Comprehensive Assessment of Ameliorative Effects of AMF in Alleviating Abiotic Stress in Tomato Plants
Abstract
1. Introduction
2. Arbuscular Mycorrhizal Fungi
3. AMF-Mediated Growth and Yield Enhancement in Tomato
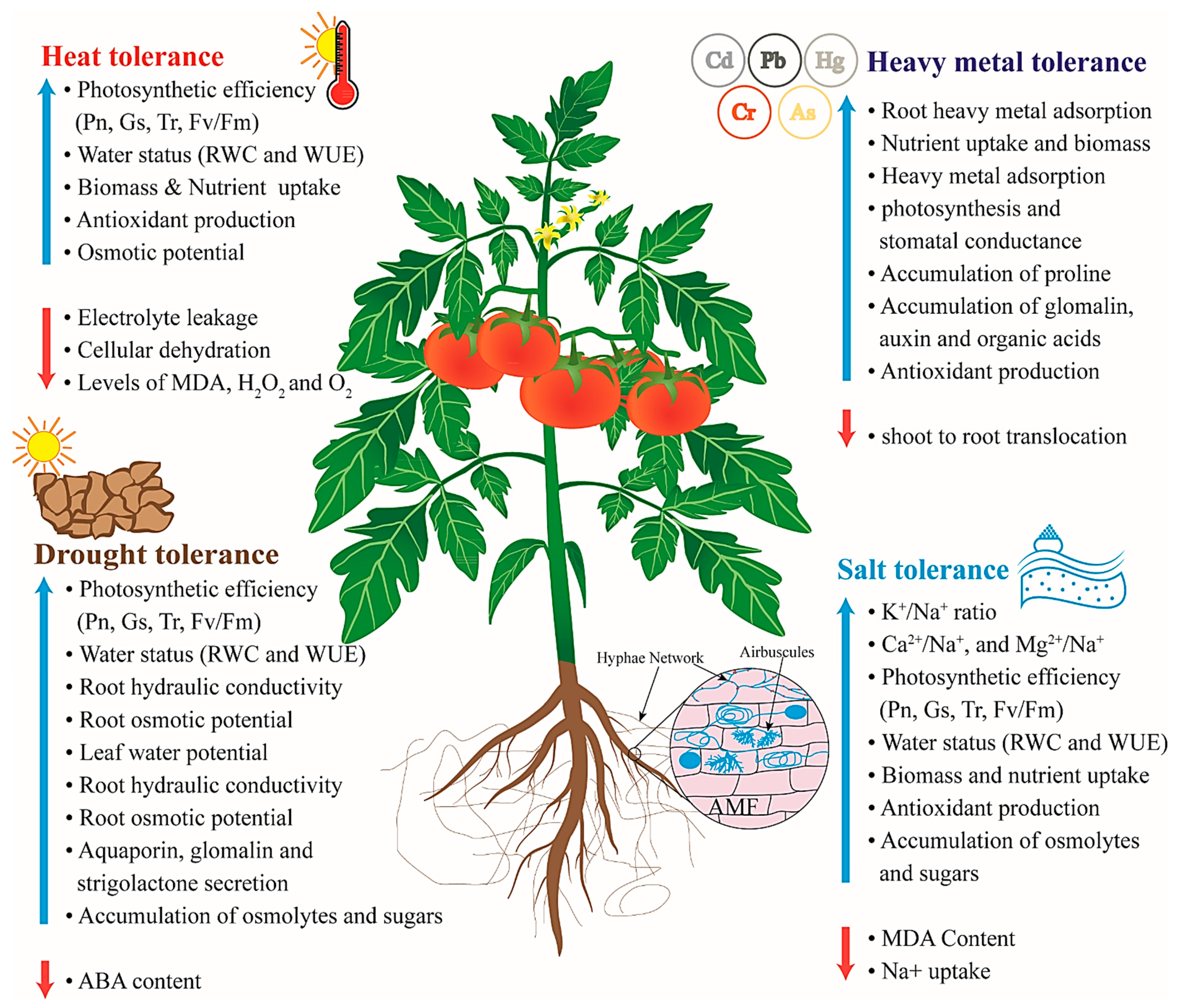
4. Drought Stress
4.1. Plant Growth and Nutrient Uptake
4.2. Photosynthesis and Water Status
4.3. Modification of Hormonal Balance
5. Salt Stress
5.1. Tomato Growth and Nutrient Uptake
5.2. K+/Na+ Ratio
5.3. Photosynthesis and Water Status
5.4. Antioxidant Enzymes
6. Temperature Stress
7. Heavy Metal Stress
8. Concluding Remarks and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Hernández-Jover, T.; Martín-Belloso, O. Carotenoid and phenolic profile of tomato juices processed by high intensity pulsed electric fields compared with conventional thermal treatments. Food Chem. 2009, 112, 258–266. [Google Scholar] [CrossRef]
- Agarwal, S.; Rao, A.V. Tomato lycopene and its role in human health and chronic diseases. Can. Med. Assoc. J. 2000, 163, 739–744. [Google Scholar]
- Rao, A.V.; Agarwal, S. Role of Antioxidant Lycopene in Cancer and Heart Disease. J. Am. Coll. Nutr. 2000, 19, 563–569. [Google Scholar] [CrossRef]
- Toor, R.K.; Lister, C.; Savage, G. Antioxidant activities of New Zealand-grown tomatoes. Int. J. Food Sci. Nutr. 2005, 56, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Schüβler, A.; Schwarzott, D.; Walker, C. A new fungal phylum, the Glomeromycota: Phylogeny and evolution. Mycol. Res. 2001, 105, 1413–1421. [Google Scholar] [CrossRef]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [PubMed]
- Redecker, D.; Schüssler, A.; Stockinger, H.; Stürmer, S.L.; Morton, J.B.; Walker, C. An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 2013, 23, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Genet. 2008, 6, 763–775. [Google Scholar] [CrossRef]
- Bonfante, P.; Genre, A. Plants and arbuscular mycorrhizal fungi: An evolutionary-developmental perspective. Trends Plant Sci. 2008, 13, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Bago, B.; Pfeffer, P.E.; Shachar-Hill, Y. Carbon Metabolism and Transport in Arbuscular Mycorrhizas. Plant Physiol. 2000, 124, 949–958. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, W.; Xie, Q.; Liu, N.; Liu, L.; Wang, D.; Zhang, X.; Yang, C.; Chen, X.; Tang, D.; et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Gianinazzi, S.; Gollotte, A.; Binet, M.N.; van Tuinen, D.; Redecker, D.; Wipf, D. Agroecology: The key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 2010, 20, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient use efficiency in plants. Commun. Soil Sci. Plant Anal. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- Giovannetti, M.; Avio, L.; Barale, R.; Ceccarelli, N.; Cristofani, R.; Iezzi, A.; Mignolli, F.; Picciarelli, P.; Pinto, B.; Reali, D.; et al. Nutraceutical value and safety of tomato fruits produced by mycorrhizal plants. Br. J. Nutr. 2011, 107, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Conversa, G.; Lazzizera, C.; Bonasia, A.; Elia, A. Yield and phosphorus uptake of a processing tomato crop grown at different phosphorus levels in a calcareous soil as affected by mycorrhizal inoculation under field conditions. Biol. Fertil. Soils 2013, 49, 691–703. [Google Scholar] [CrossRef]
- Poulton, J.L.; Bryla, D.; Koide, R.T.; Stephenson, A.G. Mycorrhizal infection and high soil phosphorus improve vegetative growth and the female and male functions in tomato. New Phytol. 2002, 154, 255–264. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J. Sci. Food Agric. 2015, 95, 1706–1715. [Google Scholar] [CrossRef]
- Kowalska, I.; Konieczny, A.; Gąstoł, M.; Sady, W.; Hanus-Fajerska, E. Effect of mycorrhiza and phosphorus content in nutrient solution on the yield and nutritional status of tomato plants grown on rockwool or coconut coir. Agric. Food Sci. 2015, 24, 39–51. [Google Scholar] [CrossRef]
- Hart, M.; Ehret, D.L.; Krumbein, A.; Leung, C.; Murch, S.; Turi, C.; Franken, P. Inoculation with arbuscular mycorrhizal fungi improves the nutritional value of tomatoes. Mycorrhiza 2015, 25, 359–376. [Google Scholar] [CrossRef]
- Ortas, I. The effect of mycorrhizal fungal inoculation on plant yield, nutrient uptake and inoculation effectiveness under long-term field conditions. Field Crop. Res. 2012, 125, 35–48. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J.; Ogaya, R. Drought’s impact on Ca, Fe, Mg, Mo and S concentration and accumulation patterns in the plants and soil of a Mediterranean evergreen Quercus ilex forest. Biogeochemistry 2007, 87, 49–69. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Li, W. Arbuscular mycorrhizal fungi infection in desert riparian forest and its environmental im-plications: A case study in the lower reach of Tarim River. Prog. Nat. Sci. 2008, 18, 983–991. [Google Scholar] [CrossRef]
- Aroca, R. (Ed.) Plant Responses to Drought Stress: From Morphological to Molecular Features; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Augé, R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Augé, R.M. Arbuscular mycorrhizae and soil/plant water relations. Can. J. Soil Sci. 2004, 84, 373–381. [Google Scholar] [CrossRef]
- Porcel, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation and oxidative stress in soybean plants subjected to drought stress. J. Exp. Bot. 2004, 55, 1743–1750. [Google Scholar] [CrossRef]
- Duc, N.H.; Csintalan, Z.; Posta, K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol. Biochem. 2018, 132, 297–307. [Google Scholar] [CrossRef]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the Impact of Arbuscular Mycorrhizal Symbiosis On Tomato Tolerance to Water Stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Nuruddin, M.M.; Madramootoo, C.A.; Dodds, G.T. Effects of Water Stress at Different Growth Stages on Greenhouse Tomato Yield and Quality. HortScience 2003, 38, 1389–1393. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Moreno, D.A.; Ferreres, F.; Rubio-Wilhelmi, M.D.M.; Ruiz, J.M. Differential responses of five cherry tomato varieties to water stress: Changes on phenolic metabolites and related enzymes. Phytochemistry 2011, 72, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Giannakoula, A.; Ilias, I. The effect of water stress and salinity on growth and physiology of tomato (Lycopersicon esculentum Mil.). Arch. Biol. Sci. 2013, 65, 611–620. [Google Scholar] [CrossRef]
- Sacco, A.; Greco, B.; Di Matteo, A.; De Stefano, R.; Barone, A. Evaluation of Tomato Genetic Resources for Response to Water Deficit. Am. J. Plant Sci. 2013, 4, 131–145. [Google Scholar] [CrossRef]
- Dell’Amico, J.; Torrecillas, A.; Rodríguez, P.; Morte, A.; Sánchez-Blanco, M.J. Responses of tomato plants associated with the arbuscular mycorrhizal fungus Glomus clarum during drought and recovery. J. Agric. Sci. 2002, 138, 387–393. [Google Scholar] [CrossRef]
- Subramanian, K.S.; Santhanakrishnan, P.; Balasubramanian, P. Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Sci. Hortic. Amst. 2006, 107, 245–253. [Google Scholar] [CrossRef]
- Aroca, R.; Del Mar Alguacil, M.; Vernieri, P.; Ruiz-Lozano, J.M. Plant responses to drought stress and exogenous ABA application are modulated differently by mycorrhization in tomato and an ABA-deficient mutant (Sitiens). Microb. Ecol. 2008, 56, 704–719. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yao, Z.; Zhao, S.; Guo, K.; Sun, J.; Zhang, J. Arbuscular mycorrhizal fungal application to improve growth and tolerance of processing tomato (Lycopersicon esculentum Miller) under drought stress. J. Food Agric. Environ. 2014, 12, 452–457. [Google Scholar]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, Á.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, J.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Jordan, D.; McDonald, G.A. Effect of phosphate-solubilizing bacteria and vesicular-arbuscular mycorrhizae on tomato growth and soil microbial activity. Biol. Fertil. Soils 1997, 26, 79–87. [Google Scholar] [CrossRef]
- Rivero, J.; Gamir, J.; Aroca, R.; Pozo, M.J.; Flors, V. Metabolic transition in mycorrhizal tomato roots. Front. Microbiol. 2015, 6, 598. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, T.; Wu, Z.; Feng, H.; Yu, M.; Zhang, X.; Chen, B. Arbuscular mycorrhiza enhances drought tolerance of tomato plants by regulating the 14-3-3 genes in the ABA signaling pathway. Appl. Soil Ecol. 2018, 125, 213–221. [Google Scholar] [CrossRef]
- Rivero, J.; Álvarez, D.; Flors, V.; Azcón-Aguilar, C.; Pozo, M.J. Root metabolic plasticity underlies functional diversity in mycorrhiza-enhanced stress tolerance in tomato. New Phytol. 2018, 220, 1322–1336. [Google Scholar] [CrossRef]
- Mannino, G.; Nerva, L.; Gritli, T.; Novero, M.; Fiorilli, V.; Bacem, M.; Bertea, C.M.; Lumini, E.; Chitarra, W.; Balestrini, R. Effects of Different Microbial Inocula on Tomato Tolerance to Water Deficit. Agronomy 2020, 10, 170. [Google Scholar] [CrossRef]
- Volpe, V.; Chitarra, W.; Cascone, P.; Volpe, M.G.; Bartolini, P.; Moneti, G.; Pieraccini, G.; Di Serio, C.; Maserti, B.; Guerrieri, E.; et al. The Association With Two Different Arbuscular Mycorrhizal Fungi Differently Affects Water Stress Tolerance in Tomato. Front. Plant Sci. 2018, 9, 1480. [Google Scholar] [CrossRef]
- Berruti, A.; Borriello, R.; Della Beffa, M.T.; Scariot, V.; Bianciotto, V. Application of nonspecific commercial AMF inocula results in poor mycorrhization in Camellia japonica L. Symbiosis 2013, 61, 63–76. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular Mycorrhizal Fungi as Natural Biofertilizers: Let’s Benefit from Past Successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia 2012, 104, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bucher, M. Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol. 2006, 173, 11–26. [Google Scholar] [CrossRef]
- Javot, H.; Penmetsa, R.V.; Terzaghi, N.; Cook, D.R.; Harrison, M.J. A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2007, 104, 1720–1725. [Google Scholar] [CrossRef]
- Smith, F.A.; Smith, S.E. What is the significance of the arbuscular mycorrhizal colonisation of many economically important crop plants? Plant Soil 2011, 348, 63–79. [Google Scholar] [CrossRef]
- Chen, A.; Chen, X.; Wang, H.; Liao, D.; Gu, M.; Qu, H. Genome-wide investigation and expression analysis suggest diverse roles and genetic redundancy of Pht1 family genes in response to Pi deficiency in tomato. BMC Plant Biol. 2014, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Nagy, R.; Drissner, D.; Amrhein, N.; Jakobsen, I.; Bucher, M. Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated. New Phytol. 2009, 181, 950–959. [Google Scholar] [CrossRef]
- Nagy, R.; Karandashov, V.; Chague, V.; Kalinkevich, K.; Tamasloukht, M.; Xu, G.; Jakobsen, I.; Levy, A.A.; Amrhein, N.; Bucher, M. The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species. Plant J. 2005, 42, 236–250. [Google Scholar] [CrossRef]
- Balestrini, R.; Gómez-Ariza, J.; Lanfranco, L.; Bonfante, P. Laser Microdissection Reveals That Transcripts for Five Plant and One Fungal Phosphate Transporter Genes Are Contemporaneously Present in Arbusculated Cells. Mol. Plant-Microbe Interact. 2007, 20, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, R.; Rosso, L.C.; Veronico, P.; Melillo, M.T.; De Luca, F.; Fanelli, E.; Colagiero, M.; Di Fossalunga, A.S.; Ciancio, A.; Pentimone, I. Transcriptomic Responses to Water Deficit and Nematode Infection in Mycorrhizal Tomato Roots. Front. Microbiol. 2019, 10, 1807. [Google Scholar] [CrossRef] [PubMed]
- Handa, Y.; Nishide, H.; Takeda, N.; Suzuki, Y.; Kawaguchi, M.; Saito, K. RNA-seq transcriptional profiling of an arbuscular mycorrhiza provides insights into regulated and coordinated gene expression in Lotus japonicus and Rhizophagus irregularis. Plant Cell Physiol. 2015, 8, 1490–1511. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xing, X.-J.; Tian, Y.-S.; Peng, R.-H.; Xue, Y.; Zhao, W.; Yao, Q.-H. Transgenic Arabidopsis Plants Expressing Tomato Glutathione S-Transferase Showed Enhanced Resistance to Salt and Drought Stress. PLoS ONE 2015, 10, e0136960. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 2003, 13, 309–317. [Google Scholar] [CrossRef]
- Pedranzani, H.E.; Rodríguez-Rivera, M.; Gutierrez, M.I.; Porcel, R.; Hause, B.; Ruizlozano, J.M. Arbuscular mycorrhizal symbiosis regulates physiology and performance of Digitaria eriantha plants subjected to abiotic stresses by modulating antioxidant and jasmonate levels. Mycorrhiza 2016, 26, 141–152. [Google Scholar] [CrossRef]
- Davies, F.T., Jr.; Svenson, S.E.; Cole, J.C.; Phavaphutanon, L.; Duray, S.A.; Olalde-Portugal, V.; Meier, C.E.; Bo, S.H. Non-nutritional stress acclimation of mycorrhizal woody plants exposed to drought. Tree Physiol. 1996, 16, 985–993. [Google Scholar] [CrossRef]
- Rodríguez, P.; Dell’Amico, J.; Morales, D.; Blanco, M.J.S.; Alarcón, J.J. Effects of salinity on growth, shoot water relations and root hydraulic conductivity in tomato plants. J. Agric. Sci. 1997, 128, 439–444. [Google Scholar] [CrossRef]
- Bárzana, G.; Aroca, R.; Bienert, G.P.; Chaumont, F.; Ruiz-Lozano, J.M. New Insights into the Regulation of Aquaporins by the Arbuscular Mycorrhizal Symbiosis in Maize Plants under Drought Stress and Possible Implications for Plant Performance. Mol. Plant-Microbe Interact. 2014, 27, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, G.; Erice, G.; Aroca, R.; Chaumont, F.; Ruiz-Lozano, J.M. Enhanced drought stress tolerance by the arbuscular mycorrhizal symbiosis in a drought-sensitive maize cultivar is related to a broader and differential regulation of host plant aquaporins than in a drought-tolerant cultivar. Front. Plant Sci. 2017, 8, 1056. [Google Scholar] [CrossRef]
- Herrera-Medina, M.J.; Steinkellner, S.; Vierheilig, H.; Bote, J.A.O.; Garrido, J.M.G. Abscisic acid determines arbuscule development and functionality in the tomato arbuscular mycorrhiza. New Phytol. 2007, 175, 554–564. [Google Scholar] [CrossRef]
- Li, W.; Wang, T.; Zhang, Y.; Li, Y. Overexpression of soybean miR172c confers tolerance to water deficit and salt stress, but increases ABA sensitivity in transgenic Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 175–194. [Google Scholar] [CrossRef]
- Thompson, A.J.; Jackson, A.C.; Symonds, R.C.; Mulholland, B.J.; Dadswell, A.R.; Blake, P.S.; Burbidge, A.; Taylor, I.B. Ectopic expression of a tomato 9-cis-epoxycarotenoid dioxygenase gene causes over-production of abscisic acid. Plant J. 2000, 23, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Martín-Rodríguez, J.A.; León-Morcillo, R.; Vierheilig, H.; Ocampo Bote, J.A.; Ludwig-Müllerm, J.; García-Garrido, J.M. Mycorrhization of the notabilis and sitiens tomato mutants in relation to abscisic acid and ethylene contents. J. Plant Physiol. 2010, 167, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Bucher, M.; Hause, B.; Krajinski, F.; Küster, H. Through the doors of perception to function in arbuscular mycorrhizal symbioses. New Phytol. 2014, 204, 833–840. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.-H.; Lee, S.C. Function of ABA in Stomatal Defense against Biotic and Drought Stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, H.; Saeid-Sar, S.; Afshari, H.; Abdel-Wahhab, M.A. Tolerance of mycorrhiza infected Pistachio (Pistacia vera L.) seedlings to drought stress under glasshouse conditions. J. Plant Physiol. 2012, 169, 704–709. [Google Scholar] [CrossRef]
- Yooyongwech, S.; Phaukinsang, N.; Cha-Um, S.; Supaibulwatana, K. Arbuscular mycorrhiza improved growth performance in Macadamia tetraphylla L. grown under water deficit stress involves soluble sugar and proline accumulation. Plant Growth Regul. 2012, 69, 285–293. [Google Scholar] [CrossRef]
- Zou, Y.N.; Wu, Q.S.; Huang, Y.M.; Ni, Q.D.; He, X.H. Mycorrhizal-mediated lower proline accumulation in Poncirus trifoliate under water deficit derives from the integration of inhibition of proline synthesis with increase of proline degradation. PLoS ONE 2013, 8, e80568. [Google Scholar] [CrossRef]
- Maurel, C.; Boursiac, Y.; Luu, D.-T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in Plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef]
- Reuscher, S.; Akiyama, M.; Mori, C.; Aoki, K.; Shibata, D.; Shiratake, K. Genome-Wide Identification and Expression Analysis of Aquaporins in Tomato. PLoS ONE 2013, 8, e79052. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops–what is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Yurtseven, E.; Kesmez, G.D.; Unlukara, A. The effects of water salinity and potassium levels on yield, fruit quality and water consumption of a native central Anatolian tomato species (Lycopersicon Esculentum). Agric. Water Manag. 2005, 78, 128–135. [Google Scholar] [CrossRef]
- He, Z.; He, C.; Zhang, Z.; Zou, Z.; Wang, H. Changes of antioxidative enzymes and cell membrane osmosis in tomato colonized by arbuscular mycorrhizae under NaCl stress. Colloids Surf. B Biointerfaces 2007, 59, 128–133. [Google Scholar] [CrossRef]
- Tanveer, K.; Gilani, S.; Hussain, Z.; Ishaq, R.; Adeel, M.; Ilyas, N. Effect of salt stress on tomato plant and the role of calcium. J. Plant Nutr. 2019, 43, 28–35. [Google Scholar] [CrossRef]
- Hajiboland, R.; Aliasgharzadeh, N.; Laiegh, S.F.; Poschenrieder, C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 2010, 331, 313–327. [Google Scholar] [CrossRef]
- Santander, C.; Sanhueza, M.; Olave, J.; Borie, F.; Valentine, A.; Cornejo, P. Arbuscular Mycorrhizal Colonization Promotes the Tolerance to Salt Stress in Lettuce Plants through an Efficient Modification of Ionic Balance. J. Soil Sci. Plant Nutr. 2019, 19, 321–331. [Google Scholar] [CrossRef]
- Juniper, S.; Abbott, L. Vesicular-arbuscular mycorrhizas and soil salinity. Mycorrhiza 1993, 4, 45–57. [Google Scholar] [CrossRef]
- Ebrahim, M.K.; Saleem, A.-R. Alleviating salt stress in tomato inoculated with mycorrhizae: Photosynthetic performance and enzymatic antioxidants. J. Taibah Univ. Sci. 2017, 11, 850–860. [Google Scholar] [CrossRef]
- Al-Karaki, G.N. Growth of mycorrhizal tomato and mineral acquisition under salt stress. Mycorrhiza 2000, 10, 51–54. [Google Scholar] [CrossRef]
- Al-Karaki, G.N.; Hammad, R.; Rusan, M. Response of two tomato cultivars differing in salt tolerance to inoculation with mycorrhizal fungi under salt stress. Mycorrhiza 2001, 11, 43–47. [Google Scholar] [CrossRef]
- Sellitto, V.M.; Golubkina, N.A.; Pietrantonio, L.; Cozzolino, E.; Cuciniello, A.; Cenvinzo, V.; Florin, I.; Caruso, G. Tomato Yield, Quality, Mineral Composition and Antioxidants as Affected by Beneficial Microorganisms Under Soil Salinity Induced by Balanced Nutrient Solutions. Agriculture 2019, 9, 110. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Chaoxing, H. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci. Hortic. 2011, 127, 228–233. [Google Scholar] [CrossRef]
- Balliu, A.; Sallaku, G.; Rewald, B. AMF Inoculation Enhances Growth and Improves the Nutrient Uptake Rates of Transplanted, Salt-Stressed Tomato Seedlings. Sustainability 2015, 7, 15967–15981. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Sonmez, O.; Aydemir, S.; Tuna, L.A.; Cullu, A.M. The influence of arbuscular mycorrhizal colonisation on key growth parameters and fruit yield of pepper plants grown at high salinity. Sci. Hort. 2009, 121, 1–6. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Azcón, R. Symbiotic efficiency and infectivity of an autochthonous arbuscular mycorrhizal Glomus sp. from saline soils and Glomus deserticola under salinity. Mycorrhiza 2000, 10, 137–143. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Cantrell, I.C.; Linderman, R.G. Preinoculation of lettuce and onion with VA mycorrhizal fungi reduces deleterious effects of soil salinity. Plant Soil 2001, 233, 269–281. [Google Scholar] [CrossRef]
- Feng, G.; Li, X.L.; Zhang, F.S.; Li, S.X. Effect of phosphorus and arbuscular mycorrhizal fungus on response of maize plant to saline environment. J. Plant Res. Environ. 2000, 9, 22–26. [Google Scholar]
- Sannazzaro, A.I.; Echeverria, M.; Alberto, E.O.; Ruiz, O.A.; Menendez, A.B. Modulation of polyamine balance in Lotus glaber by salinity and arbuscular mycorrhiza. Plant Physiol. Biochem. 2007, 45, 39–46. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef]
- Evelin, H.; Giri, B.; Kapoor, R. Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed Trigonella foenum-graecum. Mycorrhiza 2012, 22, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Gong, X.; Zhang, X.; Zhang, W.; Sun, J.; Chen, B. Effects of arbuscular mycorrhizal fungi on photosynthesis, ion balance of tomato plants under saline-alkali soil condition. J. Plant Nutr. 2020, 43, 682–698. [Google Scholar] [CrossRef]
- Dasgan, H.; Aktas, H.; Abak, K.; Cakmak, I. Determination of screening techniques to salinity tolerance in tomatoes and investigation of genotype responses. Plant Sci. 2002, 163, 695–703. [Google Scholar] [CrossRef]
- Khalloufi, M.; Martínez-Andújar, C.; Lachaâl, M.; Karray-Bouraoui, N.; Pérez-Alfocea, F.; Albacete, A. The interaction between foliar GA3 application and arbuscular mycorrhizal fungi inoculation improves growth in salinized tomato (Solanum lycopersicum L.) plants by modifying the hormonal balance. J. Plant Physiol. 2017, 214, 134–144. [Google Scholar] [CrossRef]
- Augã, R.M.; Toler, H.D.; Saxton, A.M.; Augé, R.M. Arbuscular mycorrhizal symbiosis and osmotic adjustment in response to NaCl stress: A meta-analysis. Front. Plant Sci. 2014, 5, 562. [Google Scholar] [CrossRef]
- Ouziad, F.; Wilde, P.; Schmelzer, E.; Hildebrandt, U.; Bothe, H. Analysis of expression of aquaporins and Na+/H+ transporters in tomato colonized by arbuscular mycorrhizal fungi and affected by salt stress. Environ. Exp. Bot. 2006, 57, 177–186. [Google Scholar] [CrossRef]
- Birhane, E.; Sterck, F.J.; Fetene, M.; Bongers, F.; Kuyper, T.W. Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia 2012, 169, 895–904. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Chanratana, M.; Kim, K.; Seshadri, S.; Sa, T. Impact of Arbuscular Mycorrhizal Fungi on Photosynthesis, Water Status, and Gas Exchange of Plants Under Salt Stress–A Meta-Analysis. Front. Plant Sci. 2019, 10, 457. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Y.; Sun, S.; Mu, C.; Yan, X. Effects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci. Total Environ. 2017, 576, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Riemann, M.; Dhakarey, R.; Hazman, M.; Miro, B.; Kohli, A.; Nick, P. Exploring jasmonates in the hormonal network of drought and salinity responses. Front. Plant Sci. 2015, 6, 1077. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ren, Y.; Zhang, Y.; Xu, J.; Sun, F.; Zhang, Z.; Wang, Y. Genome-wide identification and expression analysis of heat-responsive and novel microRNAs in Populus tomentosa. Gene 2012, 504, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Aroca, R.; Vernieri, P.; Irigoyen, J.J.; Sancher-Diaz, M.; Tognoni, F.; Pardosso, A. Involvement of abscisic acid in leaf and root of maize (Zea mays L.) in avoiding chilling induced water stress. Plant Sci. 2003, 165, 671–679. [Google Scholar] [CrossRef]
- Zhu, X.-C.; Song, F.-B.; Xu, H.-W. Arbuscular mycorrhizae improve low temperature stress in maize via alterations in host water status and photosynthesis. Plant Soil 2010, 331, 129–137. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Foolad, M.; Lin, G. Relationship between Cold Tolerance during Seed Germination and Vegetative Growth in Tomato: Germplasm Evaluation. J. Am. Soc. Hortic. Sci. 2000, 125, 679–683. [Google Scholar] [CrossRef]
- Ghanbari, F.; Sayyari, M. Controlled drought stress affects the chilling-hardening capacity of tomato seedlings as indicated by changes in phenol metabolisms, antioxidant enzymes activity, osmolytes concentration and abscisic acid accumulation. Sci. Hortic. 2018, 229, 167–174. [Google Scholar] [CrossRef]
- Caradonia, F.; Francia, E.; Morcia, C.; Ghizzoni, R.; Moulin, L.; Terzi, V.; Ronga, D. Arbuscular Mycorrhizal Fungi and Plant Growth Promoting Rhizobacteria Avoid Processing Tomato Leaf Damage during Chilling Stress. Agronomy 2019, 9, 299. [Google Scholar] [CrossRef]
- Liu, A.; Chen, S.; Wang, M.; Liu, D.; Chang, R.; Wang, Z.; Lin, X.; Bai, B.; Ahammed, G.J. Arbuscular Mycorrhizal Fungus Alleviates Chilling Stress by Boosting Redox Poise and Antioxidant Potential of Tomato Seedlings. J. Plant Growth Regul. 2016, 35, 109–120. [Google Scholar] [CrossRef]
- Charest, C.; Dalpe, Y.; Brown, A. The effect of vesicular arbuscular mycorrhizae and chilling on two hybrids of Zea mays L. Mycorrhiza 1993, 4, 89–92. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Chaoxing, H. Arbuscular mycorrhizal influence on growth, photosynthetic pigments, osmotic adjustment and oxidative stress in tomato plants subjected to low temperature stress. Acta Physiol. Plant. 2011, 33, 1217–1225. [Google Scholar] [CrossRef]
- Paradis, R.; Dalpe, Y.; Charest, C. The combined effect of arbuscular mycorrhizas and short-term cold exposure on wheat. New Phytol. 1995, 129, 637–642. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Xia, R.-X.; Zou, Y.-N. Reactive oxygen metabolism in mycorrhizal and non-mycorrhizal citrus (Poncirus trifoliata) seedlings subjected to water stress. J. Plant Physiol. 2006, 163, 1101–1110. [Google Scholar] [CrossRef]
- Haddidi, I.; Duc, N.; Tonk, S.; Rápó, E.; Posta, K. Defense Enzymes in Mycorrhizal Tomato Plants Exposed to Combined Drought and Heat Stresses. Agronomy 2020, 10, 1657. [Google Scholar] [CrossRef]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef]
- Cabral, L.; Soares, C.R.F.S.; Giachini, A.J.; Siqueira, J.O. Arbuscular mycorrhizal fungi in phytoremediation of contaminated areas by trace elements: Mechanisms and major benefits of their applications. World J. Microbiol. Biotechnol. 2015, 31, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, U.; Regvar, M.; Bothe, H. Arbuscular mycorrhiza and heavy metal tolerance. Phytochemistry 2007, 68, 139–146. [Google Scholar] [CrossRef]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.-M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 2003, 37, 1–16. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, B.; Ohtomo, R. Mycorrhizal effects on growth, P uptake and Cd tolerance of the host plant vary among different AM fungal species. Soil Sci. Plant Nutr. 2015, 61, 359–368. [Google Scholar] [CrossRef]
- Nayuki, K.; Chen, B.; Ohtomo, R.; Kuga, Y. Cellular Imaging of Cadmium in Resin Sections of Arbuscular Mycorrhizas Using Synchrotron Micro X-ray Fluorescence. Microbes Environ. 2014, 29, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Göhre, V.; Paszkowski, U. Contribution of the arbuscular mycorrhizal symbiosis to heavy metal phytoremediation. Planta 2006, 223, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fuego, D.; Bertrand, A.; González, A. Metal accumulation and detoxification mechanisms in mycorrhizal Betula pubescens. Environ. Pollut. 2017, 231, 1153–1162. [Google Scholar] [CrossRef]
- Hashem, A.; Abd Allah, E.F.; Alqarawi, A.A.; Al Huqail, A.A.; Egamberdieva, D.; Wirth, S. Alleviation of cadmium stress in Solanum lycopersicum L. by arbuscular mycorrhizal fungi via induction of acquired systemic tolerance. Saudi J. Biol. Sci. 2016, 23, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Fei-bo, W.; Guo-ping, Z. Effect of cadmium on growth and photosynthesis of tomato seedlings. J. Zhejiang Univ. Sci. 2005, 6, 974. [Google Scholar]
- Hayat, S.; Hasan, S.A.; Ahmad, A. Growth, nitrate reductase activity and antioxidant system in cadmium stressed tomato (Lycopersicon esculentum Mill) cultivars. Biotech. Agron. Soc. Environ. 2011, 15, 401–414. [Google Scholar]
- Li, Y.; Zeng, J.; Wang, S.; Lin, Q.; Ruan, D.; Chi, H.; Zheng, M.; Chao, Y.; Qiu, R.; Yang, Y. Effects of cadmium-resistant plant growth-promoting rhizobacteria and Funneliformis mosseae on the cadmium tolerance of tomato (Lycopersicon esculentum L.). Int. J. Phytoremediation 2019, 22, 451–458. [Google Scholar] [CrossRef]
- Ogundola, L. Effects of pre- and post-transplant inoculation with Glomus mosseae on heavy metal (cadmium) absorption by potted tomato plants. Middle East J. Sci. Res. 2006, 1, 16–22. [Google Scholar]
- Kumar, P.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Kalunke, R.M.; Colla, G. Insight into the role of grafting and arbuscular mycorrhiza on cadmium stress tolerance in tomato. Front. Plant Sci. 2015, 6, 477. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.; Chen, B.; Christie, P.; Li, X. Yield and arsenate uptake of arbuscular mycorrhizal tomato colonized by Glomus mosseae BEG167 in As spiked soil under glasshouse conditions. Environ. Int. 2005, 31, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Elahi, F.E.; Aminuzzaman, F.M.; Mridha, M.A.U.; Begum, B.; Harun, A.K.M.Y. AMF inoculation reduced arsenic toxicity and increased growth, nutrient uptake and chlorophyll content of tomato grown in arsenic amended soil. Adv. Environ. Biol. 2010, 4, 194–200. [Google Scholar]
- Malekzadeh, P.; Farshian, S.H.; Ordubadi, B. Interaction of arbuscular mycorrhizal fungus (Glomus intraradices and Glomus etunicatum) with tomato plants grown under copper toxicity. Afr. J. Biotech. 2012, 11, 10555–10567. [Google Scholar] [CrossRef]
- Burleigh, S.H.; Kristensen, B.K.; Bechmann, I.E. A plasma membrane zinc transporter from Medicago truncatula is up-regulated in roots by Zn fertilization, yet down-regulated by arbuscular mycorrhizal colonization. Plant Mol. Biol. 2003, 52, 1077–1088. [Google Scholar] [CrossRef]
- Tong, Y.; Kneer, R.; Zhu, Y. Vacuolar compartmentalization: A second-generation approach to engineering plants for phytoremediation. Trends Plant Sci. 2004, 9, 7–9. [Google Scholar] [CrossRef]
| Mycorrhizal Species | Effect | Reference |
|---|---|---|
| Septoglomus deserticola and Septoglomus constrictum | Increased root and shoot dry weight. Increased stomatal conductance, relative water content and antioxidant enzyme activities | [28] |
| Funneliformis mosseae and Rhizophagus intraradices | Increased photosynthetic rate and water use efficiency, reduction in ABA, higher proline accumulation, | [29] |
| Glomus clarum | Increased aerial biomass, photosynthetic activity and stomatal conductance | [34] |
| Rhizophagus intraradices | Increased shoot biomass, number of flowers, fruits, N and P uptake. | [35] |
| Rhizophagus intraradices | Increased shoot dry weight, stomatal conductance, photosystem II efficiency, and abscisic acid | [38] |
| Rhizophagus intraradices | Lower transpiration rate and increased water use efficiency, increased shoot biomass and P concentration | [42] |
| Funneliformis mosseae, Rhizoglomus irregulare and Rhizophagus etunicatum | Increased shoot and root dry weight. | [43] |
| Funneliformis mosseae and Rhizophagus intraradices | Rhizophagus intraradices efficient in water use efficiency, F. mosseae in volatile organic compounds | [45] |
| Level of Salinity | Mycorrhizal Species | Effect | Reference |
|---|---|---|---|
| NaCl solution (0, 0.5 and 1%) 4.2 and 7.1 dS m−1 | Funneliformis mosseae | Reduced MDA content and increased antioxidant enzymes SOD, POD, APX and CAT | [80] |
| without salt (EC = 0.63 dS m−1), with low (EC = 5 dS m−1), or high (EC = 10 dS m−1) salinity. | Rhizophagus intraradices | Increased growth, stomatal conductance, photosynthetic activity, proline and ROS enzymes | [82] |
| 1.4 (control), 4.9 (medium) and 7.1 dS m−1 (high salt stress) | Funneliformis mosseae | Shoot dry matter, yield and leaf area were higher, the contents of P, K, Zn, Cu, and Fe were higher | [87] |
| 1.5, 3.0, 4.5, 6.0 mS cm−1 EC | Rhizophagus etunicatum, Funneliformis mosseae, Glomus aggregatum, Rhizophagus intraradices | Increased yield and size of fruits | [88] |
| 0, 50 and 100 mM NaCl. | Funneliformis mosseae | Increased growth, leaf area, cholorphyll, fruit fresh weight and yield. Increased P and K uptake. Increased SOD, CAT, and POD reduced MDA content | [89] |
| 0, 50, and 100 mM, NaCl | Mixtures of Glomus sp. | Increased root biomass, P, N, Ca uptake | [90] |
| EC of 4.56 dS m−1 | Glomus clarum and Rhizophagus intraradices | Increased soluble sugar, proline accumulation and vitamin C. Increased the chlorophyll concentration, Pn, Gs and Tr of plants. | [98] |
| Type of Temperature | Mycorrhizal Species | Effect | Reference |
|---|---|---|---|
| 42 °C | Septoglomus deserticola and Septoglomus constrictum | Decreasing the lipid peroxidation, hydrogen peroxide level and improving leaf and root antioxidant enzyme activities | [28] |
| 1 °C | Funneliformis mosseae | Reduced the cell membrane injuries in term of electrolytic leakage and efficiency of photosystem II | [114] |
| 8 °C/4 °C | Funneliformis mosseae | induced activities of antioxidant enzymes, significantly reduced the level of malondialdehyde (MDA), H2O2, and O2− along with increased calcium precipitates in the apoplast and vacuole of root cells | [115] |
| 8 °C | Funneliformis mosseae | Decreased MDA content in leaves. The contents of photosynthetic pigments, sugars and soluble protein in leaves were higher, but leaf proline content was lower and increased the activities of antioxidant enzymes | [117] |
| 45 °C | Rhizophagus irregularis, Funneliformis mosseae, and Funneliformis coronatum | A decrease in hydrogen peroxide and malondialdehyde content and increased antioxidant enzyme activities | [120] |
| Level of Heavy Metals | Mycorrhizal Species | Effect | Reference |
|---|---|---|---|
| Cd concentrations (50 and 100 mg kg−1) | Funneliformis mosseae | Increased dry weights, reduction in transcolation of Cd from root to shoot | [66] |
| Cd (50 μM CdCl2) | Funneliformis mosseae Rhizophagus intraradices and Rhizophagus etunicatum | Increased antioxidant enzymes, Proline and phenol content | [129] |
| Cd (30 and 60 mg of CdSO4) | Funneliformis mosseae | Increased Cd absorption and dry weights | [133] |
| 0 and 25 μM Cd | Rhizophagus irregularis | Induced photosynthetic pigments, photosystem II efficiency, antioxidant enzyme and proline accumulation and reduced lipid peroxidation products. Increased nutritional status such as P, K, Ca, Fe, Mn, and Zn | [134] |
| As levels (0, 25, 50, 75 and 150 mg kg−1) | Funneliformis mosseae | Increased biomass and P uptake, higher shoot and root P/As ratio | [135] |
| Cu solution (0, 1.5, 3.5, 5.5, 7.5 mM CuSO4) | Rhizophagus intraradices and Rhizophagus etunicatum | Increased biomass, sugar, proline, and antioxidant enzymes | [137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandrasekaran, M.; Boopathi, T.; Manivannan, P. Comprehensive Assessment of Ameliorative Effects of AMF in Alleviating Abiotic Stress in Tomato Plants. J. Fungi 2021, 7, 303. https://doi.org/10.3390/jof7040303
Chandrasekaran M, Boopathi T, Manivannan P. Comprehensive Assessment of Ameliorative Effects of AMF in Alleviating Abiotic Stress in Tomato Plants. Journal of Fungi. 2021; 7(4):303. https://doi.org/10.3390/jof7040303
Chicago/Turabian StyleChandrasekaran, Murugesan, Thangavelu Boopathi, and Paramasivan Manivannan. 2021. "Comprehensive Assessment of Ameliorative Effects of AMF in Alleviating Abiotic Stress in Tomato Plants" Journal of Fungi 7, no. 4: 303. https://doi.org/10.3390/jof7040303
APA StyleChandrasekaran, M., Boopathi, T., & Manivannan, P. (2021). Comprehensive Assessment of Ameliorative Effects of AMF in Alleviating Abiotic Stress in Tomato Plants. Journal of Fungi, 7(4), 303. https://doi.org/10.3390/jof7040303







