A Pilot Clinical Study on Post-Operative Recurrence Provides Biological Clues for a Role of Candida Yeasts and Fluconazole in Crohn’s Disease
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient Population
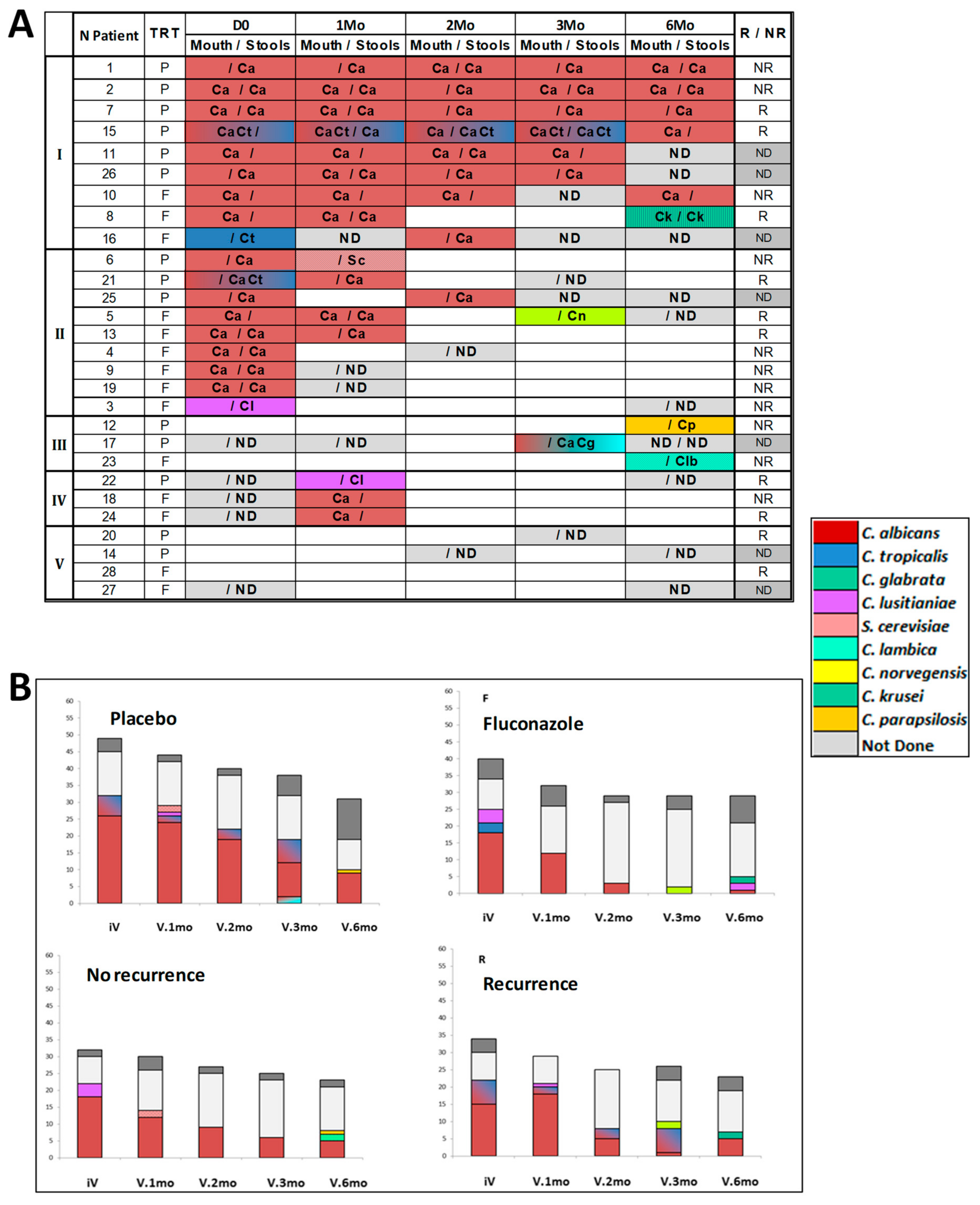
2.2. Mycological Analysis
2.3. Serological Analysis
2.3.1. Serum Markers of Inflammation
2.3.2. Serological Markers of IBD
2.3.3. Markers of Invasive Candidiasis
2.3.4. Innate Immunity Lectins Sensing C. albicans
2.4. Statistical Analysis
3. Results
3.1. Qualitative and Quantitative Evolution of Yeast Gut Colonization over the 6-Month Post-Operative Period
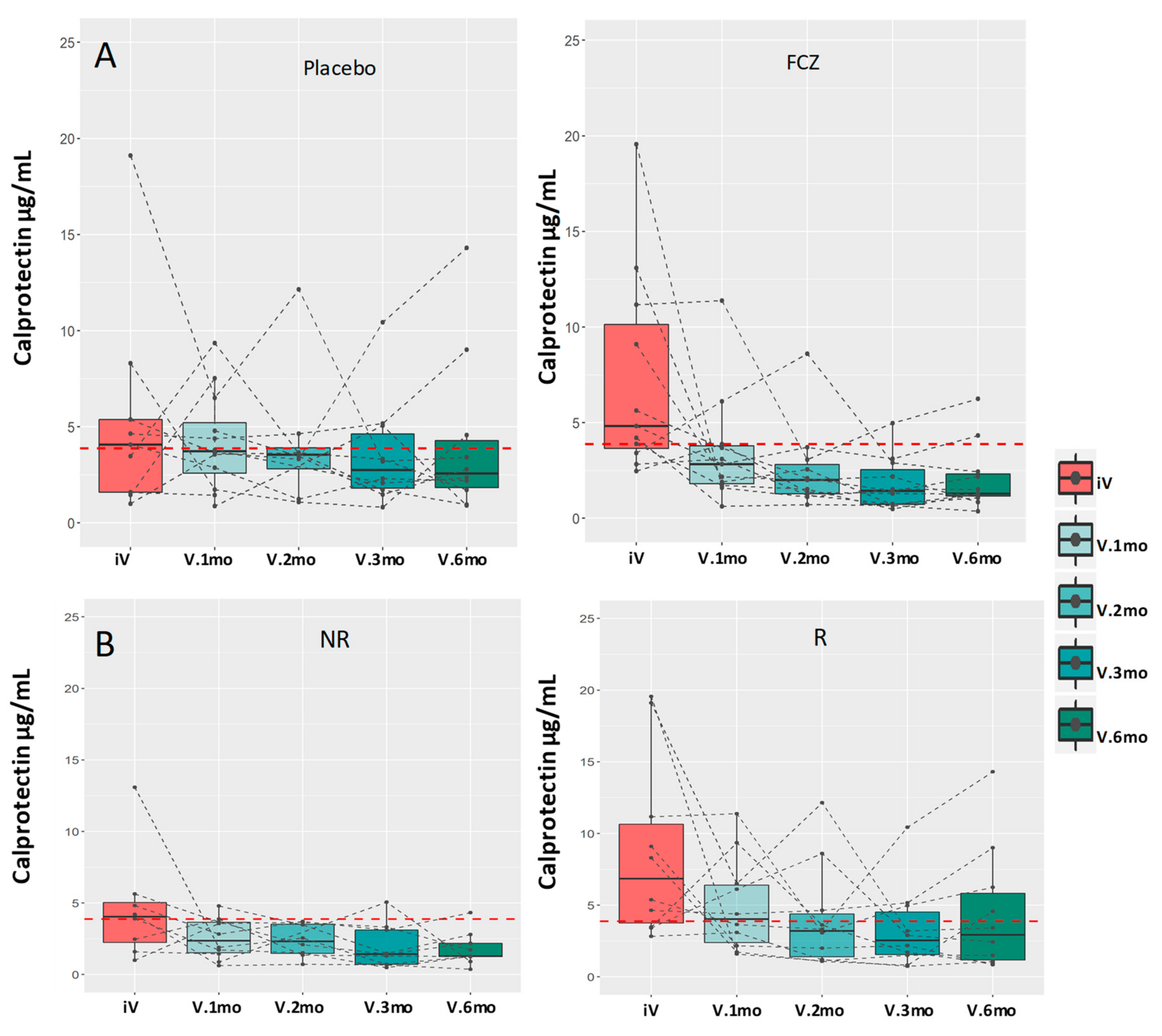
3.2. Evolution of C-Reactive Protein (CRP) and Serum Calprotectin Levels
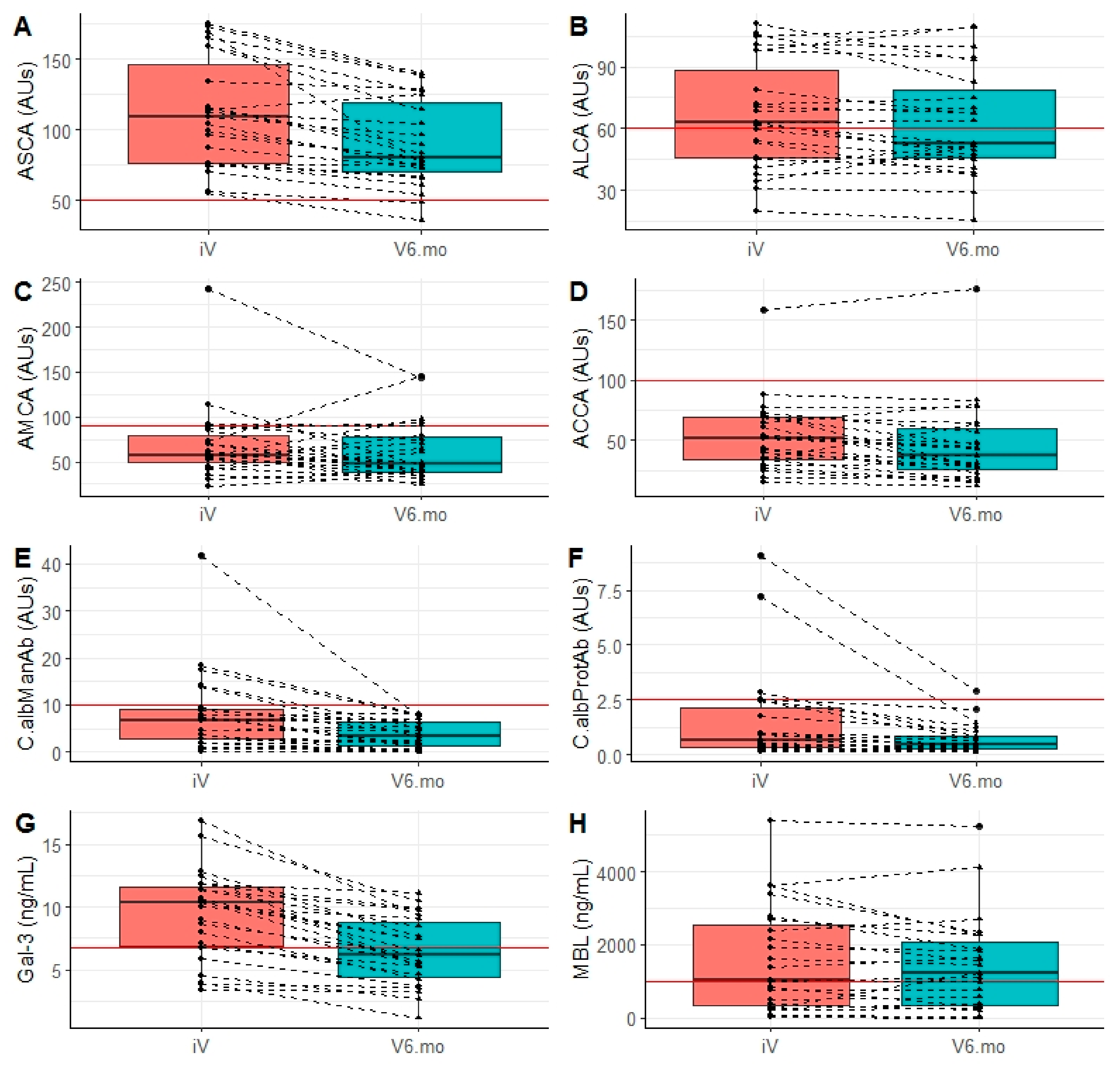
3.3. Evolution of Anti-Glycan Antibody Markers of CD
3.4. Evolution of Biomarkers of Invasive Candidiasis
3.5. Level and Evolution of Sensing of C. albicans by the Innate Immunity Lectins Gal-3 and MBL
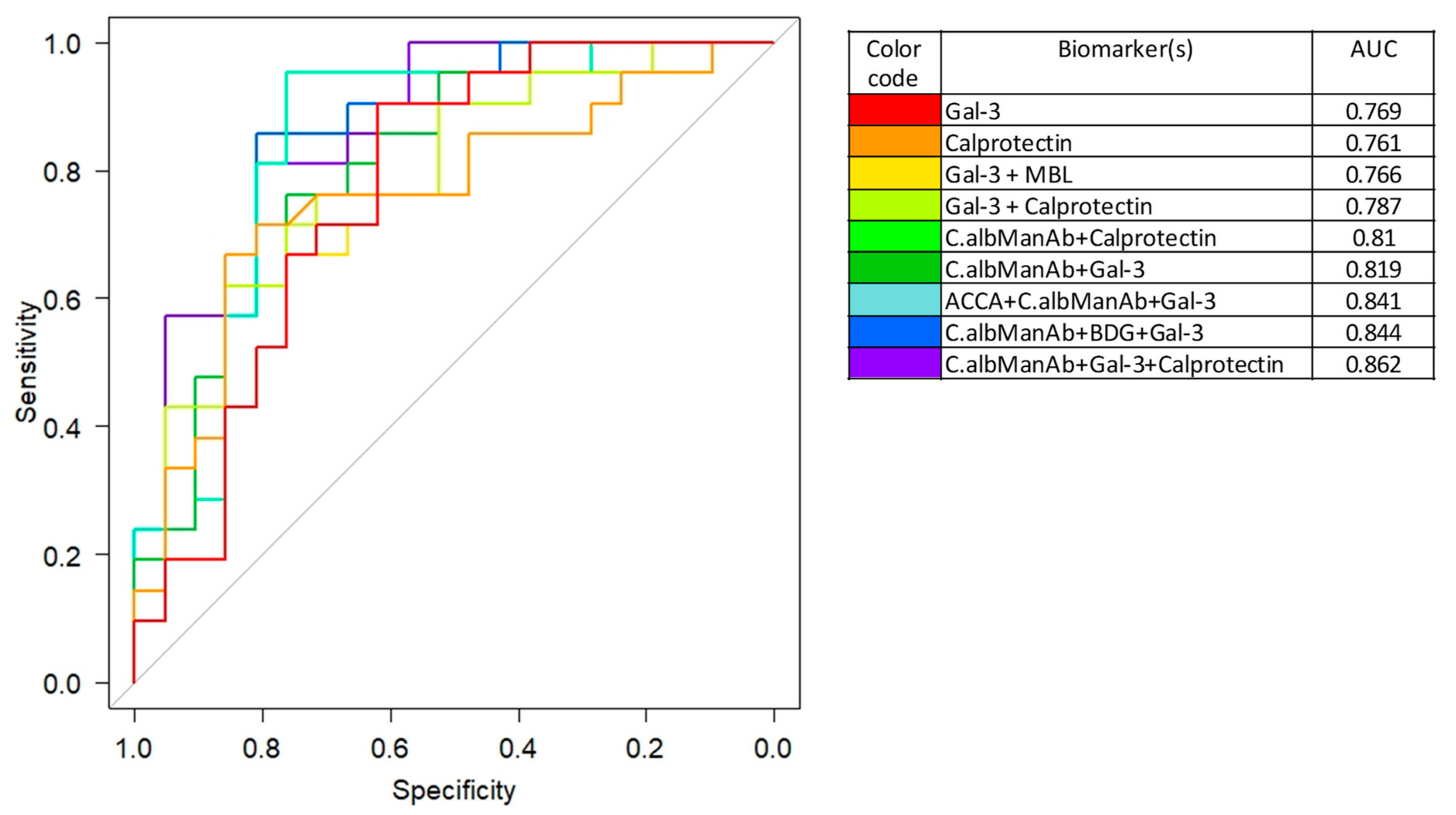
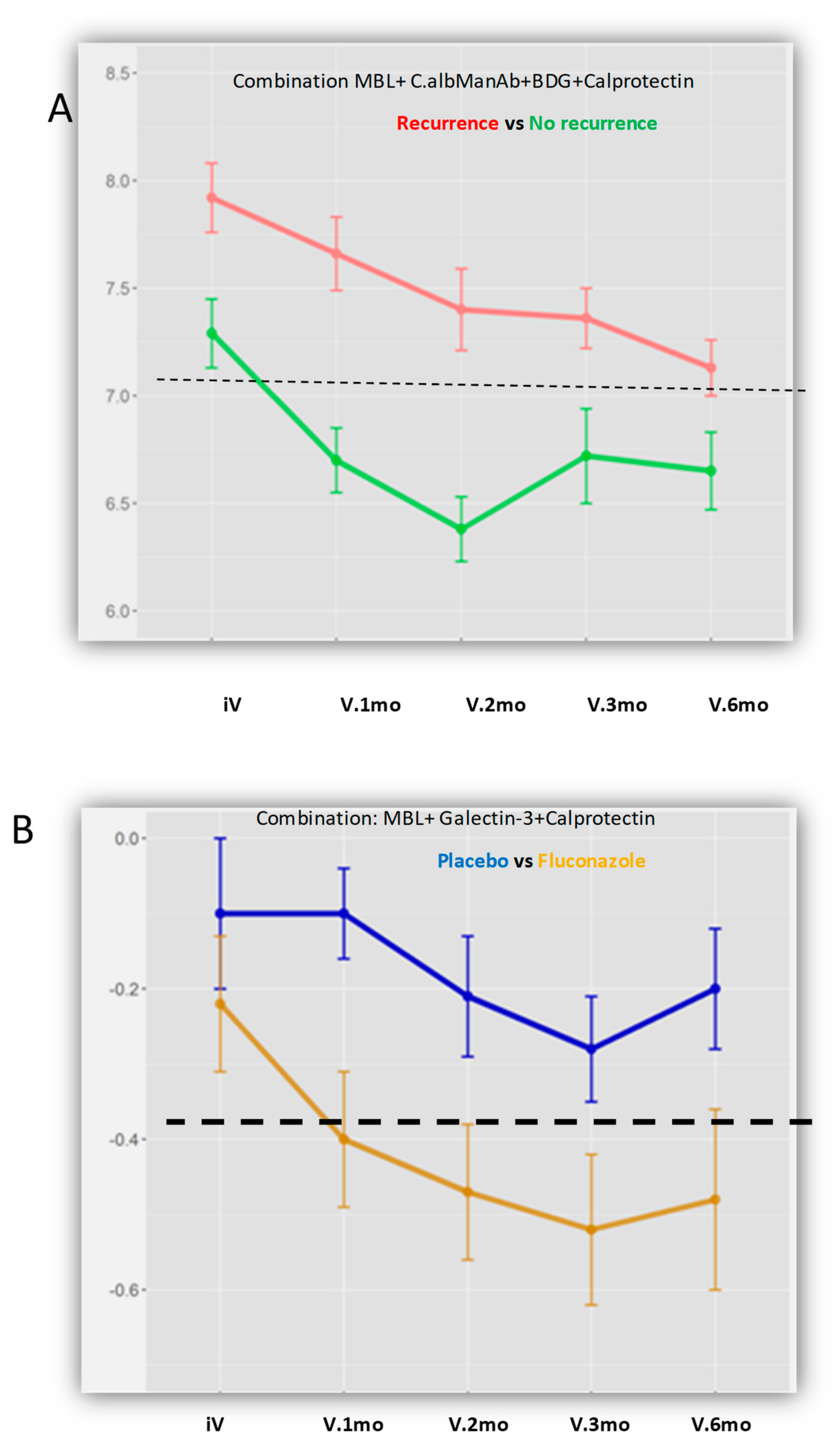
3.6. Integration of the Biomarkers Tested in Diagnostic and Prognostic Models
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pappas, P.G.; Kauffman, C.A.; Andes, D.; Benjamin, J.D.K.; Calandra, T.F.; Edwards, J.J.E.; Filler, S.G.; Fisher, J.F.; Kullberg, B.; Ostrosky-Zeichner, L.; et al. Clinical Practice Guidelines for the Management of Candidiasis: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 503–535. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef]
- Sendid, B.; Colombel, J.F.; Jacquinot, P.M.; Faille, C.; Fruit, J.; Cortot, A. Specific antibody response to oligomannosidic epitopes in Crohn’s disease. Clin. Diagn Lab. Immunol. 1996, 3, 219–226. [Google Scholar] [CrossRef]
- Nielsen, J. Yeast systems biology: Model organism and cell factory. Biotechnol. J. 2019, 14, e1800421. [Google Scholar] [CrossRef]
- Sendid, B.; Quinton, J.F.; Charrier, G.; Goulet, O.; Cortot, A.; Grandbastien, B. Anti-Saccharomyces cerevisiae mannan antibodies in familial Crohn’s disease. Am. J. Gastroenterol. 1998, 93, 1306–1310. [Google Scholar] [CrossRef]
- Quinton, J.-F.; Sendid, B.; Reumaux, D.; Duthilleul, P.; Cortot, A.; Grandbastien, B.; Charrier, G.; Targan, S.R.; Colombel, J.-F.; Poulain, D. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: Prevalence and diagnostic role. Gut 1998, 42, 788–791. [Google Scholar] [CrossRef]
- Poulain, D.; Sendid, B.; Fajardy, I.; Danze, P.M.; Colombel, J.F. Mother to child transmission of anti-Saccharomyces cerevisiae mannan antibodies (ASCA) in non-IBD families. Gut 2000, 47, 870–871. [Google Scholar] [CrossRef]
- Torres, J.; Petralia, F.; Sato, T.; Wang, P.; Telesco, S.E.; Choung, R.S.; Strauss, R.; Li, X.-J.; Laird, R.M.; Gutierrez, R.L.; et al. Serum Biomarkers Identify Patients Who Will Develop Inflammatory Bowel Diseases Up to 5 Years Before Diagnosis. Gastroenterology 2020, 159, 96–104. [Google Scholar] [CrossRef]
- Israeli, E.; Grotto, I.; Gilburd, B.; Balicer, R.D.; Goldin, E.; Wiik, A.; Shoenfeld, Y. Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease. Gut 2005, 54, 1232–1236. [Google Scholar] [CrossRef]
- Standaert-Vitse, A.; Sendid, B.; Joossens, M.; Francois, N.; Vandewalle-El Khoury, P.; Branche, J.; Van Kruiningen, H.; Jouault, T.; Rutgeerts, P.; Gower-Rousseau, C.; et al. Candida albicans colonization and ASCA in familial Crohn’s disease. Am. J. Gastroenterol. 2009, 104, 1745–1753. [Google Scholar] [CrossRef]
- Poulain, D. Candida albicans, plasticity and pathogenesis. Crit. Rev. Microbiol. 2013, 41, 208–217. [Google Scholar] [CrossRef]
- Dotan, I.; Fishman, S.; Dgani, Y.; Schwartz, M.; Karban, A.; Lerner, A.; Weishauss, O.; Spector, L.; Shtevi, A.; Altstock, R.T.; et al. Antibodies Against Laminaribioside and Chitobioside Are Novel Serologic Markers in Crohn’s Disease. Gastroenterology 2006, 131, 366–378. [Google Scholar] [CrossRef]
- Sendid, B.; Dotan, N.; Nseir, S.; Savaux, C.; Vandewalle, P.; Standaert, A.; Zerimech, F.; Guery, B.P.; Dukler, A.; Colombel, J.F.; et al. Antibodies against Glucan, Chitin, and Saccharomyces cerevisiae Mannan as New Biomarkers of Candida albicans Infection That Complement Tests Based on C. albicans Mannan. Clin. Vaccine Immunol. 2008, 15, 1868–1877. [Google Scholar] [CrossRef]
- Jawhara, S.; Thuru, X.; Standaert-Vitse, A.; Jouault, T.; Mordon, S.; Sendid, B.; Desreumaux, P.; Poulain, D. Colonization of Mice byCandida albicansIs Promoted by Chemically Induced Colitis and Augments Inflammatory Responses through Galectin-3. J. Infect. Dis. 2008, 197, 972–980. [Google Scholar] [CrossRef]
- Jouault, T.; El Abed-El Behi, M.; Martinez-Esparza, M.; Breuilh, L.; Trinel, P.A.; Chamaillard, M. Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J. Immunol. 2006, 177, 4679–4687. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.-A. The damage-response framework of microbial pathogenesis. Nat. Rev. Genet. 2003, 1, 17–24. [Google Scholar] [CrossRef]
- Wheeler, M.L.; Limon, J.J.; Bar, A.S.; Leal, C.A.; Gargus, M.; Tang, J.; Brown, J.; Funari, V.A.; Wang, H.L.; Crother, T.R.; et al. Immunological Consequences of Intestinal Fungal Dysbiosis. Cell Host. Microbe 2016, 19, 865–873. [Google Scholar] [CrossRef] [PubMed]
- A Kumamoto, C. Inflammation and gastrointestinal Candida colonization. Curr. Opin. Microbiol. 2011, 14, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Bacher, P.; Hohnstein, T.; Beerbaum, E.; Röcker, M.; Blango, M.G.; Kaufmann, S.; Röhmel, J.; Eschenhagen, P.; Grehn, C.; Seidel, K.; et al. Human Anti-fungal Th17 Immunity and Pathology Rely on Cross-Reactivity against Candida albicans. Cell 2019, 176, 1340–1355.e15. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; LeVan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Sendid, B.; Jouault, T.; Poulain, D. Secukinumab failure in Crohn’s disease: The yeast connection? Gut 2013, 62, 800–801. [Google Scholar] [CrossRef]
- Hooks, K.B.; O’Malley, M.A. Contrasting Strategies: Human Eukaryotic Versus Bacterial Microbiome Research. J. Eukaryot. Microbiol. 2020, 67, 279–295. [Google Scholar] [CrossRef]
- Tang, J.; Iliev, I.D.; Brown, J.; Underhill, D.M.; Funari, V.A. Mycobiome: Approaches to analysis of intestinal fungi. J. Immunol. Methods 2015, 421, 112–121. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Sendid, B.; Hoarau, G.; Colombel, J.-F.; Poulain, D.; Ghannoum, M.A. Mycobiota in gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Okada, S.; Kong, X.-F.; Kreins, A.Y.; Cypowyj, S.; Abhyankar, A.; Toubiana, J.; Itan, Y.; Audry, M.; Nitschke, P.; et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J. Exp. Med. 2011, 208, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.-P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal microbiota dysbiosis in IBD. Gut 2016, 66, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, G.; Mukherjee, P.K.; Gower-Rousseau, C.; Hager, C.; Chandra, J.; Retuerto, M.A.; Neut, C.; Vermeire, S.; Clemente, J.; Colombel, J.F.; et al. Bacteriome and Mycobiome Interactions Underscore Microbial Dysbiosis in Familial Crohn’s Disease. mBio 2016, 7, e01250-16. [Google Scholar] [CrossRef]
- Li, X.V.; Leonardi, I.; Iliev, I.D. Gut Mycobiota in Immunity and Inflammatory Disease. Immunity 2019, 50, 1365–1379. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Shen, S.; Hou, Y.; Chen, Y.; Wang, T. The mycobiota of the human body: A spark can start a prairie fire. Gut Microbes 2020, 11, 655–679. [Google Scholar] [CrossRef]
- Marr, K.A.; Seidel, K.; Slavin, M.A.; Bowden, R.A.; Schoch, H.G.; Flowers, M.E. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: Long-term follow-up of a randomized, placebo-controlled trial. Blood 2000, 96, 2055–2061. [Google Scholar] [CrossRef] [PubMed]
- Lille University Hospital; France Ministry of Health. Effect of Fluconazole on the Levels of Anti-Saccharomyces cerevisiae Antibodies (ASCA) After Surgical Resection for Crohn’s Disease. Multicenter, Randomized, and Controlled in Two Parallel Groups Versus Placebo; ClinicalTrialsgov ID: NCT02997059; France Ministry of Health: Paris, France, 2015.
- Sendid, B.; Ducoroy, P.; François, N.; Lucchi, G.; Spinali, S.; Vagner, O.; Damiens, S.; Bonnin, A.; Poulain, D.; Dalle, F. Evaluation of MALDI-TOF mass spectrometry for the identification of medically-important yeasts in the clinical laboratories of Dijon and Lille hospitals. Med Mycol. 2013, 51, 25–32. [Google Scholar] [CrossRef]
- Meuwis, M.A.; Vernier-Massouille, G.; Grimaud, J.C.; Bouhnik, Y.; Laharie, D.; Piver, E. Serum calprotectin as a biomarker for Crohn’s disease. J. Crohns. Colitis 2013, 7, e678–e683. [Google Scholar] [CrossRef]
- Sendid, B.; Tabouret, M.; Poirot, J.L.; Mathieu, D.; Fruit, J.; Poulain, D. New Enzyme Immunoassays for Sensitive Detection of CirculatingCandida albicans Mannan and Antimannan Antibodies: Useful Combined Test for Diagnosis of Systemic Candidiasis. J. Clin. Microbiol. 1999, 37, 1510–1517. [Google Scholar] [CrossRef]
- Laín, A.; Elguezabal, N.; Brena, S.; García-Ruiz, J.C.; Del Palacio, A.; Moragues, M.-D.; Pontón, J. Diagnosis of invasive candidiasis by enzyme-linked immunosorbent assay using the N-terminal fragment of Candida albicans hyphal wall protein 1. BMC Microbiol. 2007, 7, 35. [Google Scholar] [CrossRef]
- Poissy, J.; Sendid, B.; Damiens, S.; Ishibashi, K.I.; François, N.; Kauv, M.; Favory, R.; Mathieu, D.; Poulain, D. Presence of Candida cell wall derived polysaccharides in the sera of intensive care unit patients: Relation with candidaemia and Candida colonisation. Crit. Care 2014, 18, R135. [Google Scholar] [CrossRef]
- Chiba, M.; Mikami, K.; Iizuka, M.; Yukawa, M.; Watanabe, S.; Takazoe, M.; Fukushima, T.; Koganei, K.; Kishibe, T. Elevated plasma (1-->3)-beta-D-glucan, a fungal cell wall constituent, in a subgroup of Crohn disease. Scand. J. Gastroenterol. 2001, 36, 447–448. [Google Scholar]
- Guo, Y.; Zhou, G.; He, C.; Yang, W.; He, Z.; Liu, Z. Serum Levels of Lipopolysaccharide and 1,3-β-D-Glucan Refer to the Severity in Patients with Crohn’s Disease. Mediators Inflamm. 2015, 2015, 843089. [Google Scholar] [CrossRef]
- Fradin, C.; Poulain, D.; Jouault, T. β-1,2-Linked Oligomannosides from Candida albicans Bind to a 32-Kilodalton Macrophage Membrane Protein Homologous to the Mammalian Lectin Galectin-3. Infect. Immun. 2000, 68, 4391–4398. [Google Scholar] [CrossRef] [PubMed]
- Oever, J.T.; Giamarellos-Bourboulis, E.J.; Veerdonk, F.L.; Stelma, F.F.; Simon, A.; Janssen, M.; Johnson, M.; Pachot, A.; Kullberg, B.-J.; Joosten, L.A.B.; et al. Circulating galectin-3 in infections and non-infectious inflammatory diseases. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Damiens, S.; Poissy, J.; Francois, N.; Salleron, J.; Jawhara, S.; Jouault, T.; Poulain, D.; Sendid, B. Mannose-Binding Lectin Levels and Variation During Invasive Candidiasis. J. Clin. Immunol. 2012, 32, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Thiel, S.; Gadjeva, M. Humoral Pattern Recognition Molecules: Mannan-Binding Lectin and Ficolins. Adv. Exp. Med. Biol. 2009, 653, 58–73. [Google Scholar] [CrossRef]
- Cury, D.B.; Mizsputen, S.J.; Versolato, C.; Miiji, L.O.; Pereira, E.; Delboni, M.A.; Schor, N.; Moss, A.C. Serum calprotectin levels correlate with biochemical and histological markers of disease activity in TNBS colitis. Cell. Immunol. 2013, 282, 66–70. [Google Scholar] [CrossRef]
- Kawashima, K.; Ishihara, S.; Yoshiyuki, M.; Fukuba, N.; Oshima, N.; Kazumori, H.; Sonoyama, H.; Yamashita, N.; Tada, Y.; Kusunoki, R.; et al. Fecal calprotectin level correlated with both endoscopic severity and disease extent in ulcerative colitis. BMC Gastroenterol. 2016, 16, 1–6. [Google Scholar] [CrossRef]
- Gauss, A.; Geiss, T.; Hinz, U.; Schaefert, R.; Zwickel, P.; Zawierucha, A.; Stremmel, W.; Klute, L. Quality of Life Is Related to Fecal Calprotectin Concentrations in Colonic Crohn Disease and Ulcerative Colitis, but not in Ileal Crohn Disease. Medicine 2016, 95, e3477. [Google Scholar] [CrossRef]
- Iliev, I.D.; Funari, V.A.; Taylor, K.D.; Nguyen, Q.; Reyes, C.N.; Strom, S.P.; Brown, J.; Becker, C.A.; Fleshner, P.R.; Dubinsky, M.; et al. Interactions Between Commensal Fungi and the C-Type Lectin Receptor Dectin-1 Influence Colitis. Sciences 2012, 336, 1314–1317. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Schleder, S.; Wolf, A.; Dirmeier, A.; Strauch, U.; Obermeier, F. Association of the novel serologic anti-glycan antibodies anti-laminarin and anti-chitin with complicated Crohn’s disease behavior. Inflamm. Bowel Dis. 2010, 16, 263–274. [Google Scholar] [CrossRef]
- Moeller, J.B.; Leonardi, I.; Schlosser, A.; Flamar, A.L.; Bessman, N.J.; Putzel, G.G. Modulation of the fungal mycobiome is regulated by the chitin-binding receptor FIBCD1. J. Exp. Med. 2019, 216, 2689–2700. [Google Scholar] [CrossRef] [PubMed]
- Standaert–Vitse, A.; Jouault, T.; Vandewalle, P.; Mille, C.; Seddik, M.; Sendid, B.; Mallet, J.; Colombel, J.; Poulain, D. Candida albicans Is an Immunogen for Anti–Saccharomyces cerevisiae Antibody Markers of Crohn’s Disease. Gastroenterology 2006, 130, 1764–1775. [Google Scholar] [CrossRef]
- Staab, J.F.; Bradway, S.D.; Fidel, P.L.; Sundstrom, P. Adhesive and Mammalian Transglutaminase Substrate Properties of Candida albicans Hwp1. Sciences 1999, 283, 1535–1538. [Google Scholar] [CrossRef] [PubMed]
- Underhill, D.M.; Iliev, I.D. The mycobiota: Interactions between commensal fungi and the host immune system. Nat. Rev. Immunol. 2014, 14, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Esteban, A.; Popp, M.W.; Vyas, V.K.; Strijbis, K.; Ploegh, H.L.; Fink, G.R. Fungal recognition is mediated by the association of dectin-1 and galectin-3 in macrophages. Proc. Natl. Acad. Sci. USA 2011, 108, 14270–14275. [Google Scholar] [CrossRef]
- Frol’Ová, L.; Smetana, K.; Borovská, D.; Kitanovicova, A.; Klimešová, K.; Janatková, I.; Malickova, K.; Lukáš, M.; Drastich, P.; Beneš, Z.; et al. Detection of galectin-3 in patients with inflammatory bowel diseases: New serum marker of active forms of IBD? Inflamm. Res. 2009, 58, 503–512. [Google Scholar] [CrossRef]
- Choteau, L.; Parny, M.; François, N.; Bertin, B.; Fumery, M.; Dubuquoy, L.; Takahashi, K.; Colombel, J.-F.; Jouault, T.; Poulain, D.; et al. Role of mannose-binding lectin in intestinal homeostasis and fungal elimination. Mucosal Immunol. 2015, 9, 767–776. [Google Scholar] [CrossRef]
- Choteau, L.; Vasseur, F.; Lepretre, F.; Figeac, M.; Gower-Rousseau, C.; Dubuquoy, L.; Poulain, D.; Colombel, J.-F.; Sendid, B.; Jawhara, S. Polymorphisms in the Mannose-Binding Lectin Gene are Associated with Defective Mannose-Binding Lectin Functional Activity in Crohn’s Disease Patients. Sci. Rep. 2016, 6, 29636. [Google Scholar] [CrossRef]
- Müller, S.; Schaffer, T.; Flogerzi, B.; Seibold-Schmid, B.; Schnider, J.; Takahashi, K.; Darfeuille-Michaud, A.; Vazeille, E.; Schoepfer, A.M.; Seibold, F. Mannan-binding lectin deficiency results in unusual antibody production and excessive experimental colitis in response to mannose-expressing mild gut pathogens. Gut 2010, 59, 1493–1500. [Google Scholar] [CrossRef]
- Schoepfer, A.M.; Flogerzi, B.; Seibold-Schmid, B.; Schaffer, T.; Kun, J.F.; Pittet, V. Low Mannan-binding lectin serum levels are associated with complicated Crohn’s disease and reactivity to oligomannan (ASCA). Am. J. Gastroenterol 2009, 104, 2508–2516. [Google Scholar] [CrossRef]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B Statistical Methodol. 1964, 26, 211–243. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate—A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B-Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Su, J.Q.; Liu, J.S. Linear Combinations of Multiple Diagnostic Markers. J. Am. Stat. Assoc. 1993, 88, 1350–1355. [Google Scholar] [CrossRef]
- Chiaro, T.R.; Soto, R.; Stephens, W.Z.; Kubinak, J.L.; Petersen, C.; Gogokhia, L.; Bell, R.; Delgado, J.C.; Cox, J.; Voth, W.; et al. A member of the gut mycobiota modulates host purine metabolism exacerbating colitis in mice. Sci. Transl. Med. 2017, 9, eaaf9044. [Google Scholar] [CrossRef] [PubMed]
- Sendid, B.; Jawhara, S.; Sarter, H.; Maboudou, P.; Thierny, C.; Gower-Rousseau, C.; Colombel, J.F.; Poulain, D. Uric acid levels are independent of anti-Saccharomyces cerevisiae antibodies (ASCA) in Crohn’s disease: A reappraisal of the role of S. cerevisiae in this setting. Virulence 2018, 9, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Sendid, B.; Cotteau, A.; François, N.; D’Haveloose, A.; Standaert, A.; Camus, D.; Poulain, D. Candidaemia and antifungal therapy in a French University Hospital: Rough trends over a decade and possible links. BMC Infect. Dis. 2006, 6, 80. [Google Scholar] [CrossRef]
- Sutherland, A.D.; Gearry, R.B.; Frizelle, F.A. Review of Fecal Biomarkers in Inflammatory Bowel Disease. Dis. Colon Rectum 2008, 51, 1283–1291. [Google Scholar] [CrossRef]
- Ferrante, M.; Henckaerts, L.; Joossens, M.; Pierik, M.; Dotan, N.; Norman, G.L.; Altstock, R.T.; Van Steen, K.; Rutgeerts, P.; Van Assche, G.; et al. New serological markers in inflammatory bowel disease are associated with complicated disease behaviour. Gut 2007, 56, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Lopez, R.; Franke, A.; Wolf, A.; Schleder, S.; Dirmeier, A. Characterization of changes in serum anti-glycan antibodies in Crohn’s disease--a longitudinal analysis. PLoS ONE 2011, 6, e18172. [Google Scholar] [CrossRef]
- Duarte-Silva, M.; Afonso, P.C.; De Souza, P.R.; Peghini, B.C.; Rodrigues-Júnior, V.; Cardoso, C.R.D.B. Reappraisal of antibodies against Saccharomyces cerevisiae (ASCA) as persistent biomarkers in quiescent Crohn’s disease. Autoimmunity 2019, 52, 37–47. [Google Scholar] [CrossRef]
- Sendid, B.; Vermeire, S.; Cassagnou, M.; Joossens, S.; Collet, F.; Quandalle, P.; Poulain, D.; Peeters, M.; Rutgeerts, P.; Colombel, J.-F. Surgery is associated with fluctuations in ASCA levels suggesting antigenic stimulation. Gastroenterology 2000, 118, A1369. [Google Scholar] [CrossRef]
- Damiens, S.; Ftadin, C.; Poulain, D.; Sendid, B.; Tabouret, M. Method for Diagnosing Invasive Candida Infections. U.S. Patent 9,778,259, 3 October 2017. [Google Scholar]
- Papa Gobbi, R.; De Francesco, N.; Bondar, C.; Muglia, C.; Chirdo, F.; Rumbo, M. A galectin-specific signature in the gut delineates Crohn’s disease and ulcerative colitis from other human inflammatory intestinal disorders. Biofactors 2016, 42, 93–105. [Google Scholar]
- Lippert, E.; Falk, W.; Bataille, F.; Kaehne, T.; Naumann, M.; Goeke, M.; Herfarth, H.; Schoelmerich, J.; Rogler, G. Soluble galectin-3 is a strong, colonic epithelial-cell-derived, lamina propria fibroblast-stimulating factor. Gut 2007, 56, 43–51. [Google Scholar] [CrossRef]
- Lippert, E.; Gunckel, M.; Brenmoehl, J.; Bataille, F.; Falk, W.; Scholmerich, J.; Obermeier, F.; Rogler, G. Regulation of galectin-3 function in mucosal fibroblasts: Potential role in mucosal inflammation. Clin. Exp. Immunol. 2008, 152, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.F.; Chen, H.-Y.; Wan, L.; Wu, S.-Y.; Yu, J.-S.; Huang, A.C.; Miaw, S.-C.; Hsu, D.K.; Wu-Hsieh, B.A.; Liu, F.-T. Galectin-3 Modulates Th17 Responses by Regulating Dendritic Cell Cytokines. Am. J. Pathol. 2013, 183, 1209–1222. [Google Scholar] [CrossRef]
- Brouwer, N.; Dolman, K.M.; Van Houdt, M.; Sta, M.; Roos, D.; Kuijpers, T.W. Mannose-binding lectin (MBL) facilitates opsonophagocytosis of yeasts but not of bacteria despite MBL binding. J. Immunol. 2008, 180, 4124–4132. [Google Scholar] [CrossRef]
- Lee, S.J.; González-Aseguinolaza, G.; Nussenzweig, M.C. Disseminated Candidiasis and Hepatic Malarial Infection in Mannose-Binding-Lectin-A-Deficient Mice. Mol. Cell. Biol. 2002, 22, 8199–8203. [Google Scholar] [CrossRef] [PubMed]
- Van Till, J.W.O.; Modderman, P.W.; De Boer, M.; Hart, M.H.L.; Beld, M.G.H.M.; Boermeester, M.A. Mannose-Binding Lectin Deficiency Facilitates Abdominal Candida Infections in Patients with Secondary Peritonitis. Clin. Vaccine Immunol. 2007, 15, 65–70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Der Velden, W.J.; Plantinga, T.S.; Feuth, T.; Donnelly, J.P.; Netea, M.G.; Blijlevens, N.M. The incidence of acute graft-versus-host disease increases with Candida colonization depending the dectin-1 gene status. Clin. Immunol. 2010, 136, 302–306. [Google Scholar] [CrossRef] [PubMed]
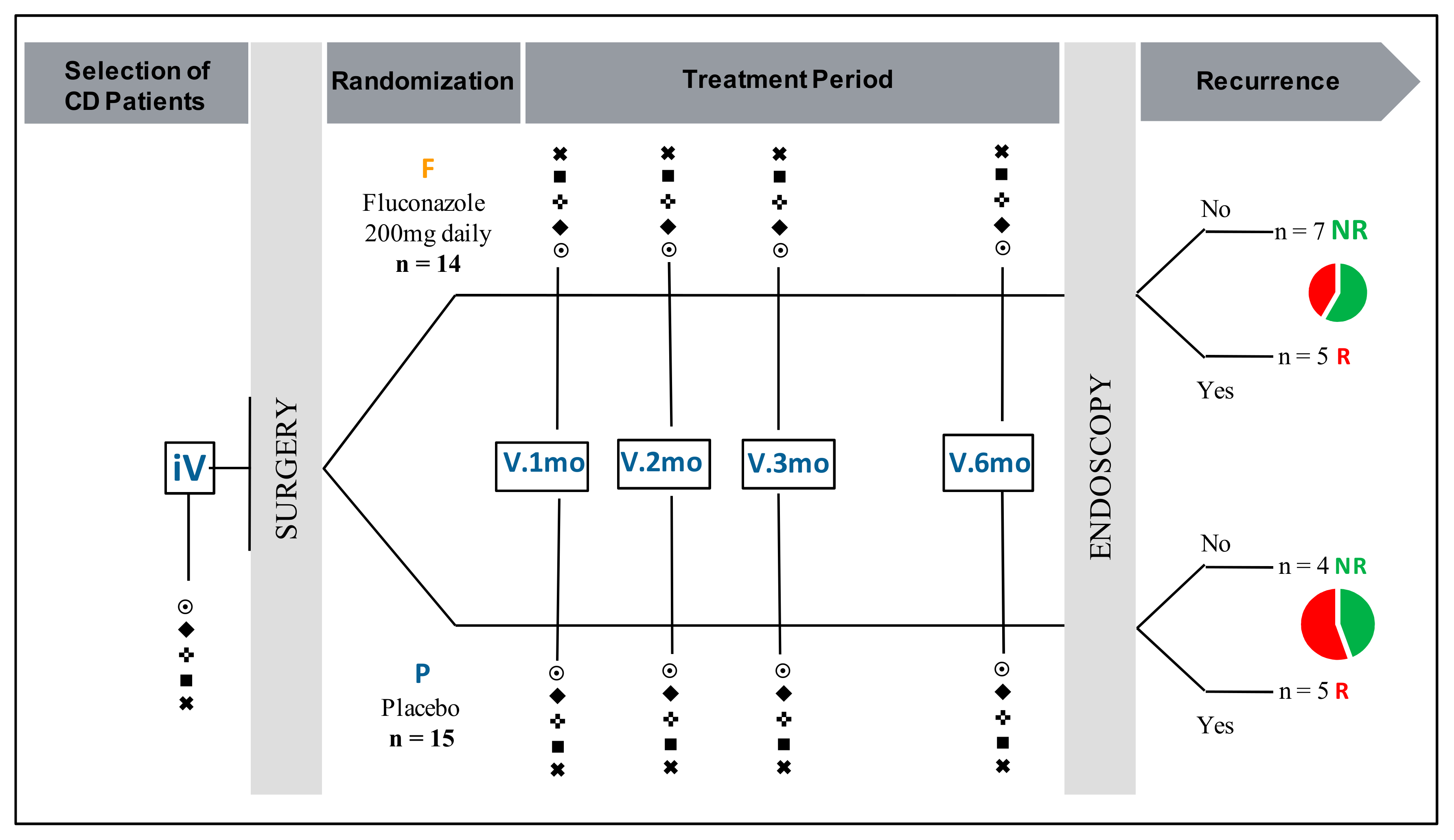
| FCZ Group (n = 14) | Placebo Group (n = 15) | |
|---|---|---|
| Sex (male) | 5 (35.7) | 4 (26.7) |
| Age at CD diagnosis (years) | 20.3 (19.4–21.8) | 23.4 (20.5–31.7) |
| Age at inclusion | 26.7 (22.8–34.2) | 29.5 (23.6–40.8) |
| Disease duration (years) | 4.9 (1.9–14.1) | 3.9 (0.5–9.1) |
| Disease type | ||
| Penetrating | 4 (28.6) | 6 (40.0) |
| Stenosing | 9 (64.3) | 9 (60.0) |
| Unclassified | 1 (7.1) | 0 (0.0) |
| Previous surgery | 0 | 0 |
| ASCA titer at inclusion | 86.5 (F70.3–114.8] | 108.4 (78.4–134.3) |
| CDAI at inclusion | 135 (86–217) | 104 (68–127) |
| Type of Serum Biomarker Analyzed/Abbreviation | General Features | Molecular Characteristics | Rationale for Longitudinal Analysis in Relation with Crohn’s Disease Evolution | Rationale for Longitudinal Analysis in Relation with Candida Colonization/Infection |
|---|---|---|---|---|
| Markers of inflammation | ||||
| Calprotectin | Described as biomarker of systemic inflammation [33,43]. Detection of feacal calprotectin in stools is recommended for IBD diagnosis and treatment follow up [44,45] | Protein from PMNs (S100 A8/A9 Family) | High levels suggested as being informative for diagnosis and disease outcome [33,43,44] | None current indication for the diagnosis of Candidiasis. |
| Serum Antibodies | ||||
| ASCA | Anti-Saccharomyces cerevisiae antibodies | Anti-oligomannose antibodies (Epitope present in S. cerevisiae and C. albicans mannan) Epitope with Man α-1,3 linked at the non reducing end of tetra or trimannose α-1,2 linked [3]. | The most potent marker of Crohn’s disease in terms of prevalence and prediction of onset [8,9] | Generated during a C. albicans invasive infection [13] In CD patients healthy relatives their presence correlates with C. albicans carriage [10]. |
| ALCA | Anti-Laminaribioside Carbohydrate Antibodies | Disaccharide sequence of α-1,3 glucans(Epitope present in S. cerevisiae and C. albicans glucans) | Adjunct of ASCA for CD diagnosis [12] | Generated during a C. albicans invasive infection [13] Antibodies targeting molecules interacting with Dectin-1, a major receptor of C. albicans innate immunity triggering IL17 pathway [46] |
| ACCA | Anti-Chitobioside Carbohydrate Antibodies | Disaccharide sequence of chitin (Epitope present in S. cerevisiae and C. albicans chitin) | Adjunct of ASCA for CD diagnosis [12,47] | Generated during a C. albicans invasive infection [13] The target of these antibodies (chitin) binding to FIBCD1 dampen Intestinal inflammation [48] |
| Platelia test® C.albManAb | Anti-Candida albicans mannan Antibodies | Detection of human polyclonal antibody response to mannan oligomannose repertoire | Levels increased [10,49] | Marker of C. albicans invasive infection used for diagnosis [13,34] |
| Anti-Hwp1 Antibodies/C.albProtAb | Anti-Hyphal Protein 1 antibody Hwp1 is a molecule specific for C. albicans hyphal (invasive) form [50] | Peptidic epitope [35] | Never explored in this setting | Marker of C. albicans invasive infection used for diagnosis [35] |
| Fungitel Assay(TM) /BDG | Circulating β-1,3 D Glucan [36] | Polymer of β-1,3 D glucan Recognized by Dectin-1 activating the Th17 pathway [29,51] | Described as circulating during CD [37] | Widely used for the diagnosis of systemic candidiasis [36] |
| Innate Immunity Lectins | ||||
| Galectin-3/Gal-3 | C-Lectin member of Galactose binding lectins Expressed on cells of the macrophage lineage [39]. Expressed on intestinal cells. Associates with dectin-1 for Fungal recognition [52] associates with TLR2 [15]. | Receptor for C. albicans β-1,2 mannoses, immunomodulatory adhesins, expressed on C. albicans mannan mannoproteins and glycolipids [14,15] | Increased during inflammatory diseases [40,53] | Increased during Candida infection [40] |
| Mannose Binding Lectin MBL | Produced by hepatic and intestinal cells [54] Combines with MASP for activating complement lectin pathway | Reacts with α-D-mannose N-acetyl-D Glucosamine L-Fucose | Role in Crohn’s disease: Impaired MBL-MASP functional activity in CD [55] Levels inversely correlates with ASCA [56,57] | Role in mucosal and systemic Candidiasis, participates to clearance of circulating C. albicans mannan in patient sera [41,42,54] |
| Biological Parameter | Visit | All Patients (n = 23) | p iV vs. V6.mo |
|---|---|---|---|
| Calprotectin | iV | 4.2 (3.3–8.7) | 0.002 |
| V6.mo | 2.2 (1.2–3.4) | ||
| ASCA | iV | 109.2 (76.7–159.0) | <0.0001 |
| V6.mo | 80.6 (67.4–125.1) | ||
| ALCA | iV | 62.6 (45.3–97.7) | 0.113 |
| V6.mo | 53.0 (45.0–82.8) | ||
| ACCA | iV | 51.6 (34.3–69.7) | 0.04 |
| V6.mo | 37.6 (23.2–63.0) | ||
| AMCA | iV | 57.3 (45.1–85.0) | 0.327 |
| V6.mo | 47.4 (38.9–79.8) | ||
| C.albManAb | iV | 6.8 (2.7–9.5) | <0.0001 |
| V6.mo | 3.3 (1.0–6.3) | ||
| C.albProtAb | iV | 0.6 (0.3–2.4) | <0.0001 |
| V6.mo | 0.4 (0.3–0.8) | ||
| Galectin-3 | iV | 10.3 (6.7–11.8) | <0.0001 |
| V6.mo | 6.2 (4.2–9.1) | ||
| MBL | iV | 1032.4 (271.5–2697.2) | 0.283 |
| V6.mo | 1216.0 (304.9–2271.4) |
| No Recurrence | Recurrence | Total | p(χ2) | |
|---|---|---|---|---|
| Gal-3 + MBL + Calprotectin | ||||
| Response to treatment | ||||
| FCZ (+) | 27 | 12 | 39 | |
| Placebo (−) | 14 | 22 | 36 | |
| Total | 41 | 34 | 75 | 0.007 |
| No response to treatment | ||||
| FCZ (−) | 7 | 13 | 20 | |
| Placebo (+) | 4 | 3 | 7 | |
| Total | 11 | 16 | 27 | |
| Overall total | 52 | 50 | 102 | |
| ACCA + CaManAb + MBL + Calprotectin | ||||
| Response to treatment | ||||
| FCZ (+) | 24 | 9 | 33 | |
| Placebo (−) | 14 | 23 | 37 | |
| Total | 38 | 32 | 70 | 0.016 |
| No response to treatment | ||||
| FCZ (−) | 10 | 16 | 26 | |
| Placebo (+) | 4 | 2 | 6 | |
| Total | 14 | 18 | 32 | |
| Overall total | 52 | 50 | 102 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sendid, B.; Salvetat, N.; Sarter, H.; Loridant, S.; Cunisse, C.; François, N.; Aijjou, R.; Gelé, P.; Leroy, J.; Deplanque, D.; et al. A Pilot Clinical Study on Post-Operative Recurrence Provides Biological Clues for a Role of Candida Yeasts and Fluconazole in Crohn’s Disease. J. Fungi 2021, 7, 324. https://doi.org/10.3390/jof7050324
Sendid B, Salvetat N, Sarter H, Loridant S, Cunisse C, François N, Aijjou R, Gelé P, Leroy J, Deplanque D, et al. A Pilot Clinical Study on Post-Operative Recurrence Provides Biological Clues for a Role of Candida Yeasts and Fluconazole in Crohn’s Disease. Journal of Fungi. 2021; 7(5):324. https://doi.org/10.3390/jof7050324
Chicago/Turabian StyleSendid, Boualem, Nicolas Salvetat, Helène Sarter, Severine Loridant, Catherine Cunisse, Nadine François, Rachid Aijjou, Patrick Gelé, Jordan Leroy, Dominique Deplanque, and et al. 2021. "A Pilot Clinical Study on Post-Operative Recurrence Provides Biological Clues for a Role of Candida Yeasts and Fluconazole in Crohn’s Disease" Journal of Fungi 7, no. 5: 324. https://doi.org/10.3390/jof7050324
APA StyleSendid, B., Salvetat, N., Sarter, H., Loridant, S., Cunisse, C., François, N., Aijjou, R., Gelé, P., Leroy, J., Deplanque, D., Jawhara, S., Weissmann, D., Desreumaux, P., Gower-Rousseau, C., Colombel, J. F., & Poulain, D. (2021). A Pilot Clinical Study on Post-Operative Recurrence Provides Biological Clues for a Role of Candida Yeasts and Fluconazole in Crohn’s Disease. Journal of Fungi, 7(5), 324. https://doi.org/10.3390/jof7050324







