Fungal Invasive Co-Infection Due to Aspergillus fumigatus and Rhizopus arrhizus: A Rhino-Orbital Presentation
Abstract
:1. Introduction
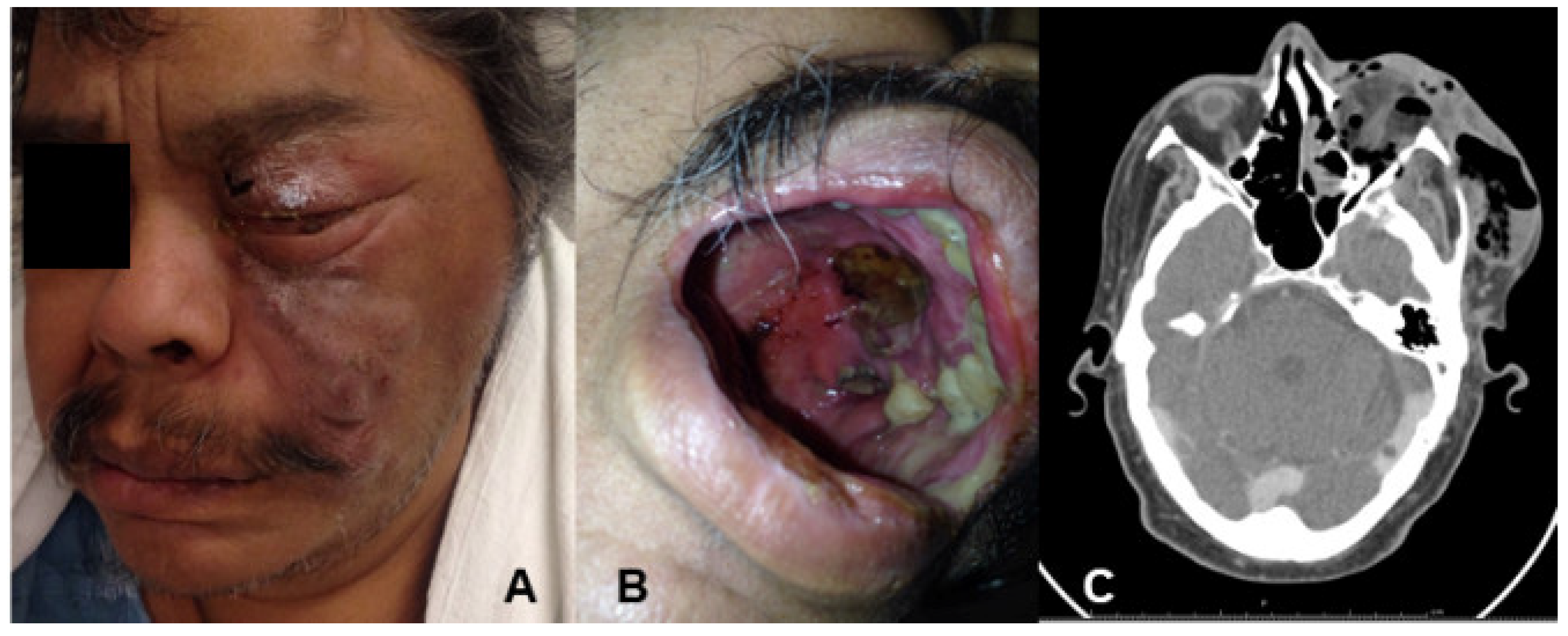
2. Case
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bouza, E.; Muñoz, P.; Guinea, J. Mucormycosis: An emerging disease? Clin. Microbiol. Infect. 2006, 12, 7–23. [Google Scholar] [CrossRef] [Green Version]
- Farmakiotis, D.; Kontoyiannis, D.P. Mucormycoses. Infect. Dis. Clin. N. Am. 2016, 30, 143–163. [Google Scholar] [CrossRef]
- Chermetz, M.; Gobbo, M.; Rupel, K.; Ottaviani, G.; Tirelli, G.; Bussani, R.; Luzzati, R.; Di Lenarda, R.; Biasotto, M. Combined Orofacial Aspergillosis and Mucormycosis: Fatal Complication of a Recurrent Paediatric Glioma—Case Report and Review of Literature. Mycopathologia 2016, 181, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Alcazar-Fuoli, L.; Mellado, E.; Alastruey-Izquierdo, A.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. Aspergillus Section Fumigati: Antifungal Susceptibility Patterns and Sequence-Based Identification. Antimicrob. Agents Chemother. 2008, 52, 1244–1251. [Google Scholar] [CrossRef] [Green Version]
- Lamoth, F. Aspergillus fumigatus-Related Species in Clinical Practice. Front. Microbiol. 2016, 7, 683. [Google Scholar] [CrossRef] [Green Version]
- Walther, G.; Wagner, L.; Kurzai, O. Updates on the Taxonomy of Mucorales with an Emphasis on Clinically Important Taxa. J. Fungi 2019, 5, 106. [Google Scholar] [CrossRef] [Green Version]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and Outcome of Zygomycosis: A Review of 929 Reported Cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef] [Green Version]
- Alfano, C.; Chiummariello, S.; Dessy, L.A.; Bistoni, G.; Scuderi, N. Combined mucormycosis and Aspergillosis of the rhinocerebral region. In Vivo 2006, 20, 311–315. [Google Scholar]
- Radowsky, J.S.; Strawn, A.A.; Sherwood, J.; Braden, A.; Liston, W. Invasive Mucormycosis and Aspergillosis in a Healthy 22-Year-Old Battle Casualty: Case Report. Surg. Infect. 2011, 12, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Korabecná, M.; Liska, V.; Fajfrlík, K. Primers ITS1, ITS2 and ITS4 detect the intraspecies variability in the internal transcribed spacers and 5.8S rRNA gene region in clinical isolates of fungi. Folia Microbiol. 2003, 48, 233–238. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols. A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Balajee, S.A.; Gribskov, J.L.; Hanley, E.; Nickle, D.; Marr, K.A. Aspergillus lentulus sp. nov., a new sibling species of A. fumigatus. Eukaryot. Cell 2005, 4, 625–632. [Google Scholar] [CrossRef] [Green Version]
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.-C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): An overview of families, genera, subgenera, sections, series and species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef]
- Lass-Flörl, C. Zygomycosis: Conventional laboratory diagnosis. Clin. Microbiol. Infect. 2009, 15, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Arndt, S.; Aschendorff, A.; Echternach, M.; Daemmrich, T.D.; Maier, W. Rhino-orbital-cerebral mucormycosis and aspergillosis: Differential diagnosis and treatment. Eur. Arch. Oto-Rhino-Laryngol. 2008, 266, 71–76. [Google Scholar] [CrossRef]
- Severo, L.C.; Guindani, C.; Geyer, G.R. Chronic sinusitis caused by zygomycosis and aspergillosis. Eur. J. Clin. Microbiol. Infect. Dis. 1989, 8, 317–318. [Google Scholar] [CrossRef]
- Maiorano, E.; Favia, G.; Capodiferro, S.; Montagna, M.T.; Muzio, L.L. Combined mucormycosis and aspergillosis of the oro-sinonasal region in a patient affected by Castleman disease. Virchows Arch. 2004, 446, 28–33. [Google Scholar] [CrossRef]
- Pellacchia, V.; Terenzi, V.; Moricca, L.M.; Buonaccorsi, S.; Indrizzi, E.; Fini, G. Brain abscess by mycotic and bacterial infection in a diabetic patient: Clinical report and review of literature. J. Craniofac. Surg. 2006, 17, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Virk, R.S.; Arora, P. Chronic Sinonasal Aspergillosis with Associated Mucormycosis. Ear Nose Throat J. 2007, 86, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishel, J.J.; Sivik, J. Breakthrough invasive fungal infection in an immunocompromised host while on posaconazole prophylaxis: An omission in patient counseling and follow-up. J. Oncol. Pharm. Pract. 2008, 14, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Rit, K.; Saha, R.; Dey, R.; Barik, G. Rhino-oculo-cerebral Aspergillus and mucor co-infections in an immunocompromised patient with type 2 diabetes mellitus. Med. J. Dr. DY Patil Univ. 2014, 7, 486. [Google Scholar] [CrossRef]
- Shashir, W.; Rupali, S.; Sujata, L.; Preeti, M. Concomitant Zygomycosis and Aspergillosis of the rhinocerebral region in a post renal transplant patient. Indian J. Transplant. 2013, 8, 25–27. [Google Scholar] [CrossRef]
- Goswami, S.; Vohra, R.; Raju, B.M.; Agarwal, A. Concomitant mucormycosis and aspergillosis of rhinocerebral region in a renal transplant patient air cooler being the culprit. Indian J. Med. Case Rep. 2016, 5, 30–34. [Google Scholar]

| Case | Clinical Presentation | Underlying Disease | Treatment | Fungal Agents | Reference |
|---|---|---|---|---|---|
| 1 | Sinus | Chronic sinusitis | Surgery | Aspergillus spp./Mucorales | [17] |
| 2 | Oro-sinonasal | Castleman disease | Amphotericin B, and itraconazole | Aspergillus spp./Mucorales | [18] |
| 3 | Rhinocerebral | Diabetes mellitus | Voriconazole, caspofungin, and amphotericin B | A. fumigatus/Mucorales | [8] |
| 4 | Sinus and brain abscess | Diabetes mellitus | Amphotericin B | A. fumigatus/Mucorales | [19] |
| 5 | Sinonasal | Sinusitis | Itraconazole, and amphotericin B | Aspergillosis/Mucorales | [20] |
| 6 | Sinus | Acute myeloid leukemia | Posaconazole | [21] | |
| 7 | Rhino-oculo-cerebral | Diabetes mellitus | Voriconazole | A. flavus/Mucorales | [22] |
| 8 | Rhinocerebral | Diabetes mellitus, and Renal transplantation | Amphotericin B | A. niger/R. arrhizus (syn. R. oryzae) | [23] |
| 9 | Orofacial | Cerebral trunk glioma | Fluconazole, amphotericin B, and micafungin | A. flavus/Mucorales | [3] |
| 10 | Rhinocerebral | Renal transplantation | Amphotericin B | A. fumigatus/ Mucorales | [24] |
| 11 | Rhino-orbital | Diabetes mellitus | Amphotericin B | A. fumigatus/R. arrhizus (syn. R. oryzae) | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Hinojosa, J.P.; Medrano-Ahumada, S.; Arenas, R.; Bravo-Escobar, A.; Paraguirre-Martínez, S.; Xicohtencatl-Cortes, J.; Martínez-Herrera, E.; Hernández-Castro, R. Fungal Invasive Co-Infection Due to Aspergillus fumigatus and Rhizopus arrhizus: A Rhino-Orbital Presentation. J. Fungi 2021, 7, 1096. https://doi.org/10.3390/jof7121096
Ramírez-Hinojosa JP, Medrano-Ahumada S, Arenas R, Bravo-Escobar A, Paraguirre-Martínez S, Xicohtencatl-Cortes J, Martínez-Herrera E, Hernández-Castro R. Fungal Invasive Co-Infection Due to Aspergillus fumigatus and Rhizopus arrhizus: A Rhino-Orbital Presentation. Journal of Fungi. 2021; 7(12):1096. https://doi.org/10.3390/jof7121096
Chicago/Turabian StyleRamírez-Hinojosa, Juan Pablo, Salvador Medrano-Ahumada, Roberto Arenas, Arturo Bravo-Escobar, Sara Paraguirre-Martínez, Juan Xicohtencatl-Cortes, Erick Martínez-Herrera, and Rigoberto Hernández-Castro. 2021. "Fungal Invasive Co-Infection Due to Aspergillus fumigatus and Rhizopus arrhizus: A Rhino-Orbital Presentation" Journal of Fungi 7, no. 12: 1096. https://doi.org/10.3390/jof7121096
APA StyleRamírez-Hinojosa, J. P., Medrano-Ahumada, S., Arenas, R., Bravo-Escobar, A., Paraguirre-Martínez, S., Xicohtencatl-Cortes, J., Martínez-Herrera, E., & Hernández-Castro, R. (2021). Fungal Invasive Co-Infection Due to Aspergillus fumigatus and Rhizopus arrhizus: A Rhino-Orbital Presentation. Journal of Fungi, 7(12), 1096. https://doi.org/10.3390/jof7121096







