Longitudinal Evaluation of Plasma Cytokine Levels in Patients with Invasive Candidiasis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Study Cohort
3.2. Cytokine Measurements
3.2.1. IL-17A
3.2.2. Other Cytokines (IL-6, IL-8, IL-10, IL-17F, IL-22, IL-23 (p19), IFN-γ, TNF-α, PTX3, TGF-β)
3.3. Kyurenine and Tryptophan Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Category | Host Factor | Clinical Presentation | Mycological Evidence |
|---|---|---|---|
| Proven IC 1 | not required | Candida species isolated from blood cultures or from other specimen obtained by a sterile procedure from a normally sterile site (including a freshly placed (<24 h ago) drain) showing a clinical or radiological abnormality consistent with an infectious disease process | |
| Probable IC | At least one of the following:
| clinical or radiological (non-pulmonary) abnormalities consistent with an infectious disease process that are otherwise unexplained | At least one of the following:
|
| Time Interval (Study Days) | Contrast | Estimate (Lower, Upper CI) | p |
|---|---|---|---|
| (−4;−2) | IC (true)—IC (other) | 0.39 (−0.22, 1.00) | 0.205 |
| IC (true)—S. aureus | −0.43 (−1.55, 0.68) | 0.436 | |
| IC (true)—E. coli | 0.38 (−0.46, 1.22) | 0.365 | |
| IC (true)—healthy | 2.30 (1.53, 3.07) | <0.001 | |
| IC (other)—S. aureus | −0.82 (−2.01, 0.37) | 0.169 | |
| IC (other)—E. coli | −0.01 (−0.94, 0.92) | 0.986 | |
| IC (other)—healthy | 1.91 (1.05, 2.78) | <0.001 | |
| S. aureus—E. coli | 0.81 (−0.51, 2.13) | 0.219 | |
| S. aureus—healthy | 2.73 (1.46, 4.01) | <0.001 | |
| E. coli—healthy | 1.92 (0.88, 2.96) | 0.001 | |
| (−1;2) | IC (true)—IC (other) | 0.93 (0.49, 1.38) | <0.001 |
| IC (true)—S. aureus | 0.11 (−0.43, 0.64) | 0.694 | |
| IC (true)—E. coli | −0.07 (−0.57, 0.43) | 0.785 | |
| IC (true)—healthy | 2.49 (1.46, 3.51) | <0.001 | |
| IC (other)—S. aureus | −0.83 (−1.37, −0.28) | 0.003 | |
| IC (other)—E. coli | −1.00 (−1.52, −0.49) | <0.001 | |
| IC (other)—healthy | 1.55 (0.52, 2.58) | 0.003 | |
| S. aureus—E. coli | −0.18 (−0.77, 0.42) | 0.559 | |
| S. aureus—healthy | 2.38 (1.31, 3.45) | <0.001 | |
| E. coli—healthy | 2.56 (1.50, 3.61) | <0.001 | |
| (3;7) | IC (true)—IC (other) | 0.99 (0.52, 1.46) | <0.001 |
| IC (true)—S. aureus | 0.29 (−0.28, 0.86) | 0.317 | |
| IC (true)—E. coli | 0.75 (0.20, 1.30) | 0.008 | |
| IC (true)—healthy | 2.38 (1.34, 3.43) | <0.001 | |
| IC (other)—S. aureus | −0.70 (−1.28, −0.12) | 0.019 | |
| IC (other)—E. coli | −0.24 (−0.80, 0.32) | 0.405 | |
| IC (other)—healthy | 1.39 (0.34, 2.44) | 0.010 | |
| S. aureus—E. coli | 0.46 (−0.18, 1.11) | 0.159 | |
| S. aureus—healthy | 2.09 (0.99, 3.19) | <0.001 | |
| E. coli—healthy | 1.63 (0.54, 2.72) | 0.004 | |
| (8;14) | IC (true)—IC (other) | 0.33 (−0.27, 0.94) | 0.276 |
| IC (true)—S. aureus | −0.25 (−0.87, 0.37) | 0.425 | |
| IC (true)—E. coli | −0.07 (−0.68, 0.54) | 0.821 | |
| IC (true)—healthy | 1.72 (0.76, 2.69) | 0.001 | |
| IC (other)—S. aureus | −0.58 (−1.19, 0.03) | 0.061 | |
| IC (other)—E. coli | −0.40 (−1.01, 0.20) | 0.187 | |
| IC (other)—healthy | 1.39 (0.43, 2.35) | 0.005 | |
| S. aureus—E. coli | 0.18 (−0.44, 0.80) | 0.563 | |
| S. aureus—healthy | 1.97 (1.01, 2.94) | <0.001 | |
| E. coli—healthy | 1.79 (0.83, 2.75) | <0.001 |
| Time Interval | Study Group | Mean | SD | Median | IQR |
|---|---|---|---|---|---|
| (−4;−2) | IC (true) | 7.6 | 5.1 | 7.1 | 4.0–10.0 |
| IC (other) | 6.7 | 4.6 | 6.6 | 2.4–10.6 | |
| S. aureus | 9.4 | 2.8 | 7.7 | 7.7–11.2 | |
| E. coli | 5.3 | 2.5 | 4.8 | 4.7–7.1 | |
| healthy controls | 0.9 | 0.6 | 0.9 | 0.2–1.4 | |
| (−1;2) | IC (true) | 11.9 | 9.9 | 8.8 | 5.4–17.3 |
| IC (other) | 6.7 | 13.2 | 3.2 | 1.5–6.6 | |
| S. aureus | 11.6 | 13.1 | 8.8 | 3.4–13.0 | |
| E. coli | 10.8 | 7.1 | 10.1 | 7.5–13.0 | |
| healthy controls | 0.9 | 0.6 | 0.9 | 0.2–1.4 | |
| (3;7) | IC (true) | 10.3 | 12.2 | 6.6 | 3.3–12.0 |
| IC (other) | 9.5 | 17.4 | 3.3 | 1.5–8.8 | |
| S. aureus | 6.8 | 4.9 | 6.3 | 3.4–9.7 | |
| E. coli | 4.7 | 3.4 | 4.7 | 1.6–6.5 | |
| healthy controls | 0.9 | 0.6 | 0.9 | 0.2–1.4 | |
| (8;14) | IC (true) | 5.6 | 4.2 | 5.4 | 2.0–7.8 |
| IC (other) | 7.1 | 9.7 | 2.5 | 1.4–7.1 | |
| S. aureus | 6.3 | 3.3 | 6.2 | 3.2–9.1 | |
| E. coli | 4.6 | 4.1 | 3.4 | 2.3–4.9 | |
| healthy controls | 0.9 | 0.6 | 0.9 | 0.2–1.4 |
| Comparison of Time Intervals | Estimate (Lower, Upper CI) | p |
|---|---|---|
| (−4;−2) vs. (−1;2) | −0.50 (−0.74, −0.26) | <0.001 |
| (−4;−2) vs. (3;7) | −0.45 (−0.71, −0.20) | 0.001 |
| (−4;−2) vs. (8;14) | −0.01 (−0.30, 0.28) | 0.961 |
| (−1;2) vs. (3;7) | 0.05 (−0.11, 0.21) | 0.553 |
| (−1;2) vs. (8;14) | 0.49 (0.28, 0.70) | <0.001 |
| (3;7) vs. (8;14) | 0.44 (0.25, 0.63) | <0.001 |

References
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Prim. 2018, 4, 18026. [Google Scholar] [CrossRef]
- Nucci, M.; Anaissie, E. Revisiting the source of candidemia: Skin or Gut? Clin. Infect. Dis. 2001, 33, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Righi, E.; Ansaldi, F.; Merelli, M.; Cecilia, T.; De Pascale, G.; Diaz-Martin, A.; Luzzati, R.; Rosin, C.; Lagunes, L.; et al. A multicenter study of septic shock due to candidemia: Outcomes and predictors of mortality. Intensive Care Med. 2014, 40, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Apostolou, K.E.; Pappas, V.D. Attributable mortality of candidemia: A systematic review of matched cohort and case-control studies. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.H. Finding the missing 50% of invasive candidiasis: How nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin. Infect. Dis. 2013, 56, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-C.; Van De Veerdonk, F.; Smeekens, S.; Joosten, L.A.; Van Der Meer, J.W.M.; Kullberg, B.-J.; Netea, M.G. Candida albicans dampens host defense by downregulating IL-17 production. J. Immunol. 2010, 185, 2450–2457. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.H.; Sherman, G.; Ward, S.; Fraser, V.J.; Kollef, M.H. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000, 118, 146–155. [Google Scholar] [CrossRef]
- Garey, K.W.; Rege, M.; Pai, M.P.; Mingo, D.E.; Suda, K.J.; Turpin, R.S.; Bearden, D.T. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: A multi-institutional study. Clin. Infect. Dis. 2006, 43, 25–31. [Google Scholar] [CrossRef]
- Knitsch, W.; Vincent, J.L.; Utzolino, S.; François, B.; Dinya, T.; Dimopoulos, G.; Özgüneş, I.; Valía, J.C.; Eggimann, P.; León, C.; et al. A randomized, placebo-controlled trial of preemptive antifungal therapy for the prevention of invasive candidiasis following gastrointestinal surgery for intra-abdominal infections. Clin. Infect. Dis. 2015, 61, 1671–1678. [Google Scholar] [CrossRef]
- León, C.; Ruiz-Santana, S.; Saavedra, P.; Galván, B.; Blanco, A.; Castro, C.; Balasini, C.; Utande-Vázquez, A.; De Molina, F.J.G.; Blasco-Navalproto, M.A.; et al. Usefulness of the ‘Candida score’ for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: A prospective multicenter study. Crit. Care Med. 2009, 37, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Estrella, M.; Verweij, P.E.; Arendrup, M.C.; Arikan-Akdagli, S.; Bille, J.; Donnelly, J.P.; Jensen, H.E.; Lass-Flörl, C.; Richardson, M.D.; Akova, M.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Diagnostic procedures. Clin. Microbiol. Infect. 2012, 18, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Reiss, E.; Obayashi, T.; Orle, K.; Yoshida, M.; Zancopé-Oliveira, R.M. Non-culture based diagnostic tests for mycotic infections. Med. Mycol. 2000, 38, 147–159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bruyère, R.; Quenot, J.P.; Prin, S.; Dalle, F.; Vigneron, C.; Aho, S.; León, C.; Quenot, J.P. Empirical antifungal therapy with an echinocandin in critically-ill patients: Prospective evaluation of a pragmatic Candida score-based strategy in one medical ICU. BMC Infect. Dis. 2014, 14, 385. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, M.; Calandra, T.; Sanguinetti, M.; Poulain, D.; Viscoli, C. The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: Recommendations from the Third European Conference on Infections in Leukemia. Crit. Care 2010, 14, R222. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, K.M.; Huffnagle, G.B.; Dickson, R.P. The significance of Candida in the human respiratory tract: Our evolving understanding. Pathog. Dis. 2017, 75, ftx029. [Google Scholar] [CrossRef]
- Pitarch, A.; Nombela, C.; Gil, C. Diagnosis of Invasive Candidiasis: From Gold Standard Methods to Promising Leading-edge Technologies. Curr. Top. Med. Chem. 2018, 18, 1375–1392. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Wissel, M.C.; Shields, R.K.; Salomoni, M.A.; Hao, B.; Press, E.G.; Cheng, S.; Mitsani, D.; Vadnerkar, A.; Silveira, F.P.; et al. Performance of Candida real-time polymerase chain reaction, β-D-glucan assay, and blood cultures in the diagnosis of invasive candidiasis. Clin. Infect. Dis. 2012, 54, 1240–1248. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. Diagnosing Invasive Candidiasis. J. Clin. Microbiol. 2018, 56, e01909–e01917. [Google Scholar] [CrossRef]
- Mylonakis, E.; Clancy, C.J.; Ostrosky-Zeichner, L.; Garey, K.W.; Alangaden, G.J.; Vazquez, J.A.; Groeger, J.S.; Judson, M.A.; Vinagre, Y.-M.; Heard, S.O.; et al. T2 Magnetic Resonance Assay for the Rapid Diagnosis of Candidemia in Whole Blood: A Clinical Trial. Clin. Infect. Dis. 2015, 60, 892–899. [Google Scholar] [CrossRef]
- Krause, R.; Zollner-Schwetz, I.; Salzer, H.J.F.; Valentin, T.; Rabensteiner, J.; Prüller, F.; Raggam, R.; Meinitzer, A.; Prattes, J.; Rinner, B.; et al. Elevated levels of interleukin 17A and kynurenine in candidemic patients, compared with levels in noncandidemic patients in the intensive care unit and those in healthy controls. J. Infect. Dis. 2015, 211, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Decker, S.O.; Sigl, A.; Grumaz, C.; Stevens, P.; Vainshtein, Y.; Zimmermann, S.; Weigand, M.A.; Hofer, S.; Sohn, K.; Brenner, T. Immune-Response Patterns and Next Generation Sequencing Diagnostics for the Detection of Mycoses in Patients with Septic Shock—Results of a Combined Clinical and Experimental Investigation. Int. J. Mol. Sci. 2017, 18, 1796. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Prüller, F.; Wagner, J.; Raggam, R.B.; Hoenigl, M.; Kessler, H.H.; Truschnig-Wilders, M.; Krause, R. Automation of serum (1→3)-beta-D-glucan testing allows reliable and rapid discrimination of patients with and without candidemia. Med. Mycol. 2014, 52, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Dambuza, M.; Levitz, S.M.; Netea, M.G.; Brown, G.D. Fungal Recognition and Host Defense Mechanisms. Microbiol Spectr. 2017, 5, 887–902. [Google Scholar]
- Ferwerda, G.; Meyer-Wentrup, F.; Kullberg, B.J.; Netea, M.G.; Adema, G.J. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell. Microbiol. 2008, 10, 2058–2066. [Google Scholar] [CrossRef]
- Luca, D.; Zelante, T.; D’Angelo, C.; Zagarella, S.; Fallarino, F.; Spreca, A.; Iannitti, R.G.; Bonifazi, P.; Renauld, J.; Bistoni, F.; et al. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. 2010, 3, 361–373. [Google Scholar] [CrossRef]
- Cheng, S.C.; Joosten, L.A.B.; Kullberg, B.J.; Netea, M.G. Interplay between Candida albicans and the mammalian innate host defense. Infect. Immun. 2012, 80, 1304–1313. [Google Scholar] [CrossRef]
- Hernández-Santos, N.; Gaffen, S. Th17 cells in immunity to Candida albicans. Cell Host Microbe 2012, 11, 425–435. [Google Scholar] [CrossRef]
- Van de Veerdonk, F.L.; Marijnissen, R.J.; jan Kullberg, B.; Koenen, H.J.P.M.; Cheng, S.; Joosten, I.; van den Berg, W.B.; Williams, D.L.; van der Meer, J.W.M.; Joosten, L.A.B.; et al. The Macrophage Mannose Receptor Induces IL-17 in Response to Candida albicans. Cell Host Microbe 2009, 5, 329–340. [Google Scholar] [CrossRef]
- Borghi, M.; Renga, G.; Puccetti, M.; Oikonomou, V.; Palmieri, M.; Galosi, C.; Romani, A.B.L. Antifungal Th Immunity: Growing up in Family. Front. Immunol. 2014, 5, 506. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, X.; Wang, C.; Muthu, S.E. The clinical value of IL-3, IL-4, IL-12p70, IL17A, IFN-γ, MIP-1β, NLR, P-selectin, and TNF-α in differentiating bloodstream infections caused by gram-negative, gram-positive bacteria and fungi in hospitalized patients: An Observational Study. Medicine 2019, 98, e17315. [Google Scholar] [CrossRef] [PubMed]
- Akin, H.; Akalin, H.; Budak, F.; Ener, B.; Ocakoglu, G.; Gürcüoğlu, E.; Göral, G.; Oral, B.H. Alterations of serum cytokine levels and their relation with inflammatory markers in candidemia. Med. Mycol. 2015, 53, 258–268. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gaffen, S.L.; Hernández-Santos, N.; Peterson, A.C. IL-17 signaling in host defense against Candida albicans. Immunol. Res. 2011, 50, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Heldt, S.; Prattes, J.; Eigl, S.; Spiess, B.; Flick, H.; Rabensteiner, J.; Johnson, G.; Prüller, F.; Wölfler, A.; Niedrist, T.; et al. Diagnosis of invasive aspergillosis in hematological malignancy patients: Performance of cytokines, Asp LFD, and Aspergillus PCR in same day blood and bronchoalveolar lavage samples. J. Infect. 2018, 77, 235–241. [Google Scholar] [CrossRef]
- Costantini, C.; Bellet, M.M.; Renga, G.; Stincardini, C.; Borghi, M.; Pariano, M.; Cellini, B.; Keller, N.; Romani, L.; Zelante, T. Tryptophan Co-Metabolism at the Host-Pathogen Interface. Front. Immunol. 2020, 11, 67. [Google Scholar] [CrossRef]
- Alvarez, Y.; Municio, C.; Hugo, E.; Zhu, J.; Alonso, S.; Hu, X.; Fernández, N.; Crespo, M.S. Notch- and transducin-like enhancer of split (TLE)-dependent histone deacetylation explain interleukin 12 (IL-12) p70 inhibition by zymosan. J. Biol. Chem. 2011, 286, 16583–16595. [Google Scholar] [CrossRef]
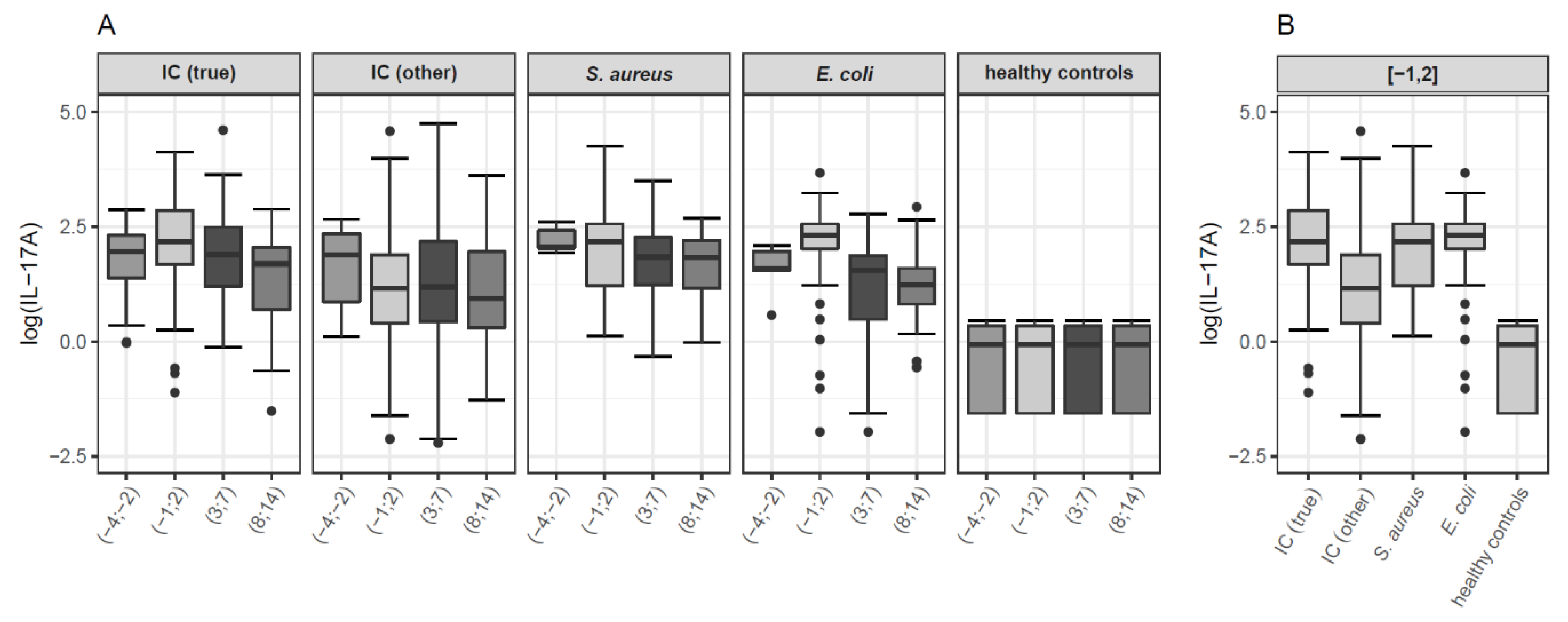

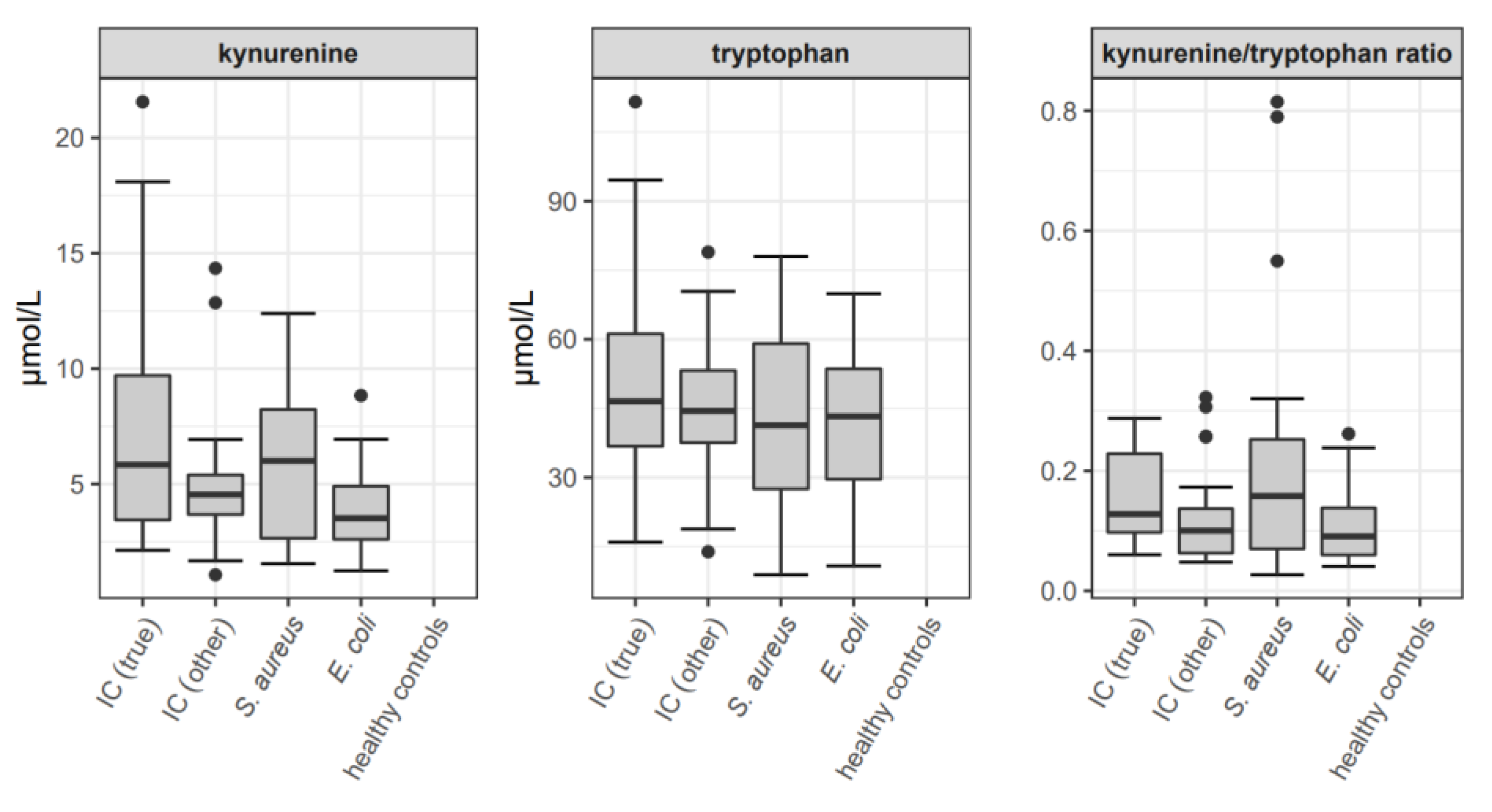
| Variable | Invasive Candidiasis | S. aureus Bacteremia | E. coli Bacteremia | Healthy Controls | p |
|---|---|---|---|---|---|
| No. of Patients | 101 | 23 | 28 | 32 | |
| Age, Years; Median (Range) | 64 (18–91) | 69 (52–87) | 79 (18–96) | 46 (19–86) | <0.001 |
| Sex | 0.012 | ||||
| Male | 64 (63.4) | 17 (73.9) | 10 (35.7) | 15 (46.9) | |
| Female | 37 (36.6) | 6 (26.1) | 18 (64.3) | 17 (53.1) | |
| Cause of Hospital Admission | |||||
| Elective Surgery | 24 (23.8) | 2 (8.7) | 0 | 32 (100) | |
| Infectious Disease | 24 (23.8) | 10 (43.5) | 14 (50) | ||
| Gastrointestinal Disease | 20 (19.8) | 1 (4.3) | 7 (25) | ||
| Malignant Disease | 16 (15.8) | 1 (4.3) | 3 (10.7) | ||
| Cardiovascular Disease | 4 (4.0) | 4 (17.4) | 3 (10.7) | ||
| Other | 13 (12.9) | 5 (21.7) | 1 (3.6) | ||
| Ward | |||||
| Internal Medicine | 13 (12.9) | 13 (56.5) | 25 (89.3) | ||
| Surgery | 27 (26.7) | 4 (17.4) | 1 (3.6) | 32 (100) | |
| Icu | 44 (43.6) | 4 (17.4) | 1 (3.6) | ||
| Other or Unknown | 17 (16.8) | 2 (8.7) | 1 (3.6) | ||
| Recent Abdominal Surgery | 50 (49.5) | 1 (4.3) | 0 | 0 | <0.001 |
| Recent Other Surgery | 42 (41.6) | 5 (21.7) | 5 (17.9) | 0 | |
| Active Hemato-oncological Disease | 41 (40.6) | 2 (8.7) | 5 (17.9) | 0 | 0.001 |
| Solid Tumor | 35/41 (85.4) | 1/2 (50) | 2/5 (40) | ||
| Hematological Malignancy | 6/41 (14.6) | 1/2 (50) | 3/5 (60) | ||
| Immunosuppressive Therapy | 18 (17.8) | 2 (8.7) | 6 (21.4) | 0 |
| Variable | No. (%) of Patients |
|---|---|
| Overall Study Cohort | 101 (100) |
| Center 1 (Graz) | 86 (85.1) |
| Center 2 (Innsbruck) | 8 (7.9) |
| Center 3 (Cologne) | 7 (6.9) |
| Category | |
| Proven IC | 62 (61.4) |
| Probable IC | 34 (33.7) |
| Possible IC | 5 (5.0) |
| Candidemia | |
| Total Number | 71 (70.3) |
| True Candidemia 1 | 56 (55.4) |
| Candida Species | |
| C. albicans | 70 (69.3) |
| C. glabrata | 13 (12.9) |
| C. parapsilosis | 8 (7.9) |
| >1 Candida spp. | 6 (5.9) |
| Other Candida spp. | 4 (4.0) |
| Follow-Up Blood Cultures 2 | |
| Candidemia (Total Number) 3 | 40/56 (71.4) |
| True Candidemia 4 | 27/41 (65.9) |
| Positivity Rate of Follow-Up Blood Cultures 2 | |
| Candidemia (Total Number) 5 | 7/40 (17.5) |
| True Candidemia 6 | 7/27 (25.9) |
| Variable | Invasive Candidiasis | S. aureus Bacteremia | E. coli Bacteremia | p |
|---|---|---|---|---|
| No. of Patients | 101 | 23 | 28 | |
| Duration of In-Hospital Stay Prior to Index Sampling, Days | 16 (0–98) | 1 (0–24) | 0 (0–52) | <0.001 |
| Duration of ICU Stay Prior to Index Sampling, Days | 5 (0–121) | 0 (0–5) | 0 (0–0) | <0.001 |
| Laboratory Values on Day of Index Sampling | ||||
| CRP (Mg/L) | 132 (3–521) | 146 (5–472) | 93 (1–399) | 0.226 |
| PCT (Ng/mL) | 1.0 (0.1–109.8) | 0.95 (0.2–358.8) | 5.2 (0.2–84.9) | 0.351 |
| Positive BDG (≥80 pg/mL) * | 31/86 (36.0) | n.a. | n.a. | |
| Within 1 Week Prior to Index Sampling 1 | 4/13 (30.8) | n.a. | n.a. | |
| On Index Sampling Day 1 | 9/15 (60.0) | n.a. | n.a. | |
| Within 1 Week after Index Sampling 1 | 21/43 (48.8) | n.a. | n.a. | |
| Vasopressors 2,* | 28/86 (32.6) | 1 (4.3) | 1 (3.6) | <0.001 |
| Proton-Pump Inhibitors * | 65/86 (75.6) | 13 (56.5) | 15 (53.6) | 0.024 |
| Sedation 3,* | 28/86 (32.6) | 4 (17.4) | 0 | <0.001 |
| Morphine * | 45/86 (52.3) | 5 (21.7) | 6 (21.4) | <0.001 |
| Parenteral Nutrition * | 38/86 (44.2) | 0 | 0 | <0.001 |
| Duration of Parenteral Nutrition Prior to Index Sampling, Days | 8 (0–65) | - | - | |
| Nasogastric Tube * | 39/86 (45.3) | 1 (4.3) | 0 | <0.001 |
| Number of Central Venous Catheters * | 1 (0–3) | 0 (0–2) | 0 (0–1) | <0.001 |
| Femoral Vascular Access * | 12/86 (14) | 0 | 0 | 0.016 |
| Urinary Catheter * | 59/86 (68.6) | 7 (30.4) | 6 (21.4) | <0.001 |
| Duration of Fever Prior to Candida spp./S. aureus/E. coli Detection, Days * | 0 (0–16) | 1 (0–3) | 1 (0–7) | 0.095 |
| Oxygenation Index * | 253 (139–523) | 280 | n.a. | |
| Respiratory Support * | ||||
| Invasive Mechanical Ventilation Prior to Index Sampling | 38/86 (44.2) | 1 (4.3) | 0 | <0.001 |
| Duration of Mechanical Ventilation Prior to Index Sampling, Days | 7 (1–49) | 1 (1) | - | |
| NIV Prior to Index Sampling | 23/86 (26.7) | 0 | 0 | <0.001 |
| Duration of NIV Prior to Index Sampling | 3 (1–15) | - | - | |
| Duration of Mechanical Ventilation Starting From Index Sampling Until end of Ventilation, Days | 0 (0–72) | 0 (0–28) | 0 (0–2) | 0.018 |
| Severity of Illness | ||||
| SAPS3 Score 4 | 54 (19–98) | 62 (31–72) | 44 (34–53) | 0.616 |
| SAPS2 Score 5 | 29 (21–53) | - | - | |
| SOFA Score Minimum 6,* | 2 (0–11) | 2 (0–8) | 2 (0–6) | 0.769 |
| Antibiotic Therapy | ||||
| Duration of Antibiotic Therapy Prior To Index Sampling, Days | 12 (0–145) | 0 (0–5) | 0 (0–51) | <0.001 |
| Antibiotic Therapy During Index Sampling | 85 (84.2) | 6 (26.1) | 3 (10.7) | <0.001 |
| Antibiotic Therapy After Index Sampling | 96 (95) | 23 (100) | 28 (100) | 1.0 |
| Antifungal Therapy | ||||
| Antifungal Therapy Within 8 Weeks Prior To Inclusion | 15 (14.9) | 0 | 0 | |
| Antifungal Therapy During Index Sampling | 11 (10.9) | 0 | 2 (7.1) | 0.308 |
| Antifungal Therapy after Index Sampling | 91 (90.1) | 0 | 0 | |
| Bacteremia | 10 (9.9) | 23 (100) | 28 (100) | <0.001 |
| Other Invasive Bacterial Infection * | 36/86 (41.9) | 2 (8.7) | 4 (14.3) | 0.001 |
| Viremia 7 | 9/33 (27.3) | 0/2 | 1/7 (14.3) | 0.327 |
| Outcome | ||||
| Intrahospital Death | 29/101 (28.7) | 9 (39.1) | 1 (3.6) | 0.008 |
| Death Related to IC | 16/29 (55.2) | - | - | |
| Death Possibly Related to IC | 1/29 (3.4) | - | - | |
| Death Within 30 Days after Index Culture | 24/101 (23.8) | 7 (30.4) | 1 (3.6) | 0.026 |
| Contrast | IL-6 | IL-8 | IL-10 | IL-17F | IL-22 | IL-23 | IFN-γ | TNF-α | PTX3 | TGF-β |
|---|---|---|---|---|---|---|---|---|---|---|
| IC (true) vs. IC (other) | 0.134 | 0.048 IC (true) > | 0.025 IC (true) > | 0.991 | 0.019 IC (true) > | 0.064 | <0.001 IC (true) > | <0.001 IC (true) > | <0.001 IC (true) > | 0.07 |
| IC (true) vs. S. aureus | 0.52 | 0.509 | 0.359 | 0.34 | 0.013 IC (true) > | 0.47 | 0.107 | 0.209 | 0.561 | 0.079 |
| IC (true) vs. E. coli | 0.354 | 0.241 | 0.862 | 0.698 | 0.857 | 0.09 | 0.06 | <0.001 E. coli> | 0.436 | 0.01 IC (true) > |
| IC (true) vs. healthy | n.d. | <0.001 IC (true) > | n.d. | 0.109 | <0.001 IC (true) > | 0.053 | <0.001 IC (true) > | <0.001 IC (true) > | <0.001 IC (true) > | 0.027 IC (true) > |
| IC (other) vs. S. aureus | 0.069 | 0.335 | 0.008 S. aureus> | 0.413 | 0.648 | 0.379 | <0.001 S. aureus> | <0.001 S. aureus> | 0.012 S. aureus> | 0.002 IC (other) > |
| IC (other) vs. E. coli | 0.03 E. coli> | 0.005 E. coli> | 0.03 E. coli> | 0.748 | 0.022 E. coli> | 0.001 E. coli> | <0.001 E. coli> | <0.001 E. coli> | <0.001 E. coli> | <0.001 IC (other) > |
| IC (other) vs. healthy | n.d. | <0.001 IC (other) > | n.d. | 0.123 | 0.002 IC (other) > | 0.33 | <0.001 IC (other) > | 0.001 IC (other) > | <0.001 IC (other) > | <0.001 IC (other) > |
| S. aureus vs. E. coli | 0.843 | 0.114 | 0.479 | 0.556 | 0.014 E. coli> | 0.035 E. coli> | 0.899 | 0.003 E. coli> | 0.239 | 0.641 |
| S. aureus vs. healthy | n.d. | <0.001 S. aureus> | n.d. | 0.054 | 0.006 S. aureus> | 0.149 | <0.001 S. aureus> | <0.001 S. aureus> | <0.001 S. aureus> | 0.869 |
| E. coli vs. healthy | n.d. | <0.001 E. coli> | n.d. | 0.086 | <0.001 E. coli> | 0.006 E. coli > | <0.001 E. coli> | <0.001 E. coli> | <0.001 E. coli> | 0.737 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wunsch, S.; Zurl, C.; Strohmaier, H.; Meinitzer, A.; Rabensteiner, J.; Posch, W.; Lass-Flörl, C.; Cornely, O.; Pregartner, G.; König, E.; et al. Longitudinal Evaluation of Plasma Cytokine Levels in Patients with Invasive Candidiasis. J. Fungi 2021, 7, 101. https://doi.org/10.3390/jof7020101
Wunsch S, Zurl C, Strohmaier H, Meinitzer A, Rabensteiner J, Posch W, Lass-Flörl C, Cornely O, Pregartner G, König E, et al. Longitudinal Evaluation of Plasma Cytokine Levels in Patients with Invasive Candidiasis. Journal of Fungi. 2021; 7(2):101. https://doi.org/10.3390/jof7020101
Chicago/Turabian StyleWunsch, Stefanie, Christoph Zurl, Heimo Strohmaier, Andreas Meinitzer, Jasmin Rabensteiner, Wilfried Posch, Cornelia Lass-Flörl, Oliver Cornely, Gudrun Pregartner, Elisabeth König, and et al. 2021. "Longitudinal Evaluation of Plasma Cytokine Levels in Patients with Invasive Candidiasis" Journal of Fungi 7, no. 2: 101. https://doi.org/10.3390/jof7020101
APA StyleWunsch, S., Zurl, C., Strohmaier, H., Meinitzer, A., Rabensteiner, J., Posch, W., Lass-Flörl, C., Cornely, O., Pregartner, G., König, E., Feierl, G., Hoenigl, M., Prattes, J., Zollner-Schwetz, I., Valentin, T., & Krause, R. (2021). Longitudinal Evaluation of Plasma Cytokine Levels in Patients with Invasive Candidiasis. Journal of Fungi, 7(2), 101. https://doi.org/10.3390/jof7020101







