fungal Infections in Allogeneic Hematopoietic Stem Cell Transplantation
Abstract
1. Introduction
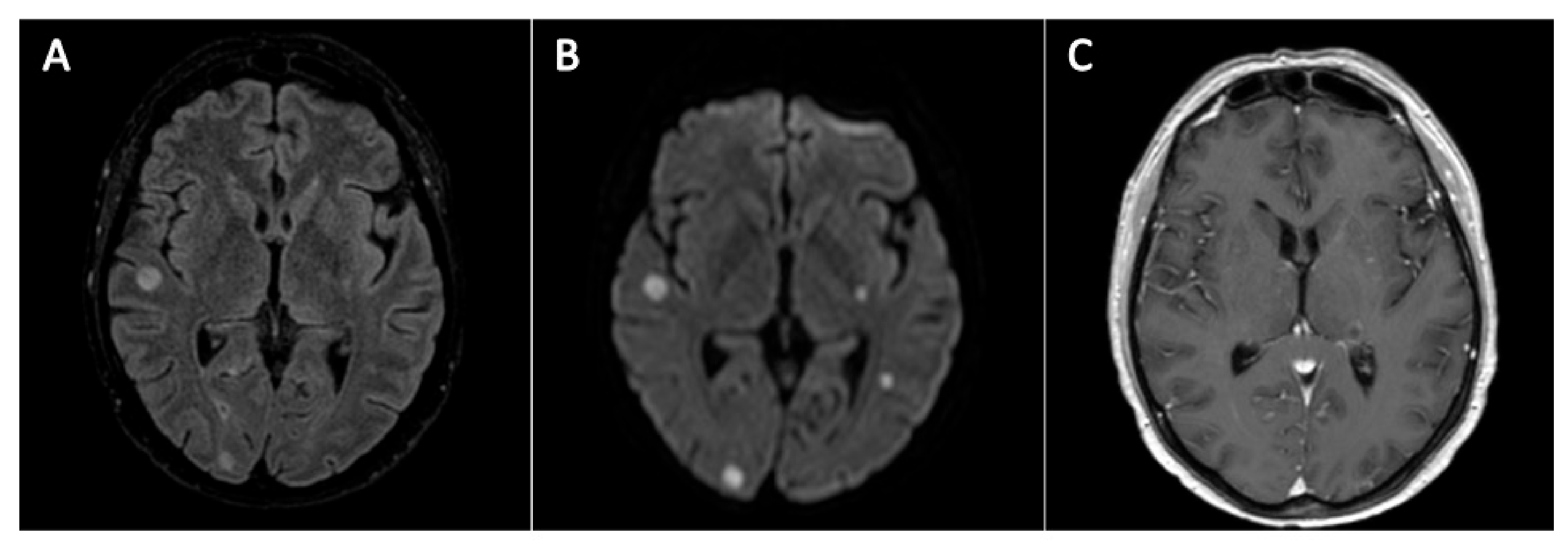
2. Case Descriptions, Methods and Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective Surveillance for Invasive Fungal Infections in Hematopoietic Stem Cell Transplant Recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Girmenia, C.; Raiola, A.M.; Piciocchi, A.; Algarotti, A.; Stanzani, M.; Cudillo, L.; Pecoraro, C.; Guidi, S.; Iori, A.P.; Montante, B.; et al. Incidence and outcome of invasive fungal diseases after allogeneic stem cell transplantation: A prospective study of the gruppo italiano trapianto midollo osseo (GITMO). Biol. Blood Marrow Transplant. 2014, 20, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.A.; Girmenia, C.; Brüggemann, R.J.; Duarte, R.F.; Kibbler, C.C.; Ljungman, P.; Racil, Z.; Ribaud, P.; Slavin, M.A.; Cornely, O.A.; et al. European guidelines for primary antifungal prophylaxis in adult haematology patients: Summary of the updated recommendations from the European Conference on Infections in Leukaemia. J. Antimicrob. Chemother. 2018, 73, 3221–3230. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Lewis, R.E.; Kontoyiannis, D.P. Breakthrough invasive mold infections in the hematology patient: Current concepts and future directions. Clin. Infect. Dis. 2018, 67, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Stern, A.; Su, Y.; Lee, Y.J.; Seo, S.; Shaffer, B.; Tamari, R.; Gyurkocza, B.; Barker, J.; Bogler, Y.; Giralt, S.; et al. A Single Center, Open-Label Trial of Isavuconazole Prophylaxis against Invasive Fungal Infection in Patients Undergoing Allogeneic Hematopoietic Cell Transplant (HCT). Biol. Blood Marrow Transplant. 2020, 26, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Wasylyshyn, A.; Linder, K.A.; Castillo, C.G.; Zhou, S.; Kauffman, C.A.; Miceli, M.H. Breakthrough Invasive Fungal Infections in Patients with Acute Myeloid Leukemia. Mycopathologia 2020, 185, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Girmenia, C.; Busca, A.; Candoni, A.; Cesaro, S.; Luppi, M.; Nosari, A.M.; Pagano, L.; Rossi, G.; Venditti, A.; Aversa, F. Breakthrough invasive fungal diseases in acute myeloid leukemia patients receiving mould active triazole primary prophylaxis after intensive chemotherapy: An Italian consensus agreement on definitions and management. Med. Mycol. 2019, 57 (Suppl. 2), S127–S137. [Google Scholar] [CrossRef]
- Hoenigl, M.; Salmanton-García, J.; Walsh, T.J.; Nucci, M.; Neoh, C.F.; Jenks, J.D.; Lackner, M.; Sprute, R.; Al-Hatmi, A.M.S.; Bassetti, M.; et al. Global guideline for the diagnosis and management of rare mould infections: An initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect. Dis. 2021, in press. [Google Scholar] [CrossRef]
- Cornely, O.A.; Maertens, J.; Winston, D.J.; Perfect, J.; Ullmann, A.J.; Walsh, T.J.; Helfgott, D.; Holowiecki, J.; Stockelberg, D.; Goh, Y.T.; et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 2007, 356, 348–359. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Lipton, J.H.; Vesole, D.H.; Chandrasekar, P.; Langston, A.; Tarantolo, S.R.; Greinix, H.; De Azevedo, W.M.; Reddy, V.; Boparai, N.; et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N. Engl. J. Med. 2007, 356, 335–347. [Google Scholar] [CrossRef]
- Ramirez-Garcia, A.; Pellon, A.; Rementeria, A.; Buldain, I.; Barreto-Bergter, E.; Rollin-Pinheiro, R.; De Meirelles, J.V.; Xisto, M.I.D.S.; Ranque, S.; Havlicek, V.; et al. Scedosporium and Lomentospora: An updated overview of underrated opportunists. Med. Mycol. 2018, 56 (Suppl. 1), 102–125. [Google Scholar] [CrossRef]
- Husain, S.; Muñoz, P.; Forrest, G.; Alexander, B.D.; Somani, J.; Brennan, K.; Wagener, M.M.; Singh, N. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: Clinical characteristics and impact of antifungal agent therapy on outcome. Clin. Infect. Dis. 2005, 40, 89–99. [Google Scholar] [CrossRef]
- Caira, M.; Girmenia, C.; Valentini, C.G.; Sanguinetti, M.; Bonini, A.; Rossi, G.; Fianchi, L.; Leone, G.; Pagano, L. Scedosporiosis in patients with acute leukemia: A retrospective multicenter report. Haematologica 2008, 93, 104–110. [Google Scholar] [CrossRef]
- Seidel, D.; Meißner, A.; Lackner, M.; Piepenbrock, E.; Salmanton-García, J.; Stecher, M.; Mellinghoff, S.; Hamprecht, A.; Durán Graeff, L.; Köhler, P.; et al. Prognostic factors in 264 adults with invasive Scedosporium spp. and Lomentospora prolificans infection reported in the literature and FungiScope®. Crit. Rev. Microbiol. 2019, 45, 1–21. [Google Scholar] [CrossRef]
- Jenks, J.D.; Seidel, D.; Cornely, O.A.; Chen, S.; Van Hal, S.; Kauffman, C.; Miceli, M.H.; Heinemann, M.; Christner, M.; Sáenz, A.J.; et al. Voriconazole plus terbinafine combination antifungal therapy for invasive Lomentospora prolificans infections: Analysis of 41 patients from the FungiScope® registry 2008–2019. Clin. Microbiol. Infect. 2020, 26, 784.e1–784.e5. [Google Scholar] [CrossRef]
- Tortorano, A.M.; Richardson, M.; Roilides, E.; Van Diepeningen, A.; Caira, M.; Munoz, P.; Johnson, E.; Meletiadis, J.; Pana, Z.D.; Lackner, M.; et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin. Microbiol. Infect. 2014, 20 (Suppl. 3), 27–46. [Google Scholar] [CrossRef]
- An Open-label Study of APX001 for Treatment of Patients with Invasive Mold Infections Caused by Aspergillus Species or Rare Molds. ClinicalTrials.gov Identifier: NCT04240886. Available online: https://clinicaltrials.gov/ct2/show/NCT04240886 (accessed on 16 March 2021).
- Evaluate F901318 Treatment of Invasive Fungal Infections in Patients Lacking Treatment Options. ClinicalTrials.gov Identifier: NCT03583164. Available online: https://clinicaltrials.gov/ct2/show/NCT03583164 (accessed on 16 March 2021).
- Marr, K.A.; Laverdiere, M.; Gugel, A.; Leisenring, W. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin. Infect. Dis. 2005, 40, 1762–1769. [Google Scholar] [CrossRef]
- Calmettes, C.; Gabriel, F.; Blanchard, E.; Servant, V.; Bouchet, S.; Kabore, N.; Forcade, E.; Leroyer, C.; Bidet, A.; Latrabe, V.; et al. Breakthrough invasive aspergillosis and diagnostic accuracy of serum galactomannan enzyme immune assay during acute myeloid leukemia induction chemotherapy with posaconazole prophylaxis. Oncotarget 2018, 9, 26724–26736. [Google Scholar] [CrossRef]
- Duarte, R.F.; Sánchez-Ortega, I.; Cuesta, I.; Arnan, M.; Patiño, B.; De Sevilla, A.F.; Gudiol, C.; Ayats, J.; Cuenca-Estrella, M. Serum galactomannan-based early detection of invasive aspergillosis in hematology patients receiving effective antimold prophylaxis. Clin. Infect. Dis. 2014, 59, 1696–1702. [Google Scholar] [CrossRef]
- Skiada, A.; Lass-Floerl, C.; Klimko, N.; Ibrahim, A.; Roilides, E.; Petrikkos, G. Challenges in the diagnosis and treatment of mucormycosis. Med. Mycol. 2018, 56 (Suppl. 1), 93–101. [Google Scholar] [CrossRef]
- Farmakiotis, D.; Kontoyiannis, D.P. Mucormycoses. Infect. Dis. Clin. N. Am. 2016, 30, 143–163. [Google Scholar] [CrossRef]
- Pagano, L.; Girmenia, C.; Mele, L.; Ricci, P.; Tosti, M.E.; Nosari, A.; Buelli, M.; Picardi, M.; Allione, B.; Corvatta, L.; et al. Infections caused by filamentous fungi in patients with hematologic malignancies. A report of 391 cases by GIMEMA infection program. Haematologica 2001, 86, 862–870. [Google Scholar]
- Miller, M.A.; Molina, K.C.; Gutman, J.A.; Scherger, S.; Lum, J.M.; Mossad, S.B.; Burgess, M.; Cheng, M.P.; Chuang, S.T.; Jacobs, S.E.; et al. Mucormycosis in Hematopoietic Cell Transplant Recipients and in Patients With Hematological Malignancies in the Era of New Antifungal Agents. Open Forum Infect. Dis. 2021, 8, ofaa646. [Google Scholar] [CrossRef]
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Lass-Florl, C.; Bouza, E.; Klimko, N.; Gaustad, P.; et al. Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011, 17, 1859–1867. [Google Scholar] [CrossRef]
- Cornely, O.; Arikan-Akdagli, S.E.V.T.A.P.; Dannaoui, E.; Groll, A.H.; Lagrou, K.; Chakrabarti, A.; Lanternier, F.; Pagano, L.I.V.I.O.; Skiada, A.; Akova, M.; et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin. Microbiol. Infect. 2014, 20 (Suppl. 3), 5–26. [Google Scholar] [CrossRef]
- Tissot, F.; Agrawal, S.; Pagano, L.; Petrikkos, G.; Groll, A.H.; Skiada, A.; Lass-Flörl, C.; Calandra, T.; Viscoli, C.; Herbrecht, R. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 2017, 102, 433–444. [Google Scholar] [CrossRef]
- Marty, F.M.; Ostrosky-Zeichner, L.; Cornely, O.A.; Mullane, K.M.; Perfect, J.R.; Thompson, G.R.; Alangaden, G.J.; Brown, J.M.; Fredricks, D.N.; Heinz, W.J.; et al. Isavuconazole treatment for mucormycosis: A single-arm open-label trial and case-control analysis. Lancet Infect. Dis. 2016, 16, 828–837. [Google Scholar] [CrossRef]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef]
| Clinical Case 1 | Clinical Case 2 | |
|---|---|---|
| Species | Lomentospora prolificans | Rhizopus microsporus |
| Sample | Peripheral blood | Soft tissue biopsy |
| Drugs | MIC | MIC |
| L-AMB | >32 mg/L | 0.5 mg/L |
| Itraconazole | >32 mg/L | 1.5 mg/L |
| Voriconazole | >32 mg/L | - |
| Posaconazole | >32 mg/L | 0.19 mg/L |
| Isavuconazole | >32 mg/L | 0.125 mg/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liberatore, C.; Farina, F.; Greco, R.; Giglio, F.; Clerici, D.; Oltolini, C.; Lupo Stanghellini, M.T.; Barzaghi, F.; Vezzulli, P.; Orsenigo, E.; et al. Breakthrough Invasive Fungal Infections in Allogeneic Hematopoietic Stem Cell Transplantation. J. Fungi 2021, 7, 347. https://doi.org/10.3390/jof7050347
Liberatore C, Farina F, Greco R, Giglio F, Clerici D, Oltolini C, Lupo Stanghellini MT, Barzaghi F, Vezzulli P, Orsenigo E, et al. Breakthrough Invasive Fungal Infections in Allogeneic Hematopoietic Stem Cell Transplantation. Journal of Fungi. 2021; 7(5):347. https://doi.org/10.3390/jof7050347
Chicago/Turabian StyleLiberatore, Carmine, Francesca Farina, Raffaella Greco, Fabio Giglio, Daniela Clerici, Chiara Oltolini, Maria Teresa Lupo Stanghellini, Federica Barzaghi, Paolo Vezzulli, Elena Orsenigo, and et al. 2021. "Breakthrough Invasive Fungal Infections in Allogeneic Hematopoietic Stem Cell Transplantation" Journal of Fungi 7, no. 5: 347. https://doi.org/10.3390/jof7050347
APA StyleLiberatore, C., Farina, F., Greco, R., Giglio, F., Clerici, D., Oltolini, C., Lupo Stanghellini, M. T., Barzaghi, F., Vezzulli, P., Orsenigo, E., Corti, C., Ciceri, F., & Peccatori, J. (2021). Breakthrough Invasive Fungal Infections in Allogeneic Hematopoietic Stem Cell Transplantation. Journal of Fungi, 7(5), 347. https://doi.org/10.3390/jof7050347






