Emerging Invasive Fungal Infections in Critically Ill Patients: Incidence, Outcomes and Prognosis Factors, a Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
2.2. Patients
2.3. Data Collection
2.4. Statistical Analysis
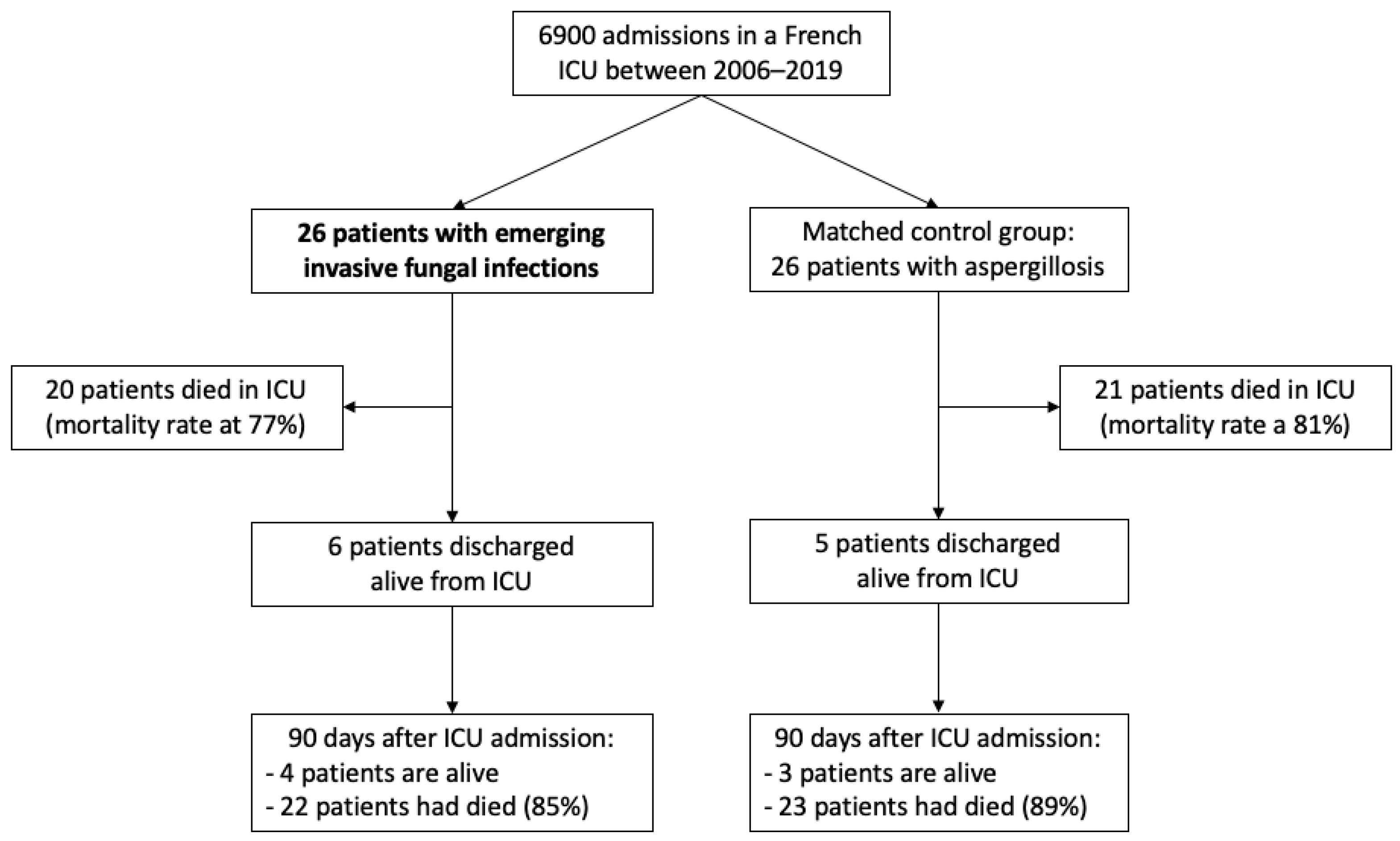
3. Results
3.1. Characteristics of the Study Population
3.2. Mold Isolates
3.3. Treatments
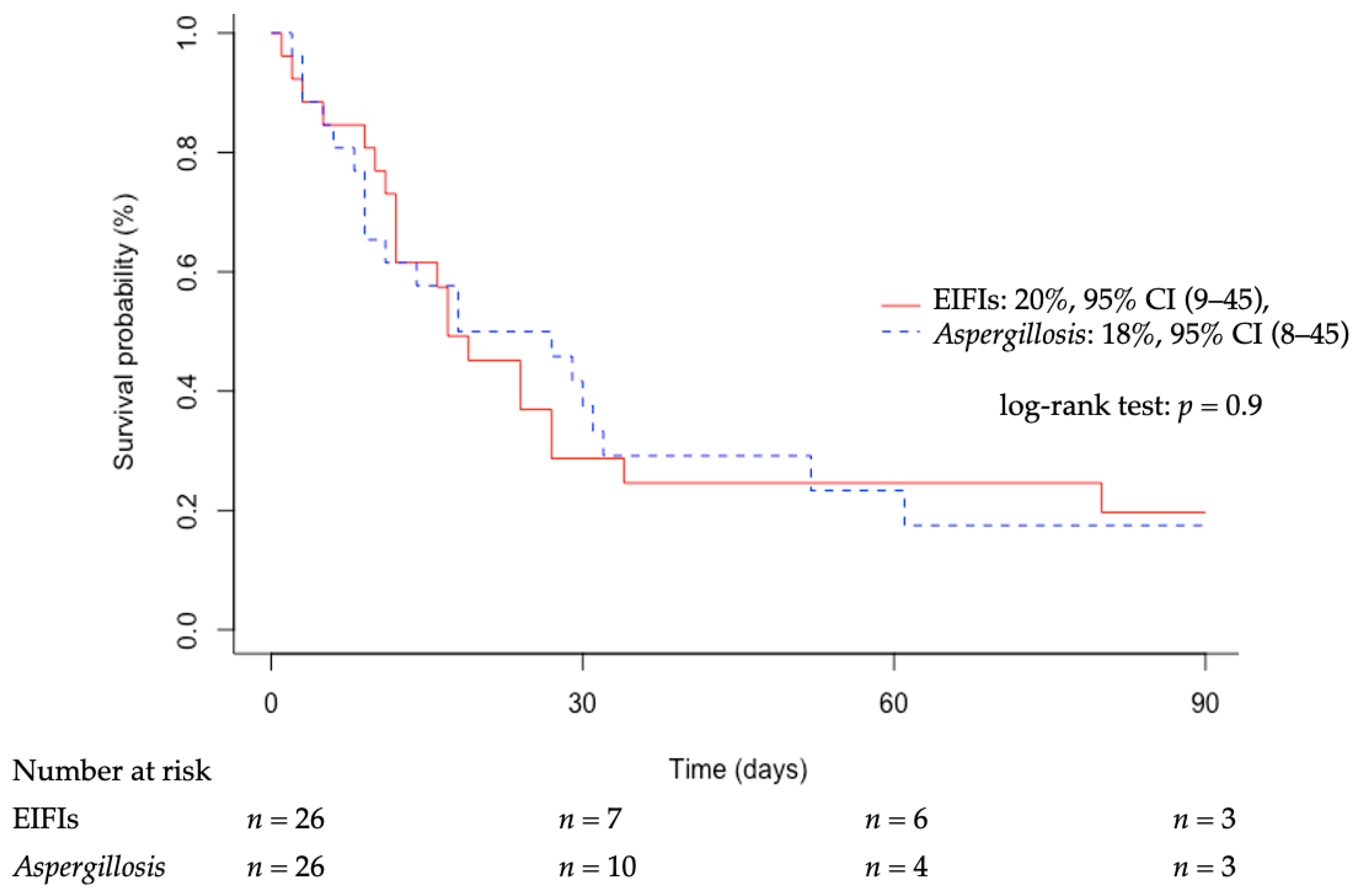
3.4. Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colombo, A.L.; de Almeida Júnior, J.N.; Slavin, M.A.; Chen, S.C.-A.; Sorrell, T.C. Candida and Invasive Mould Diseases in Non-Neutropenic Critically Ill Patients and Patients with Haematological Cancer. Lancet Infect. Dis. 2017, 17, e344–e356. [Google Scholar] [CrossRef]
- Bitar, D.; Van Cauteren, D.; Lanternier, F.; Dannaoui, E.; Che, D.; Dromer, F.; Desenclos, J.-C.; Lortholary, O. Increasing Incidence of Zygomycosis (Mucormycosis), France, 1997–2006. Emerg. Infect. Dis. 2009, 15, 1395–1401. [Google Scholar] [CrossRef]
- Wiederhold, N.P. The Antifungal Arsenal: Alternative Drugs and Future Targets. Int. J. Antimicrob. Agents 2018, 51, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Pappas, P.G.; Wannemuehler, K.A.; Alexander, B.D.; Anaissie, E.J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; Brumble, L.M.; Freifeld, A.G.; et al. Invasive Non- Aspergillus Mold Infections in Transplant Recipients, United States, 2001–2006. Emerg. Infect. Dis. 2011, 17, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Petrikkos, G.; Skiada, A.; Drogari-Apiranthitou, M. Epidemiology of Mucormycosis in Europe. Clin. Microbiol. Infect 2014, 20, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Corzo-León, D.E.; Satlin, M.J.; Soave, R.; Shore, T.B.; Schuetz, A.N.; Jacobs, S.E.; Walsh, T.J. Epidemiology and Outcomes of Invasive Fungal Infections in Allogeneic Haematopoietic Stem Cell Transplant Recipients in the Era of Antifungal Prophylaxis: A Single-Centre Study with Focus on Emerging Pathogens. Mycoses 2015, 58, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Claustre, J.; Larcher, R.; Jouve, T.; Truche, A.-S.; Nseir, S.; Cadiet, J.; Zerbib, Y.; Lautrette, A.; Constantin, J.-M.; Charles, P.-E.; et al. Mucormycosis in Intensive Care Unit: Surgery Is a Major Prognostic Factor in Patients with Hematological Malignancy. Ann. Intensive Care 2020, 10, 74. [Google Scholar] [CrossRef]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global Guideline for the Diagnosis and Management of Mucormycosis: An Initiative of the European Confederation of Medical Mycology in Cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a Combined Comorbidity Index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Jensen, G.L.; Cederholm, T.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; de Baptista, G.A.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM Criteria for the Diagnosis of Malnutrition: A Consensus Report From the Global Clinical Nutrition Community. J. Cachexia Sarcopenia Muscle 2019, 43, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Gall, J.-R.L.; Lemeshow, S.; Saulnier, F. A New Simplified Acute Physiology Score (SAPS II) Based on a European/North American Multicenter Study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-Related Organ Failure Assessment) Score to Describe Organ Dysfunction/Failure. On Behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1. [Google Scholar] [CrossRef]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef] [PubMed]
- Limper, A.H.; Knox, K.S.; Sarosi, G.A.; Ampel, N.M.; Bennett, J.E.; Catanzaro, A.; Davies, S.F.; Dismukes, W.E.; Hage, C.A.; Marr, K.A.; et al. An Official American Thoracic Society Statement: Treatment of Fungal Infections in Adult Pulmonary and Critical Care Patients. Am. J. Respir. Crit. Care Med. 2011, 183, 96–128. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Boekhout, T.; Akova, M.; Meis, J.F.; Cornely, O.A.; Lortholary, O.; European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group. European Confederation of Medical Mycology ESCMID and ECMM Joint Clinical Guidelines for the Diagnosis and Management of Rare Invasive Yeast Infections. Clin. Microbiol. Infect 2014, 20 (Suppl. 3), 76–98. [Google Scholar] [CrossRef]
- Chowdhary, A.; Meis, J.F.; Guarro, J.; de Hoog, G.S.; Kathuria, S.; Arendrup, M.C.; Arikan-Akdagli, S.; Akova, M.; Boekhout, T.; Caira, M.; et al. ESCMID and ECMM Joint Clinical Guidelines for the Diagnosis and Management of Systemic Phaeohyphomycosis: Diseases Caused by Black Fungi. Clin. Microbiol. Infect. 2014, 20 (Suppl. 3), 47–75. [Google Scholar] [CrossRef]
- Cornely, O.A.; Arikan-Akdagli, S.; Dannaoui, E.; Groll, A.H.; Lagrou, K.; Chakrabarti, A.; Lanternier, F.; Pagano, L.; Skiada, A.; Akova, M.; et al. ESCMID† and ECMM‡ Joint Clinical Guidelines for the Diagnosis and Management of Mucormycosis 2013. Clin. Microbiol. Infect. 2014, 20, 5–26. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R., III; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Arcobello, J.T.; Revankar, S.G. Phaeohyphomycosis. Semin. Respir. Crit. Care Med. 2020, 41, 131–140. [Google Scholar] [CrossRef]
- Borman, A.M.; Muller, J.; Walsh-Quantick, J.; Szekely, A.; Patterson, Z.; Palmer, M.D.; Fraser, M.; Johnson, E.M. MIC Distributions for Amphotericin B, Fluconazole, Itraconazole, Voriconazole, Flucytosine and Anidulafungin and 35 Uncommon Pathogenic Yeast Species from the UK Determined Using the CLSI Broth Microdilution Method. J. Antimicrob. Chemother. 2020, 75, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Lanternier, F.; Dannaoui, E.; Morizot, G.; Elie, C.; Garcia-Hermoso, D.; Huerre, M.; Bitar, D.; Dromer, F.; Lortholary, O.; French Mycosis Study Group. A Global Analysis of Mucormycosis in France: The RetroZygo Study (2005–2007). Clin. Infect. Dis. 2012, 54 (Suppl. 1), S35–S43. [Google Scholar] [CrossRef]
- Jestin, M.; Azoulay, E.; Pène, F.; Bruneel, F.; Mayaux, J.; Murgier, M.; Darmon, M.; Valade, S. Poor Outcome Associated with Mucormycosis in Critically Ill Hematological Patients: Results of a Multicenter Study. Ann. Intensive Care 2021, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Schellongowski, P.; Patroniti, N.; Taccone, F.S.; Reis Miranda, D.; Reuter, J.; Prodanovic, H.; Pierrot, M.; Dorget, A.; Park, S.; et al. Six-Month Outcome of Immunocompromised Severe ARDS Patients Rescued by ECMO. An International Multicenter Retrospective Study. Am. J. Respir. Crit. Care Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Platon, L.; Amigues, L.; Ceballos, P.; Fegueux, N.; Daubin, D.; Besnard, N.; Larcher, R.; Landreau, L.; Agostini, C.; Machado, S.; et al. A Reappraisal of ICU and Long-Term Outcome of Allogeneic Hematopoietic Stem Cell Transplantation Patients and Reassessment of Prognosis Factors: Results of a 5-Year Cohort Study (2009–2013). Bone Marrow Transpl. 2015. [Google Scholar] [CrossRef] [PubMed]
- Khasawneh, F.; Mohamad, T.; Moughrabieh, M.K.; Lai, Z.; Ager, J.; Soubani, A.O. Isolation of Aspergillus in Critically Ill Patients: A Potential Marker of Poor Outcome. J. Crit. Care 2006, 21, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Multani, A.; Reveron-Thornton, R.; Garvert, D.W.; Gomez, C.A.; Montoya, J.G.; Lui, N.S. Cut It out! Thoracic Surgeon’s Approach to Pulmonary Mucormycosis and the Role of Surgical Resection in Survival. Mycoses 2019, 62, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, H.; Jeon, K.; Suh, G.Y.; Shin, S.; Kim, H.K.; Kim, K.; Jeong, D.; Kim, H. Factors Affecting Surgical Resection and Treatment Outcomes in Patients with Pulmonary Mucormycosis. J. Thorac. Dis. 2019, 11, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Delaying Amphotericin B–Based Frontline Therapy Significantly Increases Mortality among Patients with Hematologic Malignancy Who Have Zygomycosis. Clin. Infect. Dis. 2008, 47, 503–509. [Google Scholar] [CrossRef]
- Goldman, C.; Akiyama, M.J.; Torres, J.; Louie, E.; Meehan, S.A. Scedosporium Apiospermum Infections and the Role of Combination Antifungal Therapy and GM-CSF: A Case Report and Review of the Literature. Med. Mycol. Case Rep. 2016, 11, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Anaissie, E.; Nelson, P.E.; Hashem, R.; Legrand, C.; Ho, D.H.; Bodey, G.P. Antifungal Susceptibility of 44 Clinical Isolates of Fusarium Species Determined by Using a Broth Microdilution Method. Antimicrob. Agents Chemother. 1989, 33, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Buchta, V.; Bolehovská, R.; Hovorková, E.; Cornely, O.A.; Seidel, D.; Žák, P. Saprochaete Clavata Invasive Infections—A New Threat to Hematological-Oncological Patients. Front. Microbiol. 2019, 10, 2196. [Google Scholar] [CrossRef]
- Vaux, S.; Criscuolo, A.; Desnos-Ollivier, M.; Diancourt, L.; Tarnaud, C.; Vandenbogaert, M.; Brisse, S.; Coignard, B.; Dromer, F.; Geotrichum Investigation Group. Multicenter Outbreak of Infections by Saprochaete Clavata, an Unrecognized Opportunistic Fungal Pathogen. mBio 2014, 5. [Google Scholar] [CrossRef]
- Kyvernitakis, A.; Torres, H.A.; Jiang, Y.; Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Initial Use of Combination Treatment Does Not Impact Survival of 106 Patients with Haematologic Malignancies and Mucormycosis: A Propensity Score Analysis. Clin. Microbiol. Infect. 2016, 22, 811.e1–811.e8. [Google Scholar] [CrossRef] [PubMed]
- Vena, A.; Muñoz, P.; Mateos, M.; Guinea, J.; Galar, A.; Pea, F.; Alvarez-Uria, A.; Escribano, P.; Bouza, E. Therapeutic Drug Monitoring of Antifungal Drugs: Another Tool to Improve Patient Outcome? Infect. Dis. Ther. 2020, 9, 137–149. [Google Scholar] [CrossRef]
- Nucci, M.; Varon, A.G.; Garnica, M.; Akiti, T.; Barreiros, G.; Trope, B.M.; Nouér, S.A. Increased Incidence of Invasive Fusariosis with Cutaneous Portal of Entry, Brazil. Emerg. Infect. Dis. 2013, 19, 1567–1572. [Google Scholar] [CrossRef]
- Lass-Flörl, C. The Changing Face of Epidemiology of Invasive Fungal Disease in Europe. Mycoses 2009, 52, 197–205. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 26) | Survivor (n = 6) | Deceased (n = 20) | p-Value | |
|---|---|---|---|---|
| Male, n (%) | 21 (81%) | 4 (67%) | 17 (85%) | 0.33 |
| Age (years), median (IQR) | 58.5 (48–69) | 63.5 (52–69) | 57 (47–68) | 0.81 |
| Charlson index, median (IQR) | 3 (2–5) | 1 (0–4) | 3 (2–4) | 0.2 |
| BMT/SOT, n (%) | 14 (54%) | 2 (33%) | 12 (60%) | 0.26 |
| Hematological mal./cancer, n (%) | 15 (58%) | 1 (17%) | 14 (70%) | 0.04 |
| Immunosuppressors, n (%) | 22 (85%) | 4 (67%) | 18 (90%) | 0.19 |
| Corticosteroids, n (%) | 18 (69%) | 4 (67%) | 14 (70%) | 0.88 |
| Diabetes, n (%) | 6 (23%) | 1 (17%) | 5 (25%) | 0.67 |
| CKD, n (%) | 4 (15%) | 3 (50%) | 1 (5%) | 0.03 |
| Burn/Trauma, n (%) | 3 (12%) | 2 (33%) | 1 (5%) | 0.09 |
| Malnutrition, n (%) | 21 (81%) | 3 (50%) | 18 (90%) | 0.047 |
| Candida colonization, n (%) | 22 (85%) | 5 (83%) | 17 (85%) | 0.92 |
| Central venous catheter, n (%) | 23 (89%) | 5 (83%) | 18 (90%) | 0.66 |
| Broad-spectrum antibiotic, n (%) | 25 (96%) | 6 (100%) | 19 (95%) | >0.99 |
| SAPS II, median (IQR) | 59 (49–76) | 65 (59–78) | 57 (48–68) | 0.48 |
| SOFA at admission, median (IQR) | 10 (8–13) | 10 (8–11) | 11 (8–13) | 0.79 |
| Invasive mechanical ventilation, n (%) | 25 (96%) | 5 (83%) | 20 (100%) | 0.99 |
| Duration (days), median (IQR) | 11 (4–24) | 30 (10–38) | 11 (5–21) | 0.13 |
| ARDS, n (%) | 16 (62%) | 1 (17%) | 15 (75%) | 0.03 |
| Vasoconstrictive drugs, n (%) | 23 (89%) | 5 (83%) | 18 (90%) | 0.66 |
| Duration (days), median (IQR) | 13 (10–28) | 30 (29–30) | 12 (9–17) | 0.2 |
| AKI, n (%) | 24 (92%) | 5 (83%) | 19 (95%) | 0.37 |
| RRT, n (%) | 18 (69%) | 4 (67%) | 14 (70%) | 0.88 |
| Duration (days), median (IQR) | 10 (6–25) | 45 (31–63) | 9 (4–18) | 0.06 |
| Hepatic dysfunction, n (%) | 18 (69%) | 2 (33%) | 16 (80%) | 0.04 |
| Myocardial dysfunction, n (%) | 14 (54%) | 1 (17%) | 13 (65%) | 0.06 |
| Mucormycosis, n (%) | 13 (50%) | 3 (50%) | 10 (50%) | >0.99 |
| Other EIFIs, n (%) | 13 (50%) | 3 (50%) | 10 (50%) | >0.99 |
| Organ involvements: | ||||
| Pulmonary, n (%) | 14 (54%) | 1 (17%) | 13 (65%) | 0.06 |
| Cutaneous, n (%) | 9 (35%) | 2 (33%) | 7 (35%) | 0.94 |
| Blood, n (%) | 8 (31%) | 3 (50%) | 5 (25%) | 0.26 |
| Sinus/orbit, n (%) | 4 (15%) | 0 (0%) | 4 (20%) | >0.99 |
| Cerebral, n (%) | 2 (8%) | 0 (0%) | 2 (10%) | >0.99 |
| Antifungal therapy | 24 (92%) | 6 (100%) | 18 (90%) | >0.99 |
| Surgery, n (%) | 5 (19%) | 2 (33%) | 3 (15%) | 0.33 |
| ICU LOS (days), median (IQR) | 17 (9–27) | 46 (15–51) | 14 (10–24) | 0.15 |
| Deceased in ICU, n (%) | 20 (77%) | 0 (0%) | 20 (100%) | >0.99 |
| Deceased at day-90, n (%) | 22 (85%) | 2 (33%) | 20 (100%) | >0.99 |
| WLST, n (%) | 9 (35%) | 2 (33%) | 7 (35%) | 0.94 |
| Total (n = 26) | Survivor (n = 6) | Deceased (n = 20) | p-Value | |
|---|---|---|---|---|
| Mucormycosis, n (%) | 13 (50%) | 3 (50%) | 10 (50%) | >0.99 |
| Rhizopus sp., n (%) | 9 (35%) | 1 (17%) | 8 (40%) | 0.31 |
| Lichtheimia corymbifera, n (%) | 2 (8%) | 2 (33%) | 0 (0%) | >0.99 |
| Rhizomucor pusillus, n (%) | 1 (4%) | 0 (0%) | 1 (5%) | 0.99 |
| Mucor circinelloides, n (%) | 1 (4%) | 0 (0%) | 1 (5%) | 0.99 |
| Other EIFIs, n (%) | 13 (50%) | 3 (50%) | 10 (50%) | >0.99 |
| Saprochaete clavata, n (%) | 1 (4%) | 1 (17%) | 1 (5%) | 0.59 |
| Saprochaete capitata, n (%) | 4 (19%) | 0 (0%) | 4 (20%) | 0.99 |
| Trichosporon asahii, n (%) | 1 (4%) | 0 (0%) | 1 (5%) | 0.99 |
| Fusarium sp., n (%) | 2 (8%) | 0 (0%) | 2 (10%) | >0.99 |
| Scedosporium apiospermum, n (%) | 2 (8%) | 0 (0%) | 2 (10%) | >0.99 |
| Chaetomium cymbiformis, n (%) | 1 (4%) | 1 (17%) | 0 (0%) | 0.99 |
| Saccharomyces cerevisiae, n (%) | 1 (4%) | 1 (17%) | 0 (0%) | 0.99 |
| Antifungal therapy | 24 (92%) | 6 (100%) | 18 (90%) | >0.99 |
| Adequate | 20 (77%) | 5 (83%) | 15 (75%) | 0.67 |
| Amphotericin B, n (%) | 18 (69%) | 3 (50%) | 15 (75%) | 0.26 |
| Echinocandin, n (%) | 6 (23%) | 1 (17%) | 5 (25%) | 0.67 |
| Fluconazole, n (%) | 1 (4%) | 1 (17%) | 0 (0%) | 0.99 |
| Voriconazole, n (%) | 2 (8%) | 0 (0%) | 2 (10%) | >0.99 |
| Isavuconazole, n (%) | 2 (8%) | 2 (33%) | 0 (0%) | >0.99 |
| Flucytosine, n (%) | 1 (4%) | 1 (17%) | 0 (0%) | 0.99 |
| Association, n (%) | 5 (19%) | 2 (33%) | 4 (20%) | 0.37 |
| Amphotericin B MIC 1 (mg/L) | Caspofungin MIC 1 (mg/L) | Fluconazole MIC 1 (mg/L) | Voriconazole MIC 1 (mg/L) | Posaconazole MIC 1 (mg/L) | Isavuconazole MIC 1 (mg/L) | Flucytosine MIC 1 (mg/L) | |
|---|---|---|---|---|---|---|---|
| S. clavata | 1–1.5 | 32 | 8 | 0.5 | 0.5 | 0.25 | |
| S. capitata | 0.5–1 | 32–32 | 0.19–24 | 0.004–0.19 | 0.016–0.38 | * | * |
| T. asahii | 2 | 32 | 0.75 | 0.023 | |||
| S. cerevisiae | * | 0.5 | 3 | * | * | * | * |
| EIFIs (n = 26) | Aspergillosis (n = 26) | p-Value | |
|---|---|---|---|
| Age (years), median (IQR) | 59 (48–69) | 57 (44–64) | 0.29 |
| Charlson index, median (IQR) | 3 (2–5) | 3 (2–4) | 0.39 |
| Malnutrition, n (%) | 21 (81%) | 20 (77%) | 0.74 |
| Hematological mal/cancer, n (%) | 15 (58%) | 16 (62%) | 0.78 |
| Diabetes, n (%) | 6 (23%) | 4 (15%) | 0.49 |
| CKD, n (%) | 4 (15%) | 4 (15%) | >0.99 |
| SAPS II, median (IQR) | 59 (49–76) | 51 (42–60) | 0.15 |
| SOFA at admission, median (IQR) | 10 (8–13) | 11 (8–13) | 0.56 |
| ARDS, n (%) | 16 (62%) | 16 (62%) | >0.99 |
| Deceased in ICU, n (%) | 20 (77%) | 21 (81%) | 0.74 |
| Deceased at day-90, n (%) | 22 (85%) | 23 (89%) | 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larcher, R.; Platon, L.; Amalric, M.; Brunot, V.; Besnard, N.; Benomar, R.; Daubin, D.; Ceballos, P.; Rispail, P.; Lachaud, L.; et al. Emerging Invasive Fungal Infections in Critically Ill Patients: Incidence, Outcomes and Prognosis Factors, a Case-Control Study. J. Fungi 2021, 7, 330. https://doi.org/10.3390/jof7050330
Larcher R, Platon L, Amalric M, Brunot V, Besnard N, Benomar R, Daubin D, Ceballos P, Rispail P, Lachaud L, et al. Emerging Invasive Fungal Infections in Critically Ill Patients: Incidence, Outcomes and Prognosis Factors, a Case-Control Study. Journal of Fungi. 2021; 7(5):330. https://doi.org/10.3390/jof7050330
Chicago/Turabian StyleLarcher, Romaric, Laura Platon, Matthieu Amalric, Vincent Brunot, Noemie Besnard, Racim Benomar, Delphine Daubin, Patrice Ceballos, Philippe Rispail, Laurence Lachaud, and et al. 2021. "Emerging Invasive Fungal Infections in Critically Ill Patients: Incidence, Outcomes and Prognosis Factors, a Case-Control Study" Journal of Fungi 7, no. 5: 330. https://doi.org/10.3390/jof7050330
APA StyleLarcher, R., Platon, L., Amalric, M., Brunot, V., Besnard, N., Benomar, R., Daubin, D., Ceballos, P., Rispail, P., Lachaud, L., Bourgeois, N., & Klouche, K. (2021). Emerging Invasive Fungal Infections in Critically Ill Patients: Incidence, Outcomes and Prognosis Factors, a Case-Control Study. Journal of Fungi, 7(5), 330. https://doi.org/10.3390/jof7050330







