Enzyme Activities of Five White-Rot Fungi in the Presence of Nanocellulose
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Growth Rate Experiments
2.2. Fungal Enzyme Experiments
2.3. Endoglucanase Assay
2.4. Laccase Assay
2.5. Urease Assay
2.6. Glucose-6-Phosphate Assay
3. Results
3.1. Growth Optima T. versicolor 159
3.2. Growth Optima T. pubescens 220
3.3. Growth Optima G. adspersum 003 and G. lipsiense 646
3.4. Growth Optima R. vitreus
3.5. Endoglucanase Assays
3.6. Laccase Assays
3.7. Urease Assay
3.8. Glucose Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pathak, P.; Kaur, P.; Bhardwaj, N.K. Chapter 6. Microbial enzymes for pulp and paper industry. In Microbial Biotechnology: An Interdiciplinary Approach; Shukla, P., Ed.; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2017; pp. 163–240. [Google Scholar]
- Wesenberg, D.; Kyriakides, I.; Agathos, S.N. White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol. Adv. 2003, 22, 161–187. [Google Scholar] [CrossRef]
- Mayolo-Deloisa, K.; González-González, M.; Rito-Palomares, M. Laccases in food industry: Bioprocessing, potential industrial and biotechnological applications. Front. Bioeng. Biotechnol. 2020, 8, 1–8. [Google Scholar] [CrossRef]
- Schwarze, F.W.M.R. Wood decay under the microscope. Fungal Biol. Rev. 2007, 21, 133–170. [Google Scholar] [CrossRef]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef]
- Manavalan, T.; Manavalan, A.; Heese, K. Characterization of lignocellulolytic enzymes from white-rot fungi. Curr. Microbiol. 2015, 70, 485–498. [Google Scholar] [CrossRef]
- Okal, E.J.; Aslam, M.M.; Karanja, J.K.; Nyimbo, W.J. Mini review: Advances in understanding regulation of cellulase enzyme in white-rot basidiomycetes. Microb. Pathog. 2020, 147, 104410. [Google Scholar] [CrossRef]
- Arora, D.S.; Gill, P.K. Laccase production by some white rot fungi under different nutritional conditions. Bioresour. Technol. 2000, 73, 283–285. [Google Scholar] [CrossRef]
- Satyamurthy, P.; Jain, P.; Karande, V.S.; Nadanathangam, V. Nanocellulose induces cellulase production in Trichoderma reesei. Process Biochem. 2016, 51, 1452–1457. [Google Scholar] [CrossRef]
- Niranjane, A.P.; Madhou, P.; Stevenson, T.W. The effect of carbohydrate carbon sources on the production of cellulase by Phlebia gigantea. Enzyme Microb. Technol. 2007, 40, 1464–1468. [Google Scholar] [CrossRef]
- Kachlishvili, E.; Penninckx, M.J.; Tsiklauri, N.; Elisashvili, V. Effect of nitrogen source on lignocellulolytic enzyme production by white-rot basidiomycetes under solid-state cultivation. World J. Microbiol. Biotechnol. 2006, 22, 391–397. [Google Scholar] [CrossRef]
- Elisashvili, V.; Kachlishvili, E.; Penninckx, M. Effect of growth substrate, method of fermentation, and nitrogen source on lignocellulose-degrading enzymes production by white-rot basidiomycetes. J. Ind. Microbiol. Biotechnol. 2008, 35, 1531–1538. [Google Scholar] [CrossRef]
- Srinivasan, C.; D’Souza, T.M.; Boominathan, K.; Reddy, C.A. Demonstration of laccase in the white rot basidiomycete Phanerochaete chrysosporium BKM-F1767. Appl. Environ. Microbiol. 1995, 61, 4274–4277. [Google Scholar] [CrossRef]
- Kobakhidze, A.; Asatiani, M.; Kachlishvili, E.; Elisashvili, V. Induction and catabolite repression of cellulase and xylanase synthesis in the selected white-rot basidiomycetes. Ann. Agrar. Sci. 2016, 14, 169–176. [Google Scholar] [CrossRef]
- Pertile, G.; Panek, J.; Oszust, K.; Siczek, A.; Oleszek, M.; Gryta, A.; Frąc, M. Effect of different organic waste on cellulose-degrading enzymes secreted by Petriella setifera in the presence of cellobiose and glucose. Cellulose 2019, 26, 7905–7922. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, R.; Zhang, Y. Production of lignocellulolytic enzymes by Trametes gallica and detection of polysaccharide hydrolase and laccase activities in polyacrylamide gels. J. Basic Microbiol. 2004, 44, 220–231. [Google Scholar] [CrossRef]
- Suzuki, H.; Igarashi, K.; Samejima, M. Real-time quantitative analysis of carbon catabolite derepression of cellulolytic genes expressed in the basidiomycete Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 2008, 80, 99–106. [Google Scholar] [CrossRef]
- Jämsä, M.; Kosourov, S.; Rissanen, V.; Hakalahti, M.; Pere, J.; Ketoja, J.A.; Tammelin, T.; Allahverdiyeva, Y. Versatile templates from cellulose nanofibrils for photosynthetic microbial biofuel production. J. Mater. Chem. A 2018, 6, 5825–5835. [Google Scholar] [CrossRef]
- Serra, A.; González, I.; Oliver-Ortega, H.; Tarrès, Q.; Delgado-Aguilar, M.; Mutjé, P. Reducing the amount of catalyst in TEMPO-oxidized cellulose nanofibers: Effect on properties and cost. Polymers 2017, 9, 557. [Google Scholar] [CrossRef]
- Mycobank: Ganoderma Lipsiense. Available online: https://www.mycobank.org/page/Name details page/name/Ganoderma applanatum (accessed on 2 March 2021).
- Atkinson, B.G.F. Ganoderma lipsiense. Ann. Mycol. 1908, 6, [MB#148315]. [Google Scholar]
- Mycobank: Physisporinus vitreus. Available online: https://www.mycobank.org/page/Name details page/name/Physisporinus vitreus (accessed on 2 March 2021).
- Donk, M.A. Notes on European polypores—I. Persoonia 1966, 4, 337–343. [Google Scholar]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, R.; Siqueira, G.; Schubert, M.; Tingaut, P.; Maniura-Weber, K.; Zimmermann, T.; Thöny-Meyer, L.; Faccio, G.; Ihssen, J. TEMPO-oxidized nanofibrillated cellulose as a high density carrier for bioactive molecules. Biomacromolecules 2015, 16, 3640–3650. [Google Scholar] [CrossRef]
- Rahman, M.S.; Fernando, S.; Ross, B.; Wu, J.; Qin, W. Endoglucanase (eg) activity assays. Methods Mol. Biol. 2018, 1796, 169–183. [Google Scholar] [CrossRef]
- Gross, K.C. A rapid and sensitive spectrophotometric method for assaying polygalacturonase using 2-cyanoacetamide. HortScience 1982, 17, 933–934. [Google Scholar] [CrossRef]
- Jurick, W.M.; Vico, I.; Whitaker, B.D.; Gaskins, V.L.; Janisiewicz, W.J. Application of the 2-cyanoacetamide method for spectrophotometric assay of cellulase enzyme activity. Plant Pathol. J. 2012, 11, 38–40. [Google Scholar] [CrossRef]
- Fu, K.; Fu, S.; Zhan, H.; Zhou, P.; Liu, M.; Liu, H. A newly isolated wood-rot fungus for laccase production in submerged cultures. BioResources 2013, 8, 1385–1397. [Google Scholar] [CrossRef]
- Rehmann, L.; Ivanova, E.; Ferguson, J.L.; Gunaratne, H.Q.N.; Seddon, K.R.; Stephens, G.M. Measuring the effect of ionic liquids on laccase activity using a simple, parallel method. Green Chem. 2012, 14, 725–733. [Google Scholar] [CrossRef]
- Berthelot, M. Correspondence-’Violet D’aniline’; Repertoire de chimie applique. Société Chimique de Paris 1859, 1, 284. [Google Scholar]
- Jo, W.-S.; Kang, M.-J.; Choi, S.-Y.; Yoo, Y.-B.; Seok, S.-J.; Jung, H.-Y. Culture conditions for mycelial growth of Coriolus versicolor. Mycobiology 2010, 38, 195. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.-S.; Cho, Y.-J.; Cho, D.-H.; Park, S.-D.; Yoo, Y.-B.; Seok, S.-J. Culture conditions for the mycelial growth of Ganoderma applanatum. Mycobiology 2009, 37, 94. [Google Scholar] [CrossRef]
- Badalyan, S.M.; Gharibyan, N.G.; Iotti, M. Morphological and ecological screening of different collections of medicinal white-rot bracket fungus Ganoderma adspersum (Schulzer) Donk (Agaricomycetes, Polyporales). Ital. J. Mycol. 2019, 48, 1–15. [Google Scholar]
- Schubert, M.; Dengler, V.; Mourad, S.; Schwarze, F.W.M.R. Determination of optimal growth parameters for the bioincising fungus physisporinus vitreus by means of response surface methodology. J. Appl. Microbiol. 2009, 106, 1734–1742. [Google Scholar] [CrossRef]
- Levin, L.; Melignani, E.; Ramos, A.M. Effect of nitrogen sources and vitamins on ligninolytic enzyme production by some white-rot fungi. Dye decolorization by selected culture filtrates. Bioresour. Technol. 2010, 101, 4554–4563. [Google Scholar] [CrossRef]
- Elisashvili, V.I.; Kachlishvili, E.T.; Wasser, S.P. Carbon and nitrogen source effects on basidiomycetes exopolysaccharide production. Appl. Biochem. Microbiol. 2009, 45, 531–535. [Google Scholar] [CrossRef]
- Merck Malt Extract. In Microbiology Manual 12 Edition; Merck: Kenilworth, NJ, USA, 2010; p. 688.
- Arora, D.S.; Gill, P.K. Effects of various media and supplements on laccase production by some white rot fungi. Bioresour. Technol. 2001, 77, 89–91. [Google Scholar] [CrossRef]
- Haars, A.; Tautz, D.; Hüttermann, A. Bioconversion of organosoluble lignins by different types of fungi. Resour. Conserv. 1986, 13, 37–51. [Google Scholar] [CrossRef]
- Cairns, T.C.; Zheng, X.; Zheng, P.; Sun, J.; Meyer, V. Moulding the mould: Understanding and reprogramming filamentous fungal growth and morphogenesis for next generation cell factories. Biotechnol. Biofuels 2019, 12, 1–18. [Google Scholar] [CrossRef]
- Basu, S.N.; Pal, P.N. An unfavourable effect of shaking on fungal cellulases. Nature 1956, 178, 312–313. [Google Scholar] [CrossRef]
- Ganesh, K.; Joshi, J.B.; Sawant, S.B. Cellulase deactivation in a stirred reactor. Biochem. Eng. J. 2000, 4, 137–141. [Google Scholar] [CrossRef]
- Reese, E.T.; Ryu, D.Y. Shear inactivation of cellulase of Trichoderma reesei. Enzyme Microb. Technol. 1980, 2, 239–240. [Google Scholar] [CrossRef]
- Sachse, H.; Kude, J.; Kerns, G.; Berger, R. Production of cellulase in a rotating disc fermenter using immobilized Trichoderma reesei cells. Acta Biotechnol. 1990, 10, 523–529. [Google Scholar] [CrossRef]
- Metreveli, E.; Kachlishvili, E.; Singer, S.W.; Elisashvili, V. Alteration of white-rot basidiomycetes cellulase and xylanase activities in the submerged co-cultivation and optimization of enzyme production by Irpex lacteus and Schizophyllum commune. Bioresour. Technol. 2017, 241, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Q.; Feng, Y.; Zhao, D.Q.; Jiang, J.X. Evaluation of cellulases produced from four fungi cultured on furfural residues and microcrystalline cellulose. Biodegradation 2012, 23, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Irbe, I.; Elisashvili, V.; Asatiani, M.D.; Janberga, A.; Andersone, I.; Andersons, B.; Biziks, V.; Grinins, J. Lignocellulolytic activity of Coniophora puteana and Trametes versicolor in fermentation of wheat bran and decay of hydrothermally modified hardwoods. Int. Biodeterior. Biodegrad. 2014, 86, 71–78. [Google Scholar] [CrossRef]
- Elisashvili, V.; Irbe, I.; Andersone, I.; Andersons, B.; Tsiklauri, N. Hydrolytic enzyme activity of EN113 standard basidiomycetes in the fermentation of lignocellulosic material and wood colonization. Holzforschung 2012, 66, 841–847. [Google Scholar] [CrossRef]
- Bollag, J.M.; Leonowicz, A. Comparative studies of extracellular fungal laccases. Appl. Environ. Microbiol. 1984, 48, 849–854. [Google Scholar] [CrossRef]
- Revankar, M.S.; Lele, S.S. Enhanced production of laccase using a new isolate of white rot fungus WR-1. Process Biochem. 2006, 41, 581–588. [Google Scholar] [CrossRef]
- Lorenzo, M.; Moldes, D.; Rodríguez Couto, S.; Sanromán, A. Improving laccase production by employing different lignocellulosic wastes in submerged cultures of Trametes versicolor. Bioresour. Technol. 2002, 82, 109–113. [Google Scholar] [CrossRef]
- Ihssen, J.; Schubert, M.; Schwarze, F.W.M.R.; Thöny-Meyer, L. Efficient production of Al(OH)3-immobilized laccase with a Heterobasidion annosum strain selected by microplate screening. J. Appl. Microbiol. 2011, 110, 924–934. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Kwiatos, N.; Szczesna-Antczak, M.; Bielecki, S. Laccases—enzymes with an unlimited potential. Biotechnol. Food Sci. 2017, 2017, 41–70. [Google Scholar]
- Mikiashvili, N.; Elisashvili, V.; Wasser, S.; Nevo, E. Carbon and nitrogen sources influence the ligninolytic enzyme activity of Trametes versicolor. Biotechnol. Lett. 2005, 27, 955–959. [Google Scholar] [CrossRef]
- Jing, D. Improving the simultaneous production of laccase and lignin peroxidase from Streptomyces lavendulae by medium optimization. Bioresour. Technol. 2010, 101, 7592–7597. [Google Scholar] [CrossRef]
- Collins, P.J.; Field, J.A.; Teunissen, P.; Dobson, A.D.W. Stabilization of lignin peroxidases in white rot fungi by tryptophan. Appl. Environ. Microbiol. 1997, 63, 2543–2548. [Google Scholar] [CrossRef]
- Kaal, E.E.J.; Field, J.A.; Joyce, T.W. Increasing ligninolytic enzyme activities in several white-rot Basidiomycetes by nitrogen-sufficient media. Bioresour. Technol. 1995, 53, 133–139. [Google Scholar] [CrossRef]
- Krajewska, B.; Ureases, I. Functional, catalytic and kinetic properties: A review. J. Mol. Catal. B Enzym. 2009, 59, 9–21. [Google Scholar] [CrossRef]
- Mobley, H.L.T.; Island, M.D.; Hausinger, R.P. Molecular biology of microbial ureases. Microbiol. Rev. 1995, 59, 451–480. [Google Scholar] [CrossRef]
- McCullough, W.; Roberts, C.F.; Osmani, S.A.; Scrutton, M.C. Chapter 9: Regulation of carbon metabolism in filamentous fungi. In Carbohydrate Metabolism in Cultured Cells; Morgan, M.J., Ed.; Plenum Press: New York, NY, USA, 1986; p. 634. ISBN 9783540773405. [Google Scholar]
- Smith, M.H.; Gold, M.H. Phanerochaete chrysosporium β-glucosidases: Induction, cellular localization, and physical characterization. Appl. Environ. Microbiol. 1979, 37, 938–942. [Google Scholar] [CrossRef]
- Manavalan, T.; Manavalan, A.; Thangavelu, K.P.; Heese, K. Characterization of a novel endoglucanase from Ganoderma lucidum. J. Basic Microbiol. 2015, 55, 761–771. [Google Scholar] [CrossRef]
- Daly, P.; Peng, M.; Di Falco, M.; Lipzen, A.; Wang, M.; Ng, V.; Grigoriev, I.V.; Tsang, A.; Mäkelä, M.R.; de Vries, R.P. Glucose-mediated repression of plant biomass utilization in the white-rot fungus. Appl. Environ. Microbiol. 2019, 85, 1–15. [Google Scholar] [CrossRef]
- Nisyzawa, T.; Suzuki, H.; Nisizawa, K. Catabolite repression of cellulase formation in Trichoderma viride. J. Biochem. 1972, 71, 999–1007. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, X.W.; Li, W.W.; Sheng, G.P.; Zang, G.L.; Cheng, Y.Y.; Shen, N.; Yang, Y.P.; Yu, H.Q. A white-rot fungus is used as a biocathode to improve electricity production of a microbial fuel cell. Appl. Energy 2012, 98, 594–596. [Google Scholar] [CrossRef]
- Magotra, V.K.; Kumar, S.; Kang, T.W.; Inamdar, A.I.; Aqueel, A.T.; Im, H.; Ghodake, G.; Shinde, S.; Waghmode, D.P.; Jeon, H.C. Compost soil microbial fuel cell to generate power using urea as fuel. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]

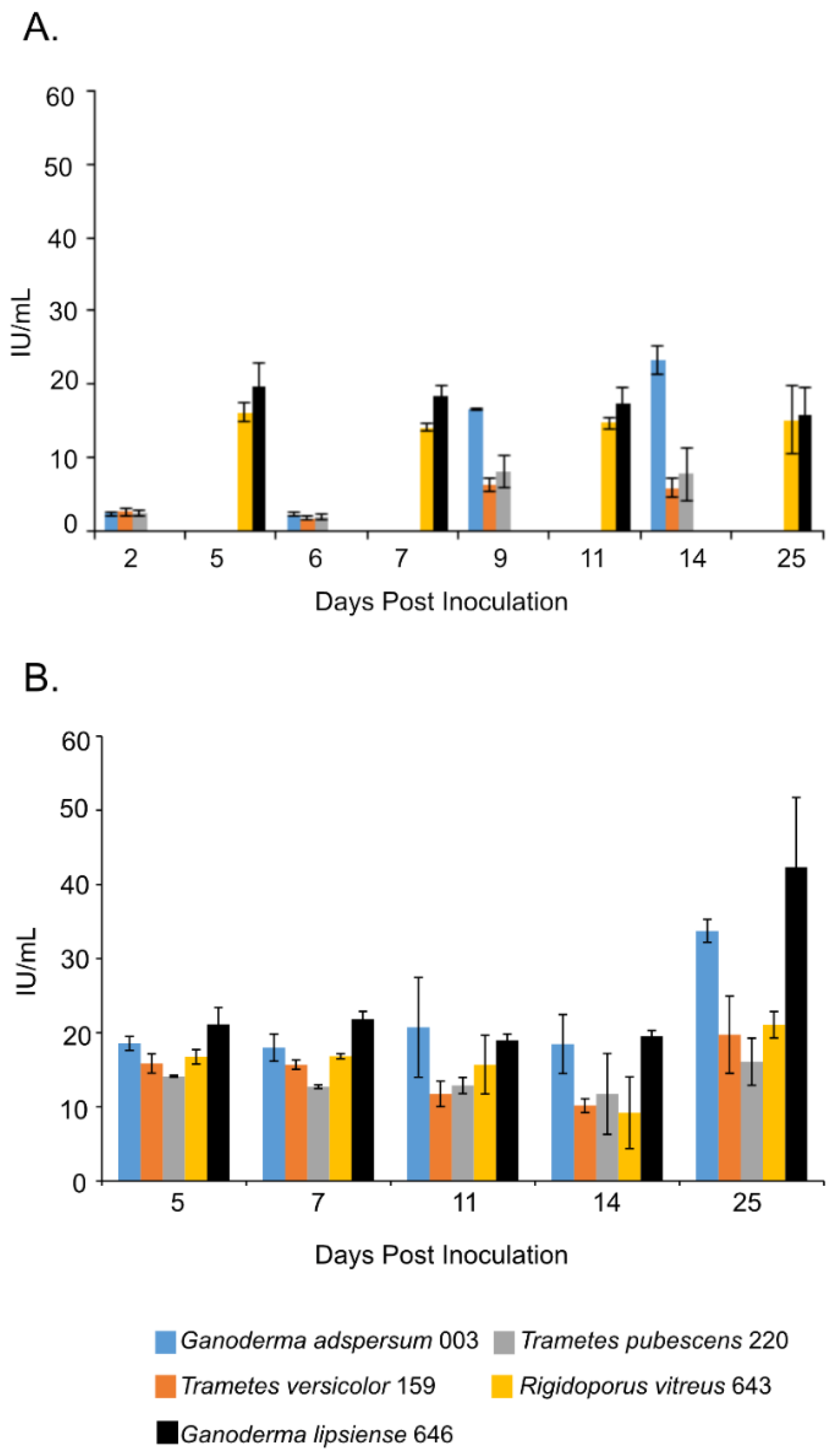
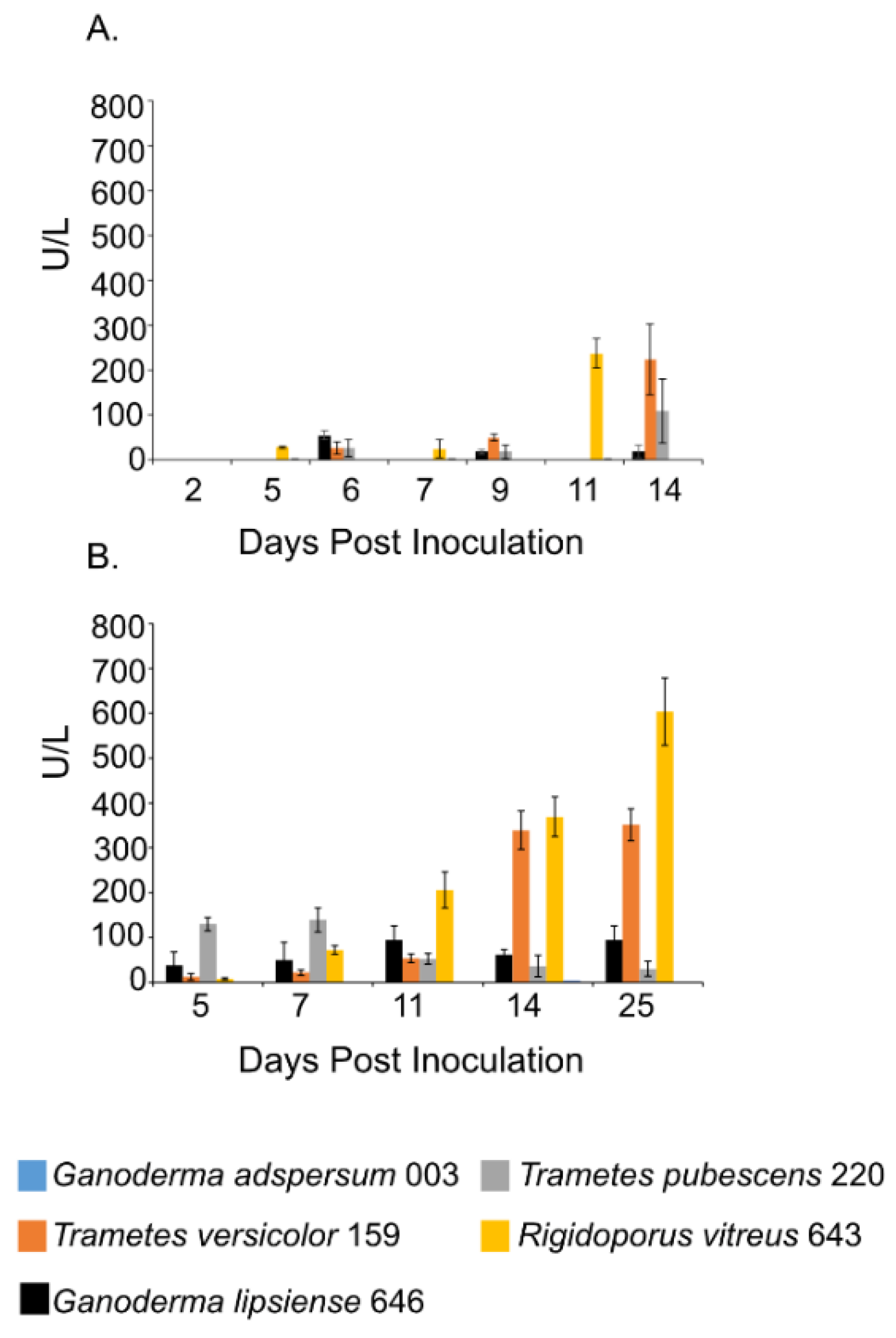
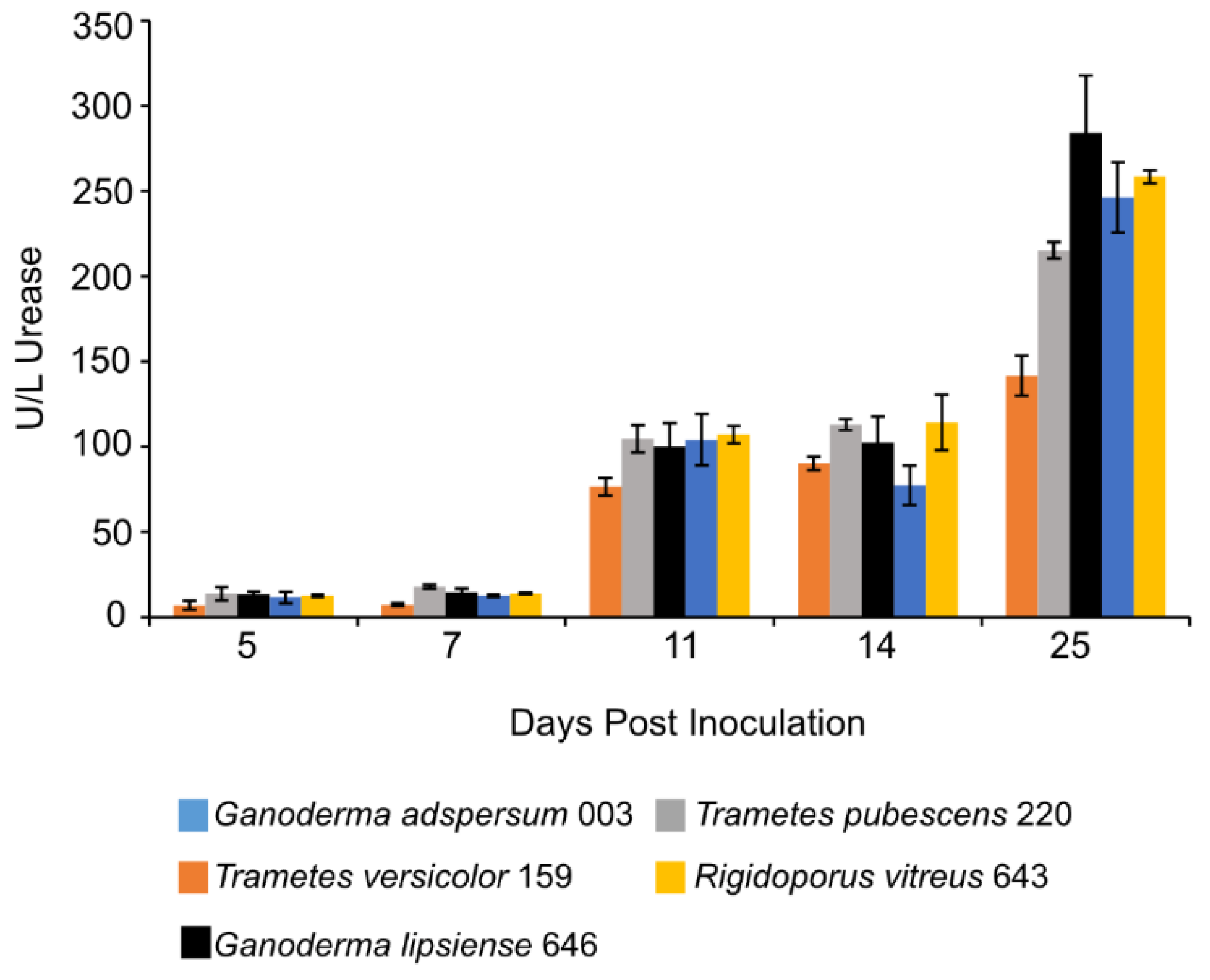
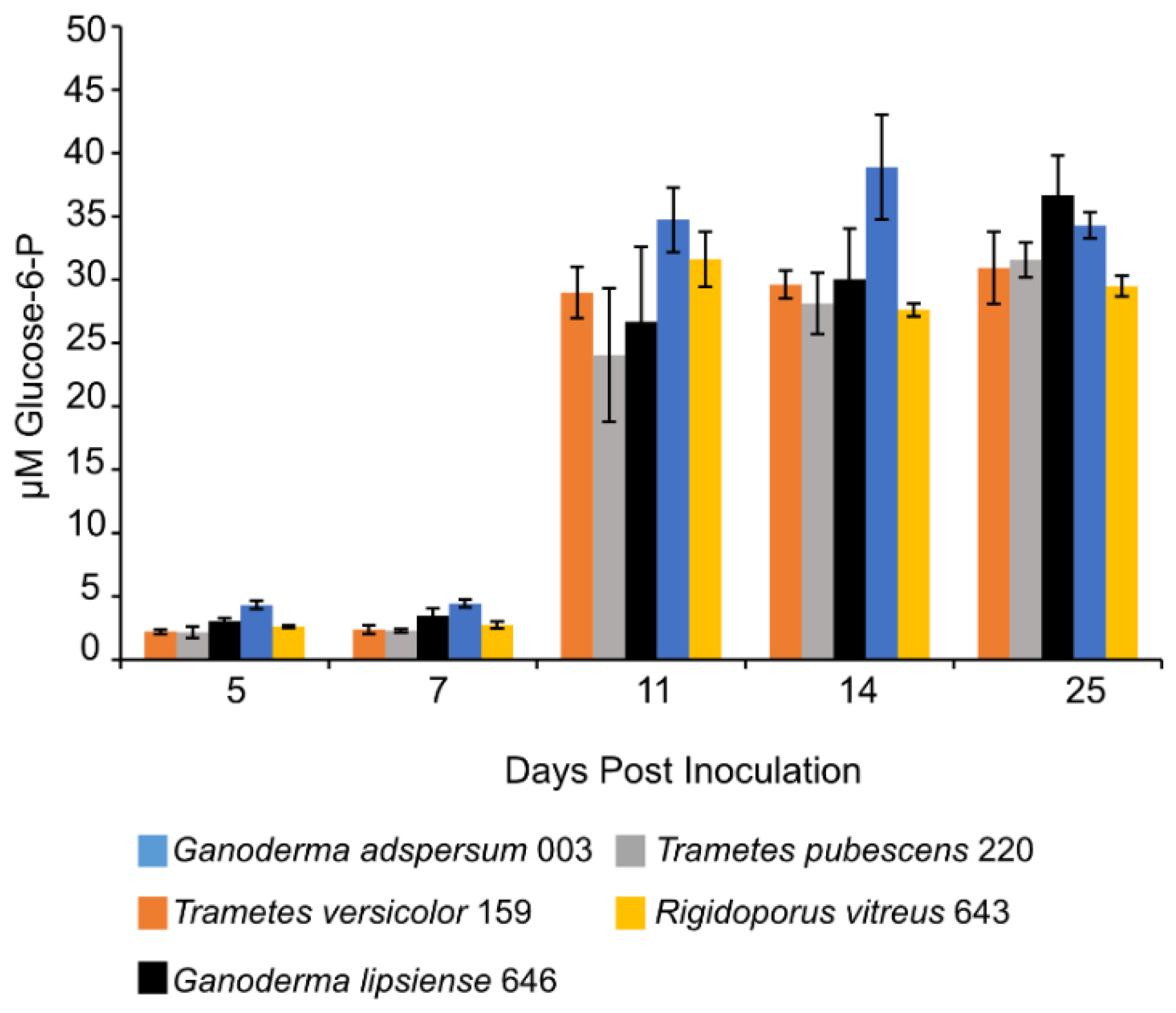
| Fungus | Growth Conditions | Endoglucanase Activity | Study |
|---|---|---|---|
| Pycnoporus. coccineus 310 (Basidiomycota) | 1.5% micro crystalline cellulose (Avicel medium) (150 rpm, 27 °C) | 65.6 ± 7.3 U/mL (14 days) | Metreveli et al. [46] |
| Schizophyllum commune 632 (Basidiomycota) | 1.5% micro crystalline cellulose (Avicel medium) (150 rpm, 27 °C) | 28 ± 3.9 U/mL (14 days) | Metreveli et al. [46] |
| Trametes hirusta 17 (Basidiomycota) | 1.5% micro crystalline cellulose (Avicel medium) (150 rpm, 27 °C) | 34.3 ± 3.1 U/mL (14 days) | Metreveli et al. [46] |
| Irepex lacteus 104 (Basidiomycota) | 1.5% micro crystalline cellulose (Avicel medium) (150 rpm, 27 °C) | 51.7 ± 4.3 U/mL (14 days) | Metreveli et al. [46] |
| Trichoderma viride (Ascomycota) | 2% commercial microcrystalline cellulose (Mandel’s medium) (150 rpm, 30 °C) | ~ 22 U/mL (15 days) | Liu et al. [47] |
| Aspergillus niger (Ascomycota) | 2% commercial microcrystalline cellulose (Mandel’s medium) (150 rpm, 30 °C) | ~ 9 U/mL (15 days) | Liu et al. [47] |
| Trichoderma koningii (Ascomycota) | 2% commercial microcrystalline cellulose (Mandel’s medium) (150 rpm, 30 °C) | ~ 32 U/mL (15 days) | Liu et al. [47] |
| Trichoderma reseei (Ascomycota) | 2% commercial microcrystalline cellulose (Mandel’s medium) (150 rpm, 30 °C) | ~5 U/mL (15 days) | Liu et al. [47] |
| Trichoderma reseei (Ascomycota) | Nanocellulose prepared by microbial hydrolysis (Mandel’s medium) | ~0.04 IU/mL (Day 1)~0.16 IU/mL (Day 5) | Satyamurthy et al. [9] |
| Trichoderma reseei (Ascomycota) | Nanofibrilliated cellulose (NFC) | ~0.02 IU/mL (Day 1) | Satyamurthy et al. [9] |
| Aspergillus niger (Ascomycota) | 0.3% nanocellulose prepared by microbial hydrolysis (Mandel’s medium) | ~0.2 IU/mL (Day 5) | Satyamurthy et al. [9] |
| Trametes versicolor (L) Loyd (CTB 863A) | Birch wood (5% malt extract, 2% agar) (22 °C, 70% RH) | 0.21 U/mL (42 days) | Irbe et al. [48] |
| Trametes versicolor (L) Loyd (CTB 863A) | Aspen wood (5% malt extract, 2% agar) (22 °C, 70% RH) | 0.30 U/mL (42 days) | Irbe et al. [48] |
| Trametes versicolor (L) Loyd (CTB 863A) | Alder wood (5% malt extract, 2% agar) (22 °C, 70% RH) | 0.29 U/mL (42 days) | Irbe et al. [48] |
| Trametes versicolor CTB 863 | Pine wood (MEA media, agar, colonized with ring) (22 °C, 70% RH) | 0.25 ± 0.02 U/mL (10 days) | Elisashvili et al. [49] |
| Trametes versicolor CTB 863 | Pine wood (WB media, agar, colonized with ring) (22 °C, 70% RH) | 0.33 ± 0.01 U/mL (20 days) | Elisashvili et al. [49] |
| Trametes versicolor 159 (Basidiomycota) | TEMPO-oxidized-CNF(0.017 wt%) and CNC(0.2 wt%) (2% (w/v) ME, 25 °C, 80% RH) (standing cultures) | ~ 20 ± 5 U/mL (25 days) | This study |
| Trametes versicolor 159 (Basidiomycota) | TEMPO-oxidized CNF(0.017 wt%) and CNC(0.2 wt%) (2% (w/v) ME, 120 rpm 25 °C, 80% RH) | ~ 6 ± 2 U/mL (14 days) | This study |
| Trametes pubescens 220 (Basidiomycota) | TEMPO-oxidized CNF(0.017 wt%) and CNC(0.2 wt%) (2% (w/v) ME, 25 °C, 80% RH) (standing cultures) | ~ 16 ± 3 U/mL (25 days) | This study |
| Trametes pubescens 220 (Basidiomycota) | TEMPO-oxidized CNF(0.017 wt%) and CNC(0.2 wt%) (2% (w/v) ME, 120 rpm, 25 °C, 80% RH) | ~ 8 ± 4 U/mL (14 days) | This study |
| Ganoderma adspersum 003 (Basidiomycota) | TEMPO-oxidized CNF(0.017 wt%) and CNC(0.2 wt%) (2% (w/v) ME, 25 °C, 80% RH) (standing cultures) | ~ 34 ± 2 U/mL (25 days) | This study |
| Ganoderma adspersum 003 (Basidiomycota) | TEMPO-oxidized CNF(0.017 wt%) and CNC(0.2 wt%) (2% (w/v) ME, 120 rpm, 25 °C, 80% RH) | ~23 ± 2 U/mL (14 days) | This study |
| Ganoderma lipsiense 646 (Basidiomycota) | TEMPO-oxidized CNF(0.017 wt%) and CNC(0.2 wt%) (2% (w/v) ME, 25 °C, 80% RH) (standing cultures) | ~ 42 ± 9 U/mL (25 days) | This study |
| Ganoderma lipsiense 646 (Basidiomycota) | TEMPO-oxidized CNF(0.017 wt%) and CNC(0.2 wt%) (2% (w/v) ME, 120 rpm, 25 °C, 80% RH) | ~ 16 ± 4 U/mL (25 days) | This study |
| Rigidoporus vitreus 643 (Basidiomycota) | TEMPO-oxidized CNF(0.017 wt%) and CNC(0.2 wt%) (2% (w/v) ME, 25 °C, 80% RH) (standing cultures) | ~ 21 ± 2 U/mL (25 days) | This study |
| Rigidoporus vitreus 643 (Basidiomycota) | TEMPO-oxidized CNF(0.017 wt%) and CNC(0.2 wt%) (2% (w/v) ME, 120 rpm, 25 °C, 80% RH) | ~ 15 ± 5 U/mL (25 days) | This study |
| Fungus | Growth Conditions | Laccase Activity | Study |
|---|---|---|---|
| Trametes versicolor IBB 897 | Glucose medium, submerged fermentation containing mandarin peels (25 °C, 150 rpm) | 3008 ± 325 U/L (10 days) | Elisashvili et al. [12] |
| Trametes versicolor IBB 897 | Glucose medium, submerged fermentation containing tree leaves (25 °C, 150 rpm) | 769 ± 84 U/L (10 days) | Ellisashvili et al. [12] |
| Trametes versicolor IBB 897 | Glucose medium, submerged fermentation containing apple peels (25 °C, 150 rpm) | 540 ± 59 U/L (10 days) | Elisashvili et al. [12] |
| Trametes versicolor IBB 897 | Glucose medium, submerged fermentation containing banana peels (25 °C, 150 rpm) | 1294 ± 149 U/L (10 days) | Elisashvili et al. [12] |
| Trametes versicolor (CBS100.29) | Glucose medium, containing barley bran (30 °C, 150 rpm) | 639 U/L (37 days) | Lorenzo et al. [52] |
| Trametes versicolor (CBS100.29) | Glucose medium, containing grape stalks (30 °C, 150 rpm) | 450 U/L (37 days) | Lorenzo et al. [52] |
| Trametes versicolor (CBS100.29) | Glucose medium, containing grape seeds (30 °C, 150 rpm) | 250 U/L (37 days) | Lorenzo et al. [52] |
| Trametes versicolor 775 | Basal synthetic medium containing CMC (180 rpm, RT) | 131 ± 3.7 U/L (5 days) 136 ± 12.9 U/L (8 days) | Mikiashvili et al. [55] |
| Trametes versicolor 775 | Basal synthetic medium containing maltose (180 rpm, RT) | 178 ± 3.4 U/L (5 days) 95 ± 3.6 U/L (8 days) | Mikiashvili et al. [55] |
| Trametes versicolor 775 | Basal synthetic medium containing Avicel (180 rpm, RT) | 48 ± 2.7 U/L (5 days) 30 ± 2.5 U/L (8 days) | Mikiashvili et al. [55] |
| Trametes versicolor 775 | Basal synthetic medium containing Cellobiose (180 rpm, RT) | 663 ± 22.2 U/L (5 days) 742 ± 29.8 U/L (8 days) | Mikiashvili et al. [55] |
| Trametes versicolor 775 | Basal synthetic medium containing mandarin peels (180 rpm, RT) | 5243 ± 113 U/L (5 days) 3438 ± 80.9 U/L (8 days) | Mikiashvili et al. [55] |
| Trametes versicolor 145 | Basal synthetic medium containing CMC (180 rpm, RT) | 27 ± 2.7 U/L (5 days) 35 ± 2.8 U/L (8 days) | Mikiashvili et al. [55] |
| Trametes versicolor 145 | Basal synthetic medium containing maltose (180 rpm, RT) | 69 ± 10.3 U/L (5 days) 48 ± 3.5 U/L (8 days) | Mikiashvili et al. [55] |
| Trametes versicolor 145 | Basal synthetic medium containing Avicel (180 rpm, RT) | 15 ± 0.1 U/L (5 days) 11 ± 0.5 U/L (8 days) | Mikiashvili et al. [55] |
| Trametes versicolor 145 | Basal synthetic medium containing Cellobiose (180 rpm, RT) | 34 ± 4.1 U/L (5 days) 26 ± 3.4 U/L (8 days) | Mikiashvili et al. [55] |
| Trametes versicolor 145 | Basal synthetic medium containing mandarin peels (180 rpm, RT) | 428 ± 19.5 U/L (5 days) 79 ± 2.2 U/L (8 days) | Mikiashvili et al. [55] |
| Trametes versicolor (L) Loyd (CTB 863A) | Birch wood (5% malt extract, 2% agar) (22 °C, 70% RH) | 0.01 U/mL (42 days) | Irbe et al. [48] |
| Trametes versicolor (L) Loyd (CTB 863A) | Aspen wood (5% malt extract, 2% agar) (22 °C, 70% RH) | 0.01 U/mL (42 days) | Irbe et at. [48] |
| Trametes versicolor (L) Loyd (CTB 863A) | Alder wood (5% malt extract, 2% agar) (22 °C, 70% RH) | 0 U/mL (42 days) | Irbe et al. [48] |
| Trametes versicolor (Empa strain 159) | Basal synthetic medium containing glucose and veratryl alcohol (25 °C, standing cultures) | 122 ± 24 U/L (9 days) | Ihssen et al. [53] |
| Trametes versicolor (Empa strain 159) | Basal synthetic medium containing wood spruce dust (25 °C, standing cultures) | 198 ± 44 U/L (9 days) | Ihssen et al. [53] |
| Trametes versicolor (Empa strain 159) | TEMPO-oxidized CNF(0.017 wt%) and CNC(0.2 wt%) (2% (w/v) ME, 25 °C, 80% RH) (standing cultures) | ~351 ± 35 U/L (25 days) | This study |
| Trametes versicolor (Empa strain 159) | TEMPO-oxidized CNF(0.017 wt%) and CNC (0.2 wt%) (2% (w/v) ME, 25 °C, 80% RH, 120 rpm) | ~225 ± 79 U/L (14 days) | This study |
| ** Trametes pubescens (Empa strain 220) | Basal synthetic medium containing glucose and veratryl alcohol (25 °C, standing cultures) | 282 ± 86 U/L (9 days) | Ihssen et al. [53] |
| ** Trametes pubescens (Empa strain 220) | Basal synthetic medium containing wood spruce dust (25 °C, standing cultures) | 53 ± 20 U/L (9 days) | Ihssen et al. [53] |
| Trametes pubescens (Empa strain 220) | TEMPO-oxidized CNF(0.017 wt%) and CNC(0.2 wt%) (2% (w/v) ME, 25 °C, 80% RH) (standing cultures) | ~30 ± 16 U/L (25 days) | This study |
| Trametes pubescens (Empa strain 220) | TEMPO-oxidized-CNF(0.017 wt%) and CNC (0.2 wt%) (2% (w/v) ME, 25 °C, 80% RH, 120 rpm) | ~110 ± 71 U/L (14 days) | This study |
| Rigidoporus vitreus (Empa strain 642) | Basal synthetic medium containing glucose and veratryl alcohol (25 °C, standing cultures) | 2128 ± 252 U/L (9 days) | Ihssen et al. [53] |
| Rigidoporus vitreus (Empa strain 642) | Basal synthetic medium containing wood spruce dust (25 °C, standing cultures) | 755 ± 148 U/L (9 days) | Ihssen et al. [53] |
| * Rigidoporus vitreus (Empa strain 643) | TEMPO-oxidized-CNF(0.017 wt%) and CNC(0.2 wt%) (2% (w/v) ME, 25 °C, 80% RH) (standing cultures) | ~603 ± 75 U/L (25 days) | This study |
| * Rigidoporus vitreus (Empa strain 643) | TEMPO-oxidized-CNF(0.017 wt%) and CNC(0.2 wt%) (2% (w/v) ME, 25 °C, 80% RH, 120 rpm) | ~238 ± 32 U/L (14 days) | This study |
| † Ganoderma lipsiense (Empa strain 646) | Basal synthetic medium containing glucose and veratryl alcohol (25 °C, standing cultures) | 104 ± 18 U/L (9 days) | Ihssen et al. [53] |
| † Ganoderma lipsiense (Empa strain 646) | Basal synthetic medium containing wood spruce dust (25 °C, standing cultures) | 18 ± 15 U/L (9 days) | Ihssen et al. [53] |
| Ganoderma lipsiense (Empa strain 646) | TEMPO-oxidized CNF(0.017 wt%) and CNC(0.2 wt%) (2% (w/v) ME, 25 °C, 80% RH) (standing cultures) | ~4 ± 0 U/L (14 days) | This study |
| Ganoderma lipsiense (Empa strain 646) | TEMPO-oxidized CNF(0.017 wt%) and CNC(0.2 wt%) (2% (w/v) ME, 25 °C, 80% RH, 120 rpm) | ~2 ± 1 U/L (11 days) | This study |
| ¥ Ganoderma adspersum (Empa strain 003) | TEMPO-oxidized CNF(0.017 wt%) and CNC(0.2 wt%) (2% (w/v) ME, 25 °C, 80% RH) (standing cultures) | ~95 ± 31 U/L (25 days) | This study |
| ¥ Ganoderma adspersum (Empa strain 003) | TEMPO-oxidized CNF(0.017 wt%) and CNC(0.2 wt%) (2% (w/v) ME, 25 °C, 80% RH, 120 rpm) | ~19 ± 14 U/L (25 days) | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes, C.; Poulin, A.; Nyström, G.; Schwarze, F.W.M.R.; Ribera, J. Enzyme Activities of Five White-Rot Fungi in the Presence of Nanocellulose. J. Fungi 2021, 7, 222. https://doi.org/10.3390/jof7030222
Reyes C, Poulin A, Nyström G, Schwarze FWMR, Ribera J. Enzyme Activities of Five White-Rot Fungi in the Presence of Nanocellulose. Journal of Fungi. 2021; 7(3):222. https://doi.org/10.3390/jof7030222
Chicago/Turabian StyleReyes, Carolina, Alexandre Poulin, Gustav Nyström, Francis W. M. R. Schwarze, and Javier Ribera. 2021. "Enzyme Activities of Five White-Rot Fungi in the Presence of Nanocellulose" Journal of Fungi 7, no. 3: 222. https://doi.org/10.3390/jof7030222
APA StyleReyes, C., Poulin, A., Nyström, G., Schwarze, F. W. M. R., & Ribera, J. (2021). Enzyme Activities of Five White-Rot Fungi in the Presence of Nanocellulose. Journal of Fungi, 7(3), 222. https://doi.org/10.3390/jof7030222






