Nonionizing Electromagnetic Field: A Promising Alternative for Growing Control Yeast
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Growth Conditions
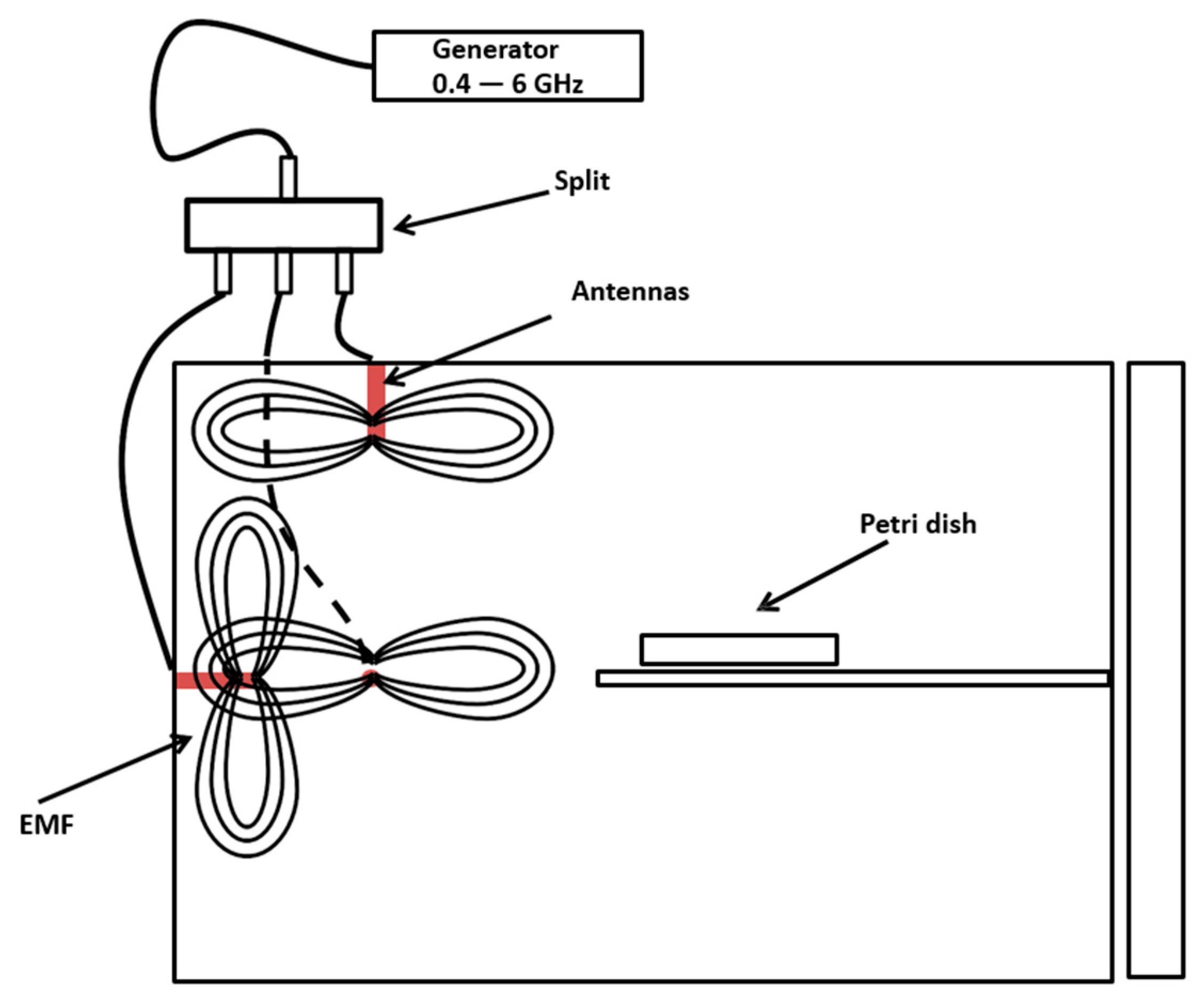
2.2. Exposure of S. cerevisiae to Several EMF Frequencies at Different Distances
2.3. Determination of the Percent Viability Reduction of S. cerevisiae after Irradiation
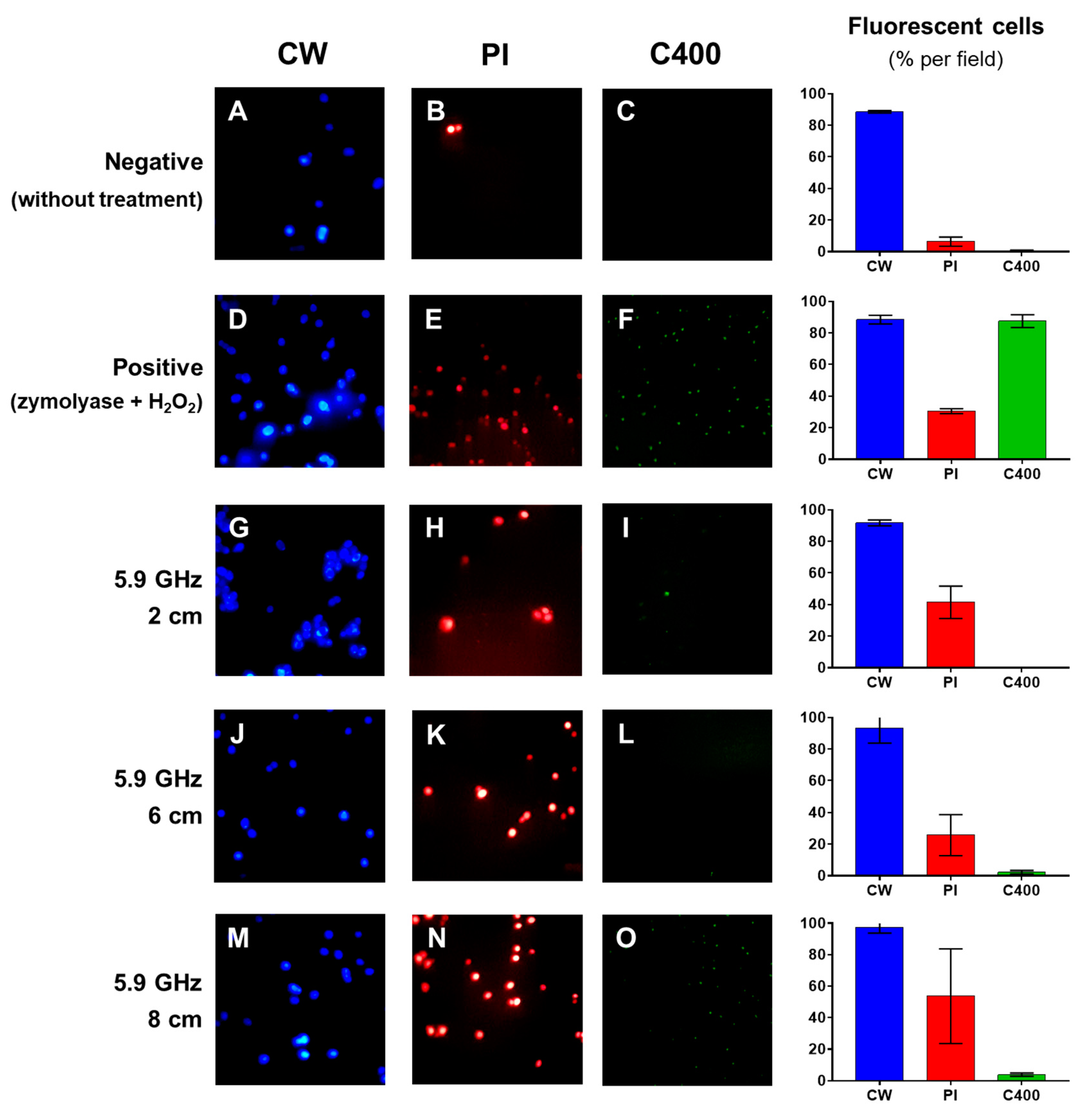
2.4. Exploration of the Antifungal Action Mechanism of the EMF on S. cerevisiae
2.5. Observation by Transmission Electron Microscopy (TEM)
2.6. Statistical Analysis
3. Results
3.1. Study of the Effect of EMF on the Viability of S. cerevisiae
3.2. Effect of the EMF on the Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajauria, G.; Tiwari, B.K. Fruit Juices: An Overview. Fruit Juices 2018, 2018, 3–13. [Google Scholar] [CrossRef]
- Ağçam, E.; Akyıldız, A.; Dündar, B. Thermal Pasteurization and Microbial Inactivation of Fruit Juices. Fruit Juices 2018, 2018, 309–339. [Google Scholar] [CrossRef]
- Hussain, M.A. Food Contamination: Major Challenges of the Future. Foods 2016, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Negi, P.S. Use of Natural Preservatives for Shelf Life Extension of Fruit Juices. Fruit Juices 2018, 2018, 571–605. [Google Scholar] [CrossRef]
- Salomão, B.D.C.M. Pathogens and Spoilage Microorganisms in Fruit Juice: An Overview. Fruit Juices 2018, 2018, 291–308. [Google Scholar] [CrossRef]
- Tournas, V.; Heeres, J.; Burgess, L. Moulds and yeasts in fruit salads and fruit juices. Food Microbiol. 2006, 23, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; Pérez-Nevado, F.; Ruiz-Moyano, S.; Serradilla, M.; Villalobos, M.; Martín, A.; Córdoba, M. Spoilage yeasts: What are the sources of contamination of foods and beverages? Int. J. Food Microbiol. 2018, 286, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Asokapandian, S.; Periasamy, S.; Swamy, G.J. Ozone for Fruit Juice Preservation. Fruit Juices 2018, 2018, 511–527. [Google Scholar] [CrossRef]
- Augusto, P.E.; Tribst, A.A.; Cristianini, M. High Hydrostatic Pressure and High-Pressure Homogenization Processing of Fruit Juices. Fruit Juices 2018, 2018, 393–421. [Google Scholar] [CrossRef]
- Baysal, A.H. Short-Wave Ultraviolet Light Inactivation of Pathogens in Fruit Juices. Fruit Juices 2018, 463–510. [Google Scholar] [CrossRef]
- Kalaiselvan, R.R.; Sugumar, A.; Radhakrishnan, M. Gamma Irradiation Usage in Fruit Juice Extraction. Fruit Juices 2018, 2018, 423–435. [Google Scholar] [CrossRef]
- Koubaa, M.; Barba, F.J.; Kovačević, D.B.; Putnik, P.; Santos, M.D.; Queirós, R.P.; Moreira, S.A.; Inácio, R.S.; Fidalgo, L.G.; Saraiva, J.A. Pulsed Electric Field Processing of Fruit Juices. Fruit Juices 2018, 2018, 437–449. [Google Scholar] [CrossRef]
- Sun, X.; Baldwin, E.; Bai, J. Applications of gaseous chlorine dioxide on postharvest handling and storage of fruits and vegetables—A review. Food Control. 2019, 95, 18–26. [Google Scholar] [CrossRef]
- Swamy, G.J.; Muthukumarappan, K.; Asokapandian, S. Ultrasound for Fruit Juice Preservation. Fruit Juices 2018, 2017, 451–462. [Google Scholar] [CrossRef]
- Rodriguez, A.B.; Ganga, A.; Godoy, L. Campos Electromagnéticos No Ionizantes para la Inocuidad Alimentaria. Inf. Technol. 2018, 29, 229–236. [Google Scholar] [CrossRef][Green Version]
- Al-Harbi, F.F.; Alkhalifah, D.H.M.; Elqahtani, Z.M.; Ali, F.M.; Mohamed, S.A.; Abdelbacki, A.M.M. Nonthermal control of Escherichia coli growth using extremely low frequency electromagnetic (ELF-EM) waves. BioMed. Mater. Eng. 2018, 29, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Bayir, E.; Bilgi, E.; Sendemir-Urkmez, A.; Hameş-Kocabaş, E.E.; Bayır, E. The effects of different intensities, frequencies and exposure times of extremely low-frequency electromagnetic fields on the growth of Staphylococcus aureus and Escherichia coli O157:H7. Electromagn. Biol. Med. 2013, 34, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.P.; Shamis, Y.; Croft, R.J.; Wood, A.; McIntosh, R.L.; Crawford, R.J.; Ivanova, E.P. 18 GHz electromagnetic field induces permeability of Gram-positive cocci. Sci. Rep. 2015, 5, 10980. [Google Scholar] [CrossRef]
- Nguyen, T.H.P.; Pham, V.T.H.; Nguyen, S.H.; Baulin, V.; Croft, R.J.; Phillips, B.; Crawford, R.J.; Ivanova, E.P. The Bioeffects Resulting from Prokaryotic Cells and Yeast Being Exposed to an 18 GHz Electromagnetic Field. PLoS ONE 2016, 11, e0158135. [Google Scholar] [CrossRef]
- Oncul, S.; Cuce, E.M.; Aksu, B.; Garip, A.I. Effect of extremely low frequency electromagnetic fields on bacterial membrane. Int. J. Radiat. Biol. 2015, 92, 42–49. [Google Scholar] [CrossRef]
- Torgomyan, H.; Trchounian, A. Bactericidal effects of low-intensity extremely high frequency electromagnetic field: An overview with phenomenon, mechanisms, targets and consequences. Crit. Rev. Microbiol. 2012, 39, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Akbal, A.; Balik, H. Investigation of antibacterial effects of electromagnetic waves emitted by mobile phones. Pol. J. Environ. Stud. 2013, 22, 1589–1594. [Google Scholar]
- Latif, I.; Al-Azzawie, A.F.; Al-Assie, A. Assessment of Genetic Effects of Bacterial Cells after Exposure to Mobile Phone Radia-tion Using RAPD Technique. Iraqui J. Biotechnol. 2013, 12, 63–74. [Google Scholar]
- American Society Standard Testing and Materials (E2149-1). Standard Methods for Determining the Antimicrobial Activity of Immobilized Antimicrobial Agents under Dynamic Contact Conditions. AMST Am. Soc. Stand. Test. Mater. 2001, 1–4. [Google Scholar] [CrossRef]
- Harrington, B.J.; Hageage, J.G.J. Calcofluor White: A Review of its Uses and Applications in Clinical Mycology and Parasitology. Lab. Med. 2003, 34, 361–367. [Google Scholar] [CrossRef]
- Villalba, M.L.; Sáez, J.S.; del Monaco, S.; Lopes, C.A.; Sangorrín, M.P. TdKT, a new killer toxin produced by Torulaspora delbrueckii effective against wine spoilage yeasts. Int. J. Food Microbiol. 2016, 217, 94–100. [Google Scholar] [CrossRef]
- Kaiserer, L.; Oberparleiter, C.; Burgstaller, W.; Leiter, E.; Marx, F.; Weiler-Görz, R. Characterization of the Penicillium chrysogenum antifungal protein PAF. Arch. Microbiol. 2003, 180, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Ştefănoiu, G.-A.; Tănase, E.E.; Miteluţ, A.C.; Popa, M.E. Unconventional Treatments of Food: Microwave vs. Radiofrequency. Agric. Agric. Sci. Procedia 2016, 10, 503–510. [Google Scholar] [CrossRef]
- Song, K.; Taghipour, F.; Mohseni, M. Microorganisms inactivation by continuous and pulsed irradiation of ultraviolet light-emitting diodes (UV-LEDs). Chem. Eng. J. 2018, 343, 362–370. [Google Scholar] [CrossRef]
- Monyethabeng, M.M.; Krügel, M. The effect of UV-C treatment on various spoilage microorganisms inoculated into Rooibos iced tea. LWT 2016, 73, 419–424. [Google Scholar] [CrossRef]
- Shamis, Y.; Taube, A.; Mitik-Dineva, N.; Croft, R.; Crawford, R.J.; Ivanova, E.P. Specific Electromagnetic Effects of Microwave Radiation on Escherichia coli. Appl. Environ. Microbiol. 2011, 77, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Mulye, K.; Thorat, V.; Talker, J.; Talker, E.; Raj, A.; Pawar, J. Effect of electromagnetic radiations emitted by mobile towers on sur-vival of E. coli. IOSR J. Environ. Sci. Toxicol. Food Technol. 2015, 9, 31–34. [Google Scholar] [CrossRef]
- Zeng, S.-W.; Huang, Q.-L.; Zhao, S.-M. Effects of microwave irradiation dose and time on Yeast ZSM-001 growth and cell membrane permeability. Food Control. 2014, 46, 360–367. [Google Scholar] [CrossRef]
- Gründler, W.; Keilmann, F.; Frohlich, H. Resonant growth rate response of yeast cells irradiated by weak microwaves. Phys. Lett. A 1977, 62, 463–466. [Google Scholar] [CrossRef]
- Dreyfuss, M.S.; Chipley, J.R. Comparison of Effects of Sublethal Microwave Radiation and Conventional Heating on the Metabolic Activity of Staphylococcus aureus. Appl. Environ. Microbiol. 1980, 39, 13–16. [Google Scholar] [CrossRef]
- Novickij, V.; Lastauskienė, E.; Švedienė, J.; Grainys, A.; Staigvila, G.; Paškevičius, A.; Girkontaitė, I.; Zinkevičienė, A.; Markovskaja, S.; Novickij, J. Membrane Permeabilization of Pathogenic Yeast in Alternating Sub-microsecond Electromagnetic Fields in Combination with Conventional Electroporation. J. Membr. Biol. 2017, 251, 189–195. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riffo, B.; Henríquez, C.; Chávez, R.; Peña, R.; Sangorrín, M.; Gil-Duran, C.; Rodríguez, A.; Ganga, M.A. Nonionizing Electromagnetic Field: A Promising Alternative for Growing Control Yeast. J. Fungi 2021, 7, 281. https://doi.org/10.3390/jof7040281
Riffo B, Henríquez C, Chávez R, Peña R, Sangorrín M, Gil-Duran C, Rodríguez A, Ganga MA. Nonionizing Electromagnetic Field: A Promising Alternative for Growing Control Yeast. Journal of Fungi. 2021; 7(4):281. https://doi.org/10.3390/jof7040281
Chicago/Turabian StyleRiffo, Byron, Consuelo Henríquez, Renato Chávez, Rubén Peña, Marcela Sangorrín, Carlos Gil-Duran, Arturo Rodríguez, and María Angélica Ganga. 2021. "Nonionizing Electromagnetic Field: A Promising Alternative for Growing Control Yeast" Journal of Fungi 7, no. 4: 281. https://doi.org/10.3390/jof7040281
APA StyleRiffo, B., Henríquez, C., Chávez, R., Peña, R., Sangorrín, M., Gil-Duran, C., Rodríguez, A., & Ganga, M. A. (2021). Nonionizing Electromagnetic Field: A Promising Alternative for Growing Control Yeast. Journal of Fungi, 7(4), 281. https://doi.org/10.3390/jof7040281





