Ustilago maydis Secreted Endo-Xylanases Are Involved in Fungal Filamentation and Proliferation on and Inside Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids, and Growth Conditions
2.2. Molecular Biology and Genetics Methods
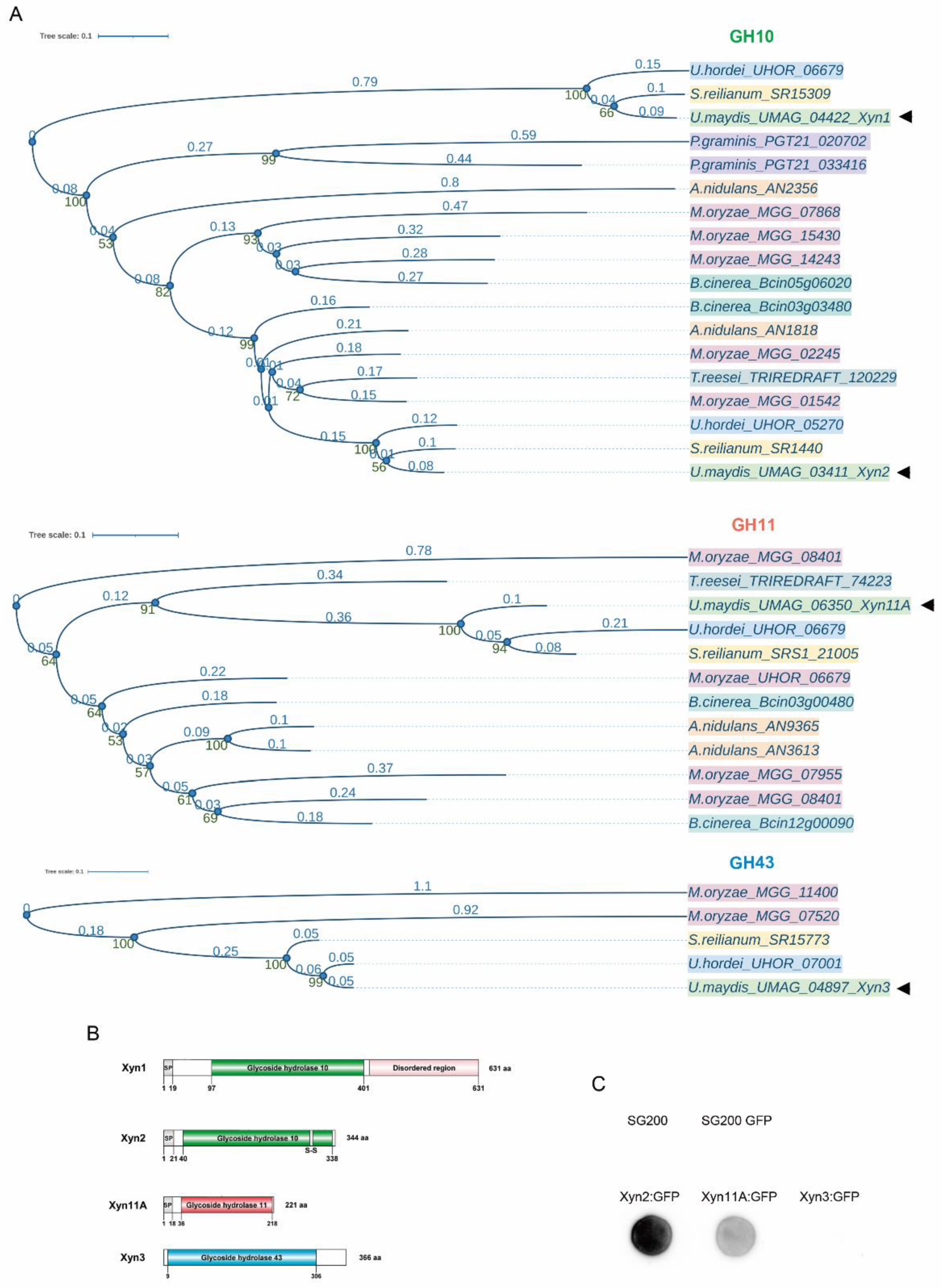
2.3. Sequence Alignment and Phylogenetic Analysis
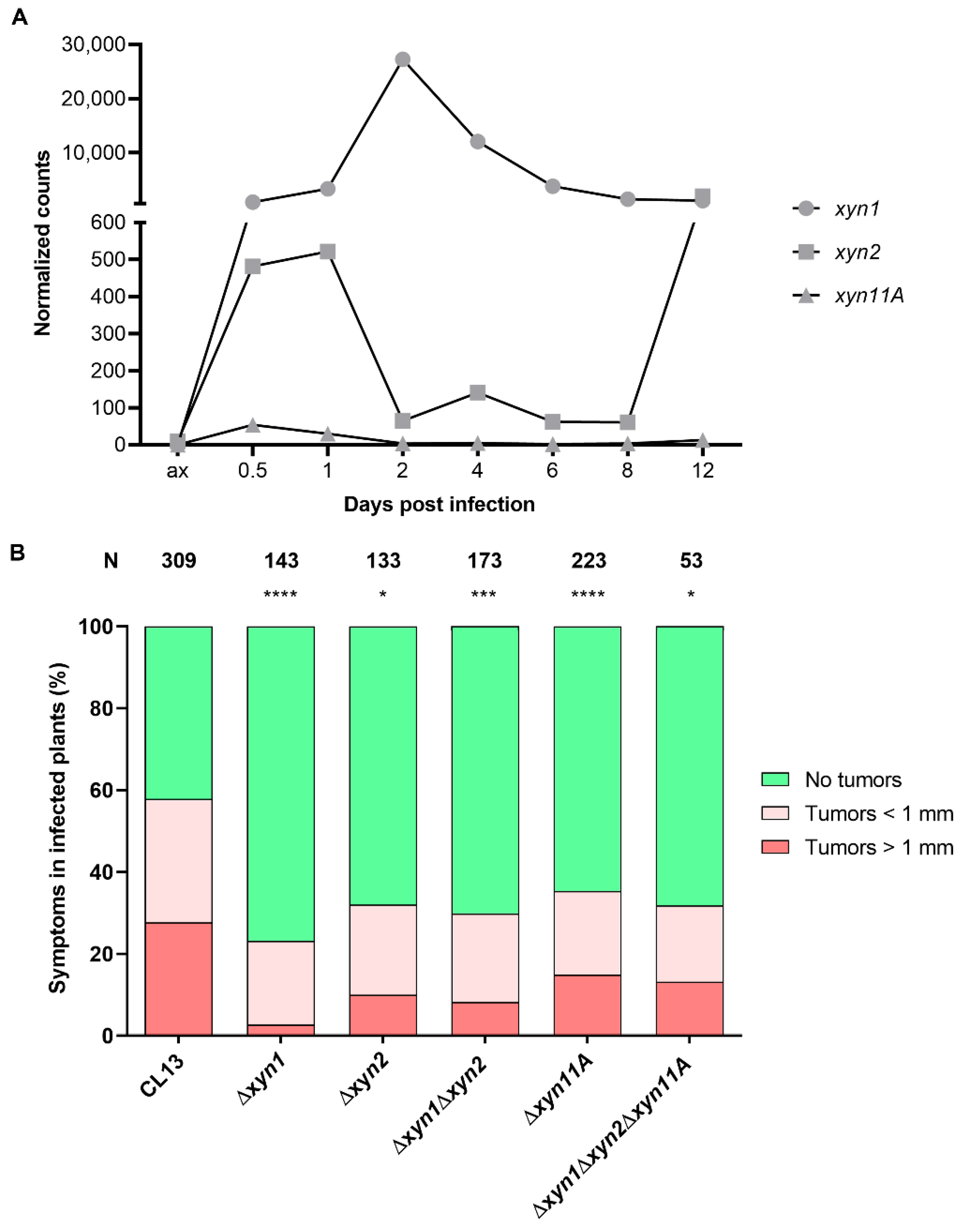
2.4. Infection Stage Analysis
2.5. Protein and Blotting Assays
2.6. Microscopy
3. Results and Discussion
3.1. Ustilago maydis Xylanases Are Secreted and Required for Full Virulence
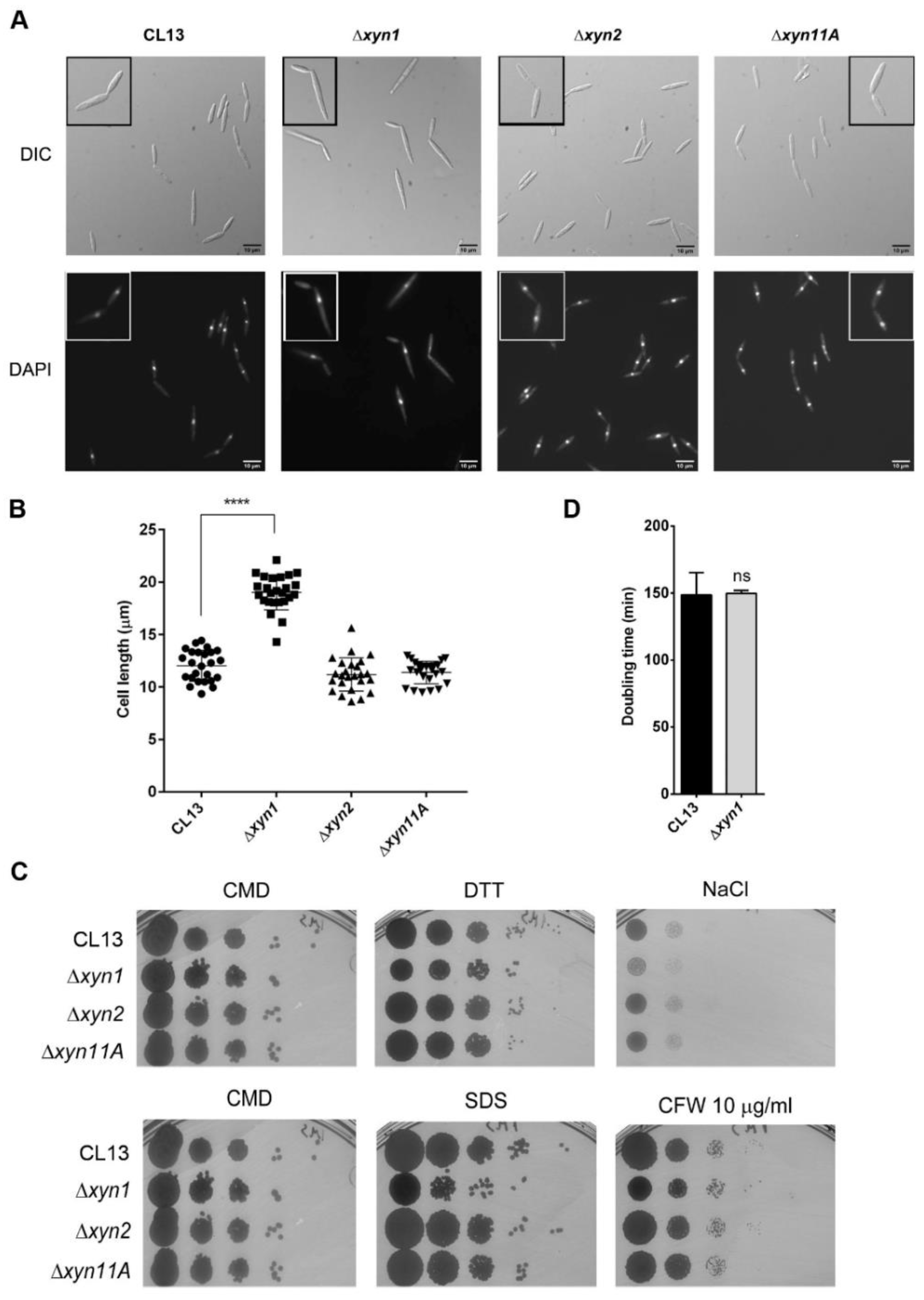
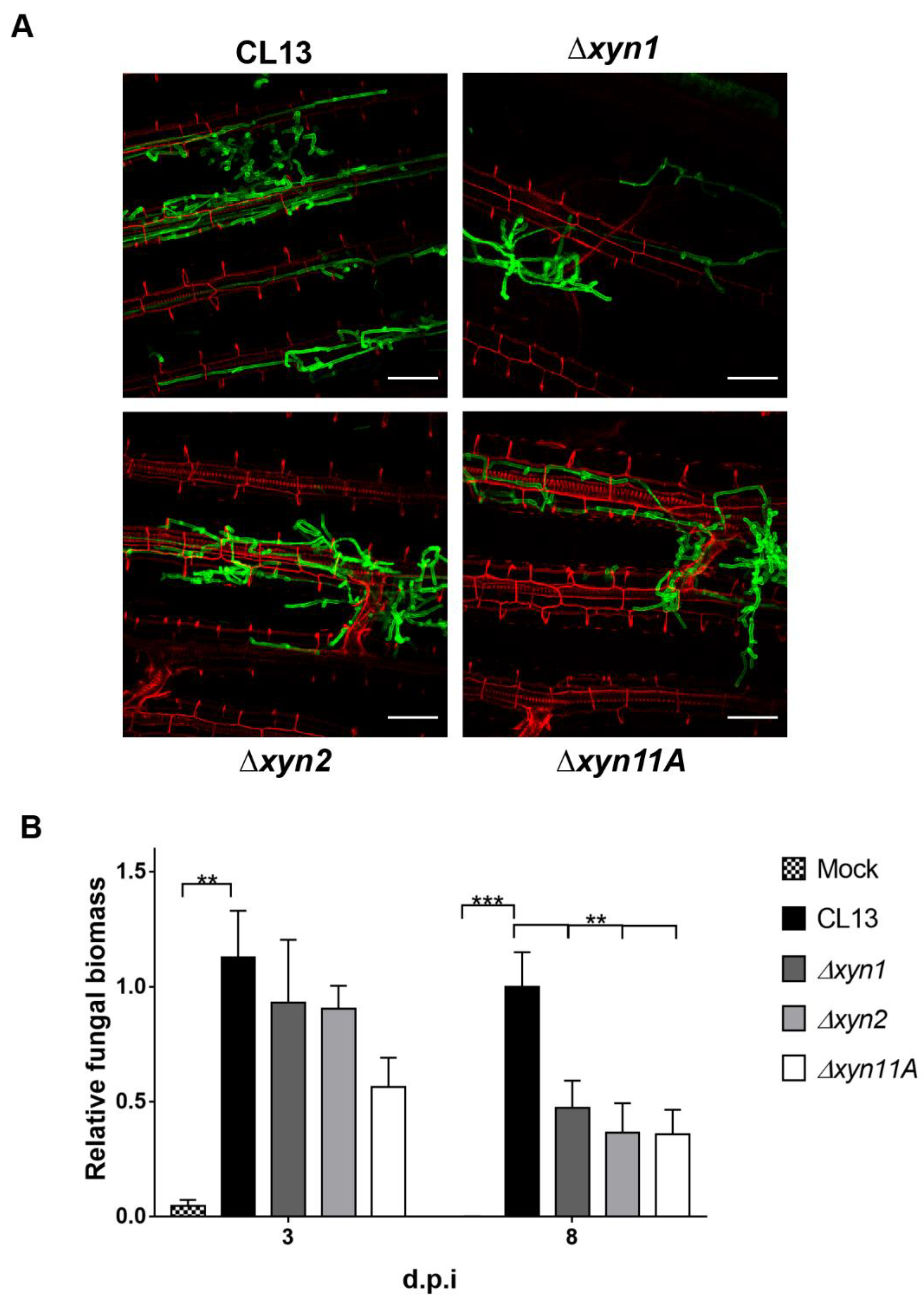
3.2. Xylanases Are Necessary to Assure Proper Fungal Filamentation and Progression Inside the Plant
3.3. Xyn11A Is Secreted to the Apoplast during Fungal Progression Inside the Plant
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van der Linde, K.; Göhre, V. How Do Smut Fungi Use Plant Signals to Spatiotemporally Orientate on and In Planta? J. Fungi 2021, 7, 107. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Kamper, J.; Kahmann, R.; Bolker, M.; Ma, L.J.; Brefort, T.; Saville, B.J.; Banuett, F.; Kronstad, J.W.; Gold, S.E.; Muller, O.; et al. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 2006, 444, 97–101. [Google Scholar] [CrossRef]
- Okmen, B.; Doehlemann, G. Inside plant: Biotrophic strategies to modulate host immunity and metabolism. Curr. Opin. Plant Biol. 2014, 20, 19–25. [Google Scholar] [CrossRef]
- Feldbrügge, M.; Kämper, J.; Steinberg, G.; Kahmann, R. Regulation of mating and pathogenic development in Ustilago maydis. Curr. Opin. Microbiol. 2004, 7, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, S.; Pérez-Martín, J. Appressorium formation in the corn smut fungus Ustilago maydis requires a G2 cell cycle arrest. Plant Signal. Behav. 2015, 10, e1001227. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Mendoza, A.; Berndt, P.; Djamei, A.; Weise, C.; Linne, U.; Marahiel, M.; Vranes, M.; Kamper, J.; Kahmann, R. Physical-chemical plant-derived signals induce differentiation in Ustilago maydis. Mol. Microbiol. 2009, 71, 895–911. [Google Scholar] [CrossRef] [PubMed]
- Vollmeister, E.; Schipper, K.; Baumann, S.; Haag, C.; Pohlmann, T.; Stock, J.; Feldbrügge, M. Fungal development of the plant pathogen Ustilago maydis. FEMS Microbiol. Rev. 2012, 36, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Matei, A.; Doehlemann, G. Cell biology of corn smut disease—Ustilago maydis as a model for biotrophic interactions. Curr. Opin. Microbiol. 2016, 34, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Lanver, D.; Tollot, M.; Schweizer, G.; Lo Presti, L.; Reissmann, S.; Ma, L.-S.; Schuster, M.; Tanaka, S.; Liang, L.; Ludwig, N.; et al. Ustilago maydis effectors and their impact on virulence. Nat. Rev. Microbiol. 2017, 15, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Redkar, A.; Matei, A.; Doehlemann, G. Insights into Host Cell Modulation and Induction of New Cells by the Corn Smut Ustilago maydis. Front. Plant Sci. 2017, 8, 899. [Google Scholar] [CrossRef]
- Horbach, R.; Navarro-Quesada, A.R.; Knogge, W.; Deising, H.B. When and how to kill a plant cell: Infection strategies of plant pathogenic fungi. J. Plant Physiol. 2011, 168, 51–62. [Google Scholar] [CrossRef]
- Lo Presti, L.; Lanver, D.; Schweizer, G.; Tanaka, S.; Liang, L.; Tollot, M.; Zuccaro, A.; Reissmann, S.; Kahmann, R. Fungal effectors and plant susceptibility. Annu. Rev. Plant Biol. 2015, 66, 513–545. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y. Trick or Treat: Microbial Pathogens Evolved Apoplastic Effectors Modulating Plant Susceptibility to Infection. Mol. Plant-Microbe Interact. 2018, 31, 6–12. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Starr, T.L.; Glass, N.L. Plant Cell Wall–Degrading Enzymes and Their Secretion in Plant-Pathogenic Fungi. Annu. Rev. Phytopathol. 2014, 52, 427–451. [Google Scholar] [CrossRef]
- Snetselaar, K.M.; Mims, C.W. Sporidial Fusion and Infection of Maize Seedlings by the Smut Fungus Ustilago maydis. Mycologia 1992, 84, 193. [Google Scholar] [CrossRef]
- Snetselaar, K.M.; Mims, C.W. Infection of Maize Stigmas by Ustilago maydis: Light and Electron Microscopy. Phytopathology 1993, 83, 843. [Google Scholar] [CrossRef]
- Snetselaar, K.M.; Carfioli, M.A.; Cordisco, K.M. Pollination can protect maize ovaries from infection by Ustilago maydis, the corn smut fungus. Can. J. Bot. 2001, 79, 1390–1399. [Google Scholar] [CrossRef]
- Schirawski, J.; Bohnert, H.U.; Steinberg, G.; Snetselaar, K.; Adamikowa, L.; Kahmann, R. Endoplasmic Reticulum Glucosidase II Is Required for Pathogenicity of Ustilago maydis. Plant Cell 2005, 17, 3532–3543. [Google Scholar] [CrossRef] [PubMed]
- Lanver, D.; Berndt, P.; Tollot, M.; Naik, V.; Vranes, M.; Warmann, T.; Munch, K.; Rossel, N.; Kahmann, R. Plant surface cues prime Ustilago maydis for biotrophic development. PLoS Pathog. 2014, 10, e1004272. [Google Scholar] [CrossRef]
- Lanver, D.; Muller, A.N.; Happel, P.; Schweizer, G.; Haas, F.B.; Franitza, M.; Pellegrin, C.; Reissmann, S.; Altmuller, J.; Rensing, S.A.; et al. The biotrophic development of Ustilago maydis studied by RNAseq analysis. Plant Cell 2018, 30, tpc.00764.2017. [Google Scholar] [CrossRef]
- Marín-Menguiano, M.; Moreno-Sánchez, I.; Barrales, R.R.; Fernández-Álvarez, A.; Ibeas, J. N-glycosylation of the protein disulfide isomerase Pdi1 ensures full Ustilago maydis virulence. PLoS Pathog. 2019, 15, e1007687. [Google Scholar] [CrossRef] [PubMed]
- Doehlemann, G.; Wahl, R.; Vranes, M.; de Vries, R.P.; Kämper, J.; Kahmann, R. Establishment of compatibility in the Ustilago maydis/maize pathosystem. J. Plant Physiol. 2008, 165, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.; Gerday, C.; Feller, G. Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol. Rev. 2005, 29, 3–23. [Google Scholar] [CrossRef]
- Shallom, D.; Shoham, Y. Microbial hemicellulases. Curr. Opin. Microbiol. 2003, 6, 219–228. [Google Scholar] [CrossRef]
- Biely, P. Microbial xylanolytic systems. Trends Biotechnol. 1985, 3, 286–290. [Google Scholar] [CrossRef]
- Fontes, C.M.G.A.; Gilbert, H.J.; Hazlewood, G.P.; Clarke, J.H.; Prates, J.A.M.; McKie, V.A.; Nagy, T.; Fernandes, T.H.; Ferreira, L.M.A. A novel Cellvibrio mixtus family 10 xylanase that is both intracellular and expressed under non-inducing conditions. Microbiology 2000, 146, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Shulami, S.; Gat, O.; Sonenshein, A.L.; Shoham, Y. The glucuronic acid utilization gene cluster from Bacillus stearothermophilus T-6. J. Bacteriol. 1999, 181, 3695–3704. [Google Scholar] [CrossRef] [PubMed]
- Teplitsky, A.; Shulami, S.; Moryles, S.; Shoham, Y.; Shoham, G. Crystallization and preliminary X-ray analysis of an intracellular xylanase from Bacillus stearothermophilus T-6. Acta Crystallogr. Sect. D Biol. Crystallogr. 2000, 56, 181–184. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- Lagaert, S.; Beliën, T.; Volckaert, G. Plant cell walls: Protecting the barrier from degradation by microbial enzymes. Semin. Cell Dev. Biol. 2009, 20, 1064–1073. [Google Scholar] [CrossRef]
- Biely, P.; Vršanská, M.; Tenkanen, M.; Kluepfel, D. Endo-β-1,4-xylanase families: Differences in catalytic properties. J. Biotechnol. 1997, 57, 151–166. [Google Scholar] [CrossRef]
- Misas-Villamil, J.C.; van der Hoorn, R. AL Enzyme-inhibitor interactions at the plant-pathogen interface. Curr. Opin. Plant Biol. 2008, 11, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Brito, N.; Espino, J.J.; González, C. The Endo-β-1,4-Xylanase Xyn11A Is Required for Virulence in Botrytis cinerea. Mol. Plant-Microbe Interact. 2006, 19, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Liu, Y.; Zhang, Z. Global characterization of gh10 family xylanase genes in rhizoctonia cerealis and functional analysis of xylanase RcXYN1 during fungus infection in wheat. Int. J. Mol. Sci. 2020, 21, 1812. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gómez, E.; Ruíz-Roldán, M.C.; Di Pietro, A.; Roncero, M.I.G.; Hera, C. Role in pathogenesis of two endo-β-1,4-xylanase genes from the vascular wilt fungus Fusarium oxysporum. Fungal Genet. Biol. 2002, 35, 213–222. [Google Scholar] [CrossRef]
- Wu, S.C.; Ham, K.S.; Darvill, A.G.; Albersheim, P. Deletion of two endo-β-1,4-xylanase genes reveals additional isozymes secreted by the rice blast fungus. Mol. Plant-Microbe Interact. 1997, 10, 700–708. [Google Scholar] [CrossRef]
- Wu, S.C.; Halley, J.E.; Luttig, C.; Fernekes, L.M.; Gutiérrez-Sanchez, G.; Darvill, A.G.; Albersheim, P. Identification of an endo-β-1,4-D-xylanase from Magnaporthe grisea by gene knockout analysis, purification, and heterologous expression. Appl. Environ. Microbiol. 2006, 72, 986–993. [Google Scholar] [CrossRef][Green Version]
- Sambrook, J.; Frisch, E.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Gillissen, B.; Bergemann, J.; Sandmann, C.; Schroeer, B.; Bolker, M.; Kahmann, R. A two-component regulatory system for self/non-self recognition in Ustilago maydis. Cell 1992, 68, 647–657. [Google Scholar] [CrossRef]
- Banuett, F.; Herskowitz, I. Different a alleles of Ustilago maydis are necessary for maintenance of filamentous growth but not for meiosis. Proc. Natl. Acad. Sci. USA 1989, 86, 5878–5882. [Google Scholar] [CrossRef]
- Bölker, M.; Böhnert, H.U.; Braun, K.H.; Görl, J.; Kahmann, R. Tagging pathogenicity genes in Ustilago maydis by restriction enzyme-mediated integration (REMI). Mol. Gen. Genet. 1995, 248, 547–552. [Google Scholar] [CrossRef]
- Doehlemann, G.; Reissmann, S.; Assmann, D.; Fleckenstein, M.; Kahmann, R. Two linked genes encoding a secreted effector and a membrane protein are essential for Ustilago maydis-induced tumour formation. Mol. Microbiol. 2011, 81, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Banuett, F.; Dahl, M.; Schlesinger, R.; Schafer, W.; Martin, T.; Herskowitz, I.; Kahmann, R. The b alleles of U. maydis, whose combinations program pathogenic development, code for polypeptides containing a homeodomain-related motif. Cell 1990, 60, 295–306. [Google Scholar] [CrossRef]
- Wedlich-Soldner, R.; Bolker, M.; Kahmann, R.; Steinberg, G. A putative endosomal t-SNARE links exo- and endocytosis in the phytopathogenic fungus Ustilago maydis. EMBO J. 2000, 19, 1974–1986. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Fernandez-Alvarez, A.; Elias-Villalobos, A.; Ibeas, J.I. The O-mannosyltransferase PMT4 is essential for normal appressorium formation and penetration in Ustilago maydis. Plant Cell 2009, 21, 3397–3412. [Google Scholar] [CrossRef]
- Fernandez-Alvarez, A.; Elias-Villalobos, A.; Jimenez-Martin, A.; Marin-Menguiano, M.; Ibeas, J.I. Endoplasmic reticulum glucosidases and protein quality control factors cooperate to establish biotrophy in Ustilago maydis. Plant Cell 2013, 25, 4676–4690. [Google Scholar] [CrossRef] [PubMed]
- Grigoriev, I.V.; Nikitin, R.; Haridas, S.; Kuo, A.; Ohm, R.; Otillar, R.; Riley, R.; Salamov, A.; Zhao, X.; Korzeniewski, F.; et al. MycoCosm portal: Gearing up for 1000 fungal genomes. Nucleic Acids Res. 2014, 42, D699–D704. [Google Scholar] [CrossRef] [PubMed]
- Geiser, E.; Wierckx, N.; Zimmermann, M.; Blank, L.M. Identification of an endo-1,4-beta-xylanase of Ustilago maydis. BMC Biotechnol. 2013, 13, 59. [Google Scholar] [CrossRef]
- Geiser, E.; Reindl, M.; Blank, L.M.; Feldbrügge, M.; Wierckx, N.; Schipper, K. Activating Intrinsic Carbohydrate-Active Enzymes of the Smut Fungus Ustilago maydis for the Degradation of Plant Cell Wall Components. Appl. Environ. Microbiol. 2016, 82, 5174–5185. [Google Scholar] [CrossRef]
- Babu, M.M. The contribution of intrinsically disordered regions to protein function, cellular complexity, and human disease. Biochem. Soc. Trans. 2016, 44, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Van Der Lee, R.; Buljan, M.; Lang, B.; Weatheritt, R.J.; Daughdrill, G.W.; Dunker, A.K.; Fuxreiter, M.; Gough, J.; Gsponer, J.; Jones, D.T.; et al. Classification of Intrinsically Disordered Regions and Proteins. Chem. Rev. 2014, 114, 6589–6631. [Google Scholar] [CrossRef]
- Wang, R.; Arioka, M. Functional analyses of xylanolytic enzymes involved in xylan degradation and utilization in Neurospora crassa. Int. J. Biol. Macromol. 2021, 169, 302–310. [Google Scholar] [CrossRef]
- Nguyen, Q.B.; Itoh, K.; Van Vu, B.; Tosa, Y.; Nakayashiki, H. Simultaneous silencing of endo-β-1,4 xylanase genes reveals their roles in the virulence of Magnaporthe oryzae. Mol. Microbiol. 2011, 81, 1008–1019. [Google Scholar] [CrossRef]
- Van Vu, B.; Itoh, K.; Nguyen, Q.B.; Tosa, Y.; Nakayashiki, H. Cellulases Belonging to Glycoside Hydrolase Families 6 and 7 Contribute to the Virulence of Magnaporthe oryzae. Mol. Plant-Microbe Interact. 2012, 25, 1135–1141. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Park, S.Y.; Kim, S.G.; Yoo, J.S.; Park, S.; Gupta, R.; Kang, K.Y.; Kim, S.T. Secreted alpha-N-arabinofuranosidase B protein is required for the full virulence of Magnaporthe oryzae and triggers host defences. PLoS ONE 2016, 11, e0165149. [Google Scholar] [CrossRef]
- García, N.; González, M.A.; González, C.; Brito, N. Simultaneous Silencing of Xylanase Genes in Botrytis cinerea. Front. Plant Sci. 2017, 8, 2174. [Google Scholar] [CrossRef]
- Kema, G.H.J.; van der Lee, T.A.J.; Mendes, O.; Verstappen, E.C.P.; Lankhorst, R.K.; Sandbrink, H.; van der Burgt, A.; Zwiers, L.-H.; Csukai, M.; Waalwijk, C. Large-scale gene discovery in the septoria tritici blotch fungus Mycosphaerella graminicola with a focus on in planta expression. Mol. Plant Microb. Interact. 2008, 21, 1249–1260. [Google Scholar] [CrossRef]
- Higa, A.; Taouji, S.; Lhomond, S.; Jensen, D.; Fernandez-Zapico, M.E.; Simpson, J.C.; Pasquet, J.-M.; Schekman, R.; Chevet, E. Endoplasmic Reticulum Stress-Activated Transcription Factor ATF6 Requires the Disulfide Isomerase PDIA5 To Modulate Chemoresistance. Mol. Cell. Biol. 2014, 34, 1839–1849. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, C.J.; Sorgo, A.G.; Mohammadi, S.; Sosinsk, G.J.; de Koster, C.G.; Brul, S.; de Koning, L.J.; Klis, F.M. Surface stress induces a conserved cell wall stress response in the pathogenic fungus Candida albicans. Eukaryot. Cell 2013, 12, 254–264. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.J.; Panstruga, R. Tete a tete inside a plant cell: Establishing compatibility between plants and biotrophic fungi and oomycetes. New Phytol. 2006, 171, 699–718. [Google Scholar] [CrossRef]
- Tucker, S.L.; Talbot, N.J. Surface attachment and pre-penetration stage development by plant pathogenic fungi. Annu. Rev. Phytopathol. 2001, 39, 385–417. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, C.; Giebel, K.F.; Schnell, M.; Leiderer, P.; Deising, H.B.; Bastmeyer, M. Optical measurements of invasive forces exerted by appressoria of a plant pathogenic fungus. Science 1999, 285, 1896–1899. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.N.; Ziemann, S.; Treitschke, S.; Aßmann, D.; Doehlemann, G. Compatibility in the Ustilago maydis-maize interaction requires inhibition of host cysteine proteases by the fungal effector Pit2. PLoS Pathog. 2013, 9, e1003177. [Google Scholar] [CrossRef]



| Strain | Relevant Genotype | Reference |
|---|---|---|
| FB1 | a1 b1 | [41] |
| FB2 | a2 b2 | [41] |
| FB1 Δxyn1 | a1 b1 Δxyn1::nat | [22] |
| FB2 Δxyn1 | a2 b2 Δxyn1::nat | [22] |
| FB1 Δxyn2 | a1 b1 Δxyn2::gen | This work |
| FB2 Δxyn2 | a2 b2 Δxyn2::gen | This work |
| FB1 Δxyn11A | a1 b1 Δxyn11A::hyg | This work |
| FB2 Δxyn11A | a2 b2 Δxyn11A::hyg | This work |
| SG200 | a1 mfa2 bW2 bE1 | [42] |
| SG200 3xGFP | a1 mfa2 bW2 bE1 Potef:3xGFP:cbx | [22] |
| SG200 xyn2:GFP | a1 mfa2 bW2 bE1 Potef:xyn2:GFP:cbx | This work |
| SG200 xyn11A:GFP | a1 mfa2 bW2 bE1 Potef:xyn11A:GFP:cbx | This work |
| SG200 xyn3:GFP | a1 mfa2 bW2 bE1 Potef:xyn3:GFP:cbx | This work |
| SG200 Ppit2:mcherry-HA | a1 mfa2 bW2 bE1 Ppit2:mcherry-HA:cbx | [43] |
| SG200 Ppit2:xyn1:mcherry-HA | a1 mfa2 bW2 bE1 Ppit2:xyn1:mcherry-HA:cbx | This work |
| SG200 Ppit2:xyn2:mcherry-HA | a1 mfa2 bW2 bE1 Ppit2:xyn2:mcherry-HA:cbx | This work |
| SG200 Ppit2:xyn11A:mcherry-HA | a1 mfa2 bW2 bE1 Ppit2:xyn11A:mcherry-HA:cbx | This work |
| CL13 | a1 bE1 bW2 | [42] |
| CL13 Δxyn1 | a1 bE1 bW2 Δxyn1::nat | [22] |
| CL13 Δxyn2 | a1 bE1 bW2 Δxyn2::gen | This work |
| CL13 Δxyn1Δxyn2 | a1 bE1 bW2 Δxyn1::nat Δxyn2::gen | This work |
| CL13 Δxyn11A | a1 bE1 bW2 Δxyn11A::hyg | This work |
| CL13 Δxyn1Δxyn2Δxyn11A | a1 bE1 bW2 Δxyn1::nat Δxyn2::gen Δxyn11A::hyg | This work |
| CL13 Δxyn1 Pxyn1:xyn1 | a1 bE1 bW2 Δxyn1:gen Pxyn1:xyn1:cbx | This work |
| CL13 Δxyn2 Pxyn2:xyn2 | a1 bE1 bW2 Δxyn2:gen Pxyn2:xyn2:cbx | This work |
| CL13 Δxyn11A Pxyn11A:xyn11A | a1 bE1 bW2 Δxyn11A:hyg Pxyn11A:xyn11A:cbx | This work |
| CL13 CFP | a1 bE1 bW2 Potef:CFP:cbx | This work |
| CL13 2xRFP Δxyn1 | a1 bE1 bW2 Potef:2xRFP:cbx Δxyn1::nat | This work |
| CL13 2xRFP Δxyn2 | a1 bE1 bW2 Potef:2xRFP:cbx Δxyn2::gen | This work |
| CL13 2xRFP Δxyn11A | a1 bE1 bW2 Potef:2xRFP:cbx Δxyn11A::hyg | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Sánchez, I.; Pejenaute-Ochoa, M.D.; Navarrete, B.; Barrales, R.R.; Ibeas, J.I. Ustilago maydis Secreted Endo-Xylanases Are Involved in Fungal Filamentation and Proliferation on and Inside Plants. J. Fungi 2021, 7, 1081. https://doi.org/10.3390/jof7121081
Moreno-Sánchez I, Pejenaute-Ochoa MD, Navarrete B, Barrales RR, Ibeas JI. Ustilago maydis Secreted Endo-Xylanases Are Involved in Fungal Filamentation and Proliferation on and Inside Plants. Journal of Fungi. 2021; 7(12):1081. https://doi.org/10.3390/jof7121081
Chicago/Turabian StyleMoreno-Sánchez, Ismael, María Dolores Pejenaute-Ochoa, Blanca Navarrete, Ramón R. Barrales, and José I. Ibeas. 2021. "Ustilago maydis Secreted Endo-Xylanases Are Involved in Fungal Filamentation and Proliferation on and Inside Plants" Journal of Fungi 7, no. 12: 1081. https://doi.org/10.3390/jof7121081
APA StyleMoreno-Sánchez, I., Pejenaute-Ochoa, M. D., Navarrete, B., Barrales, R. R., & Ibeas, J. I. (2021). Ustilago maydis Secreted Endo-Xylanases Are Involved in Fungal Filamentation and Proliferation on and Inside Plants. Journal of Fungi, 7(12), 1081. https://doi.org/10.3390/jof7121081






