Bile Acid Regulates the Colonization and Dissemination of Candida albicans from the Gastrointestinal Tract by Controlling Host Defense System and Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Reagents
2.2. Fungal Colonization and Dissemination in the Immunosuppressed Mouse Model
2.3. Effect of TCA on Fungal Colonization and Dissemination in Mice
2.4. FITC-Dextran Permeability Assay
2.5. RNA Sequencing and Analysis
2.6. Microbiome Sequencing and Analysis
2.7. Immunofluorescence Staining
2.8. Metabolomics
2.9. Statistical Analysis
3. Results
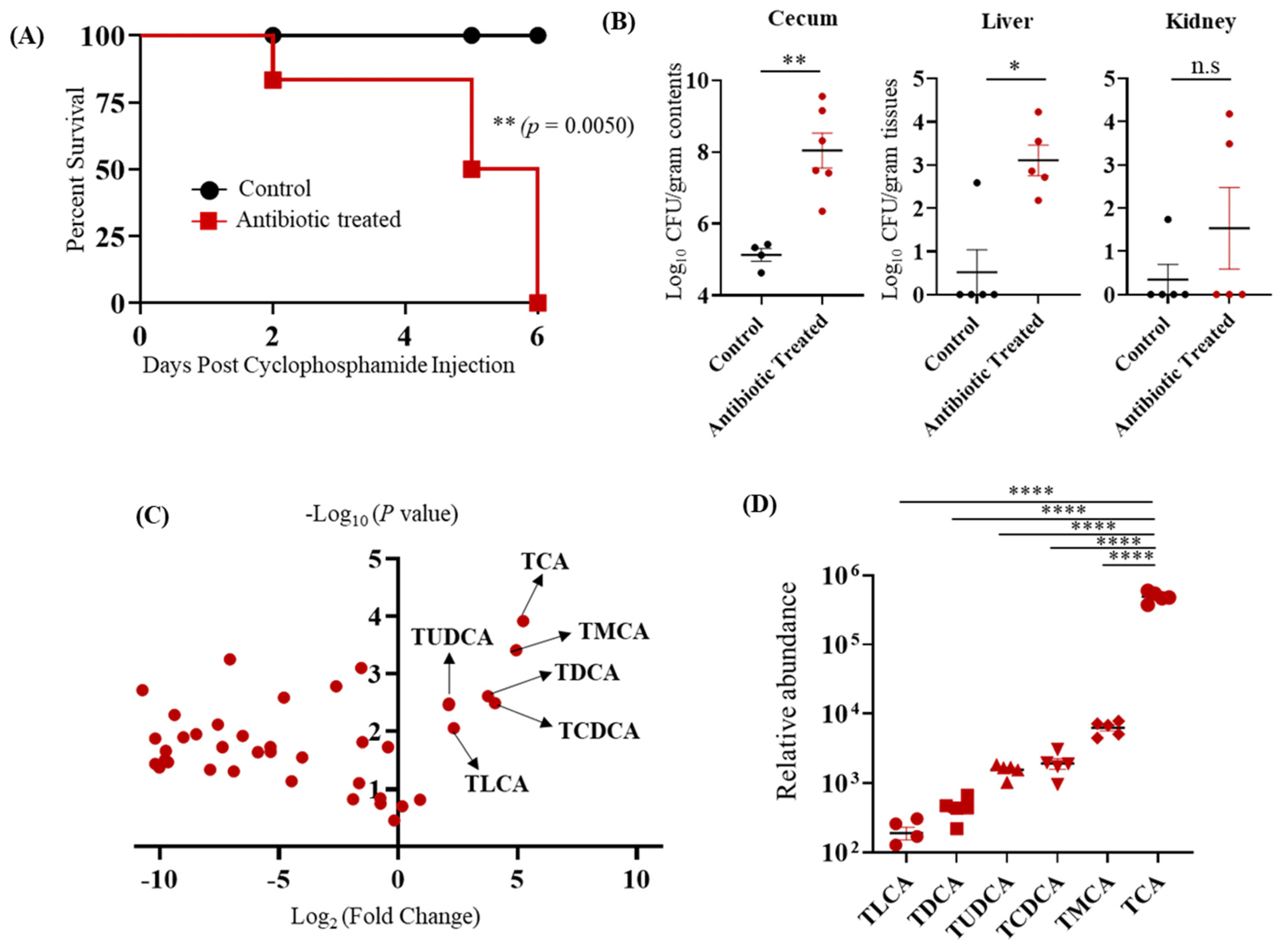
3.1. TCA Is the Major Bile Acid Metabolite Up-Regulated in the Cefoperazone-Treated Mice Susceptible to CA Infection
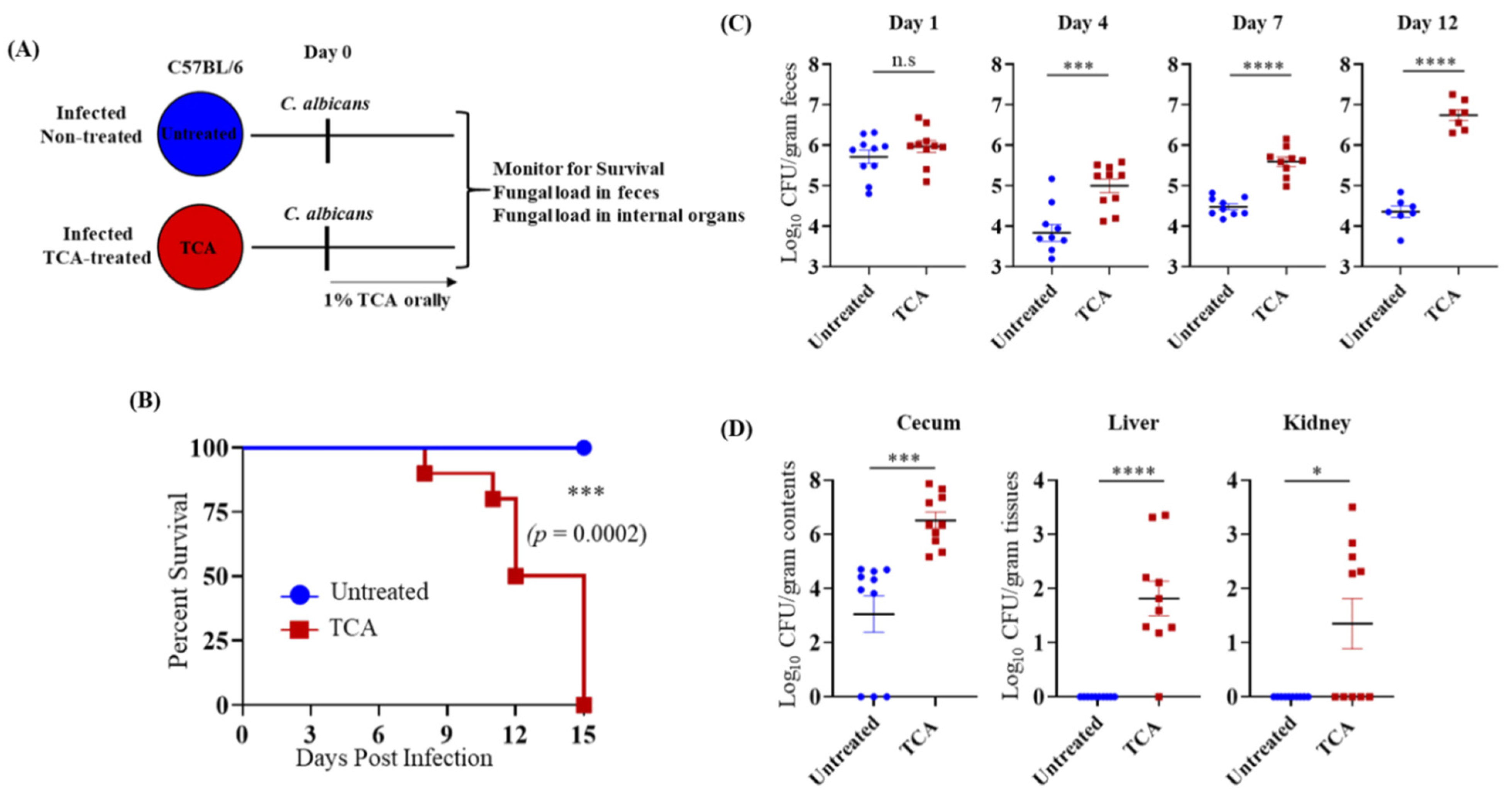
3.2. TCA Alone Induces Fungal Colonization and Dissemination from the GI Tract in the Absence of Antibiotics and Immunosuppressive Agents
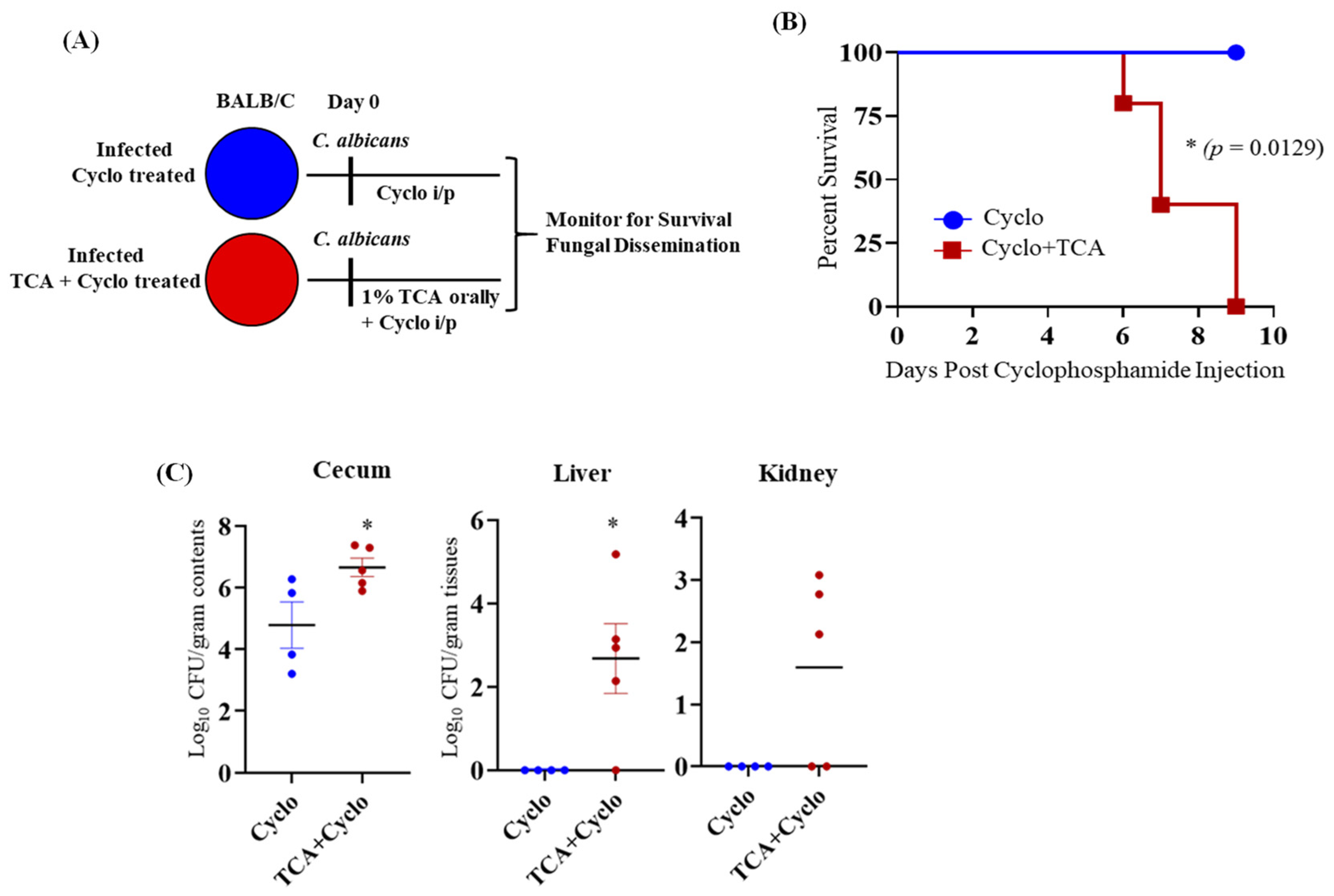
3.3. TCA Induces Fungal Colonization and Dissemination from the GI Tract of Immunosuppressed Mice in the Absence of Antibiotic Treatment
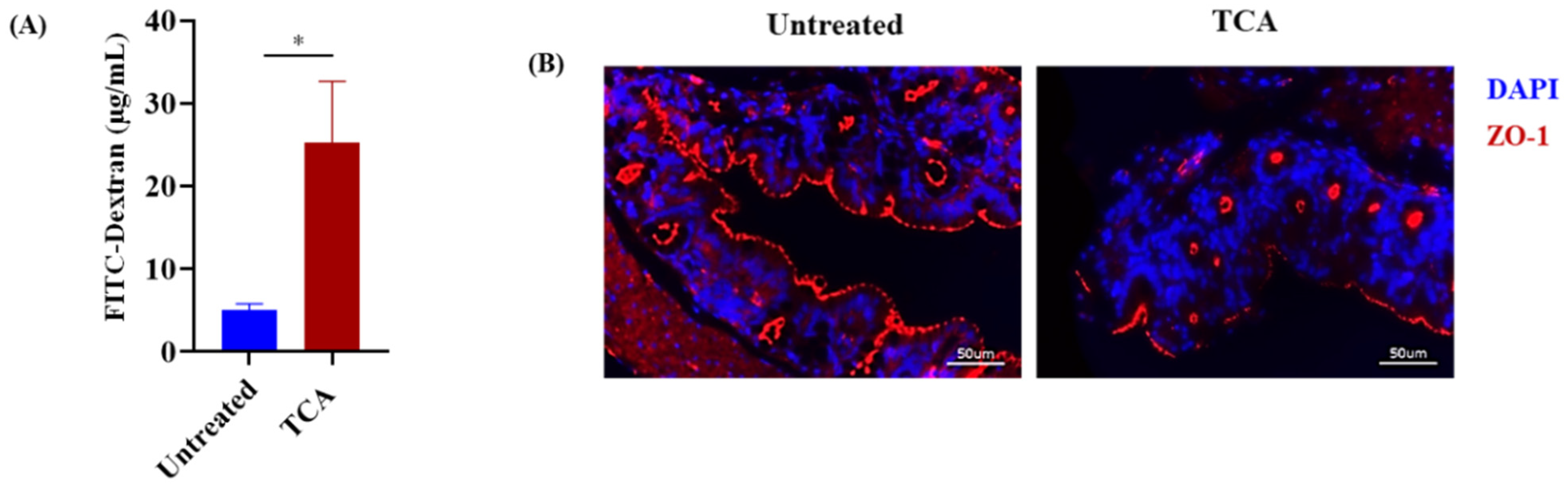
3.4. TCA Enhanced Intestinal Permeability and Reduced the Expression of a Tight Junction Protein
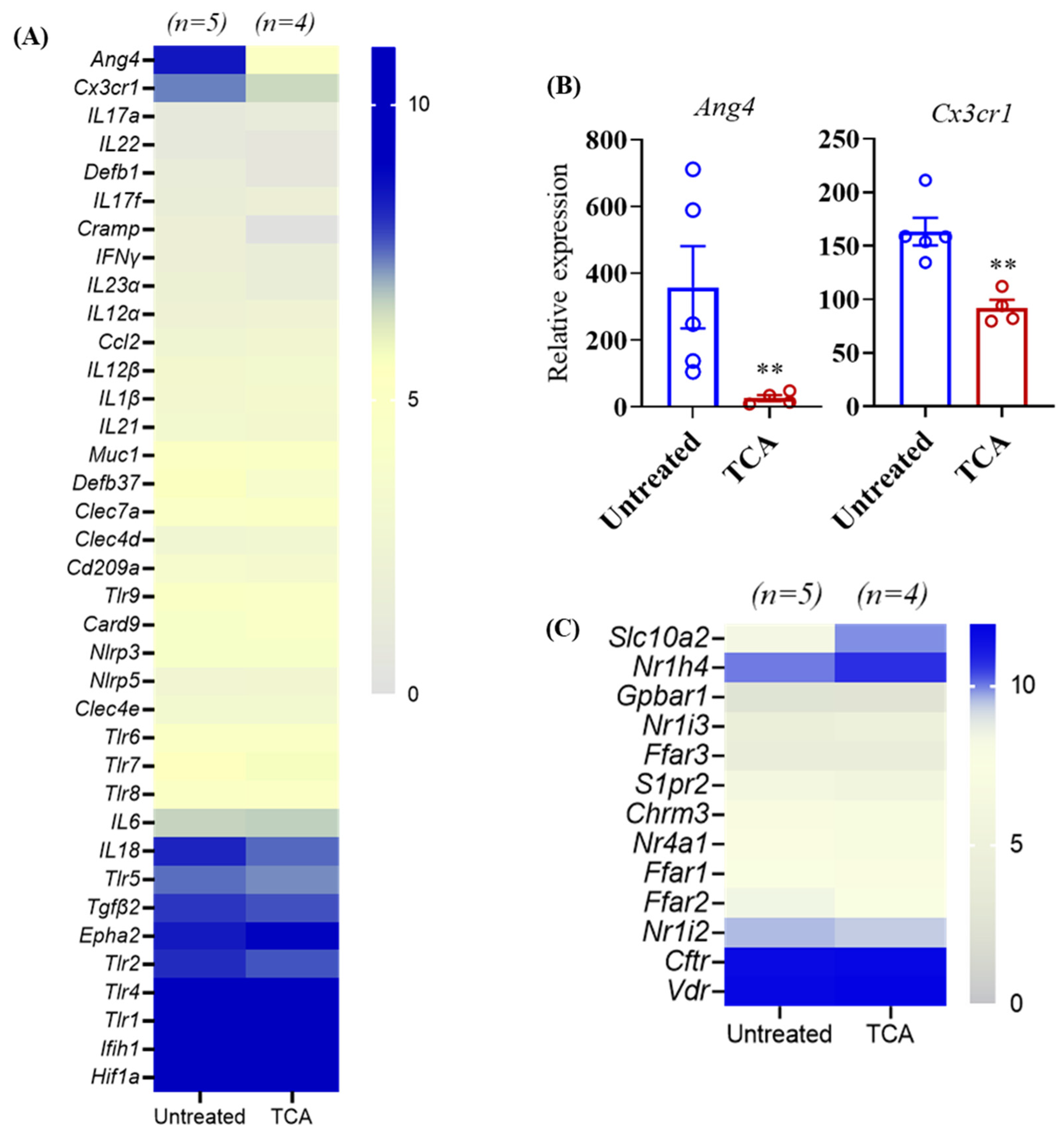
3.5. TCA Down-Regulates ang4 and Cx3cr1 Expression in the Colon Tissue
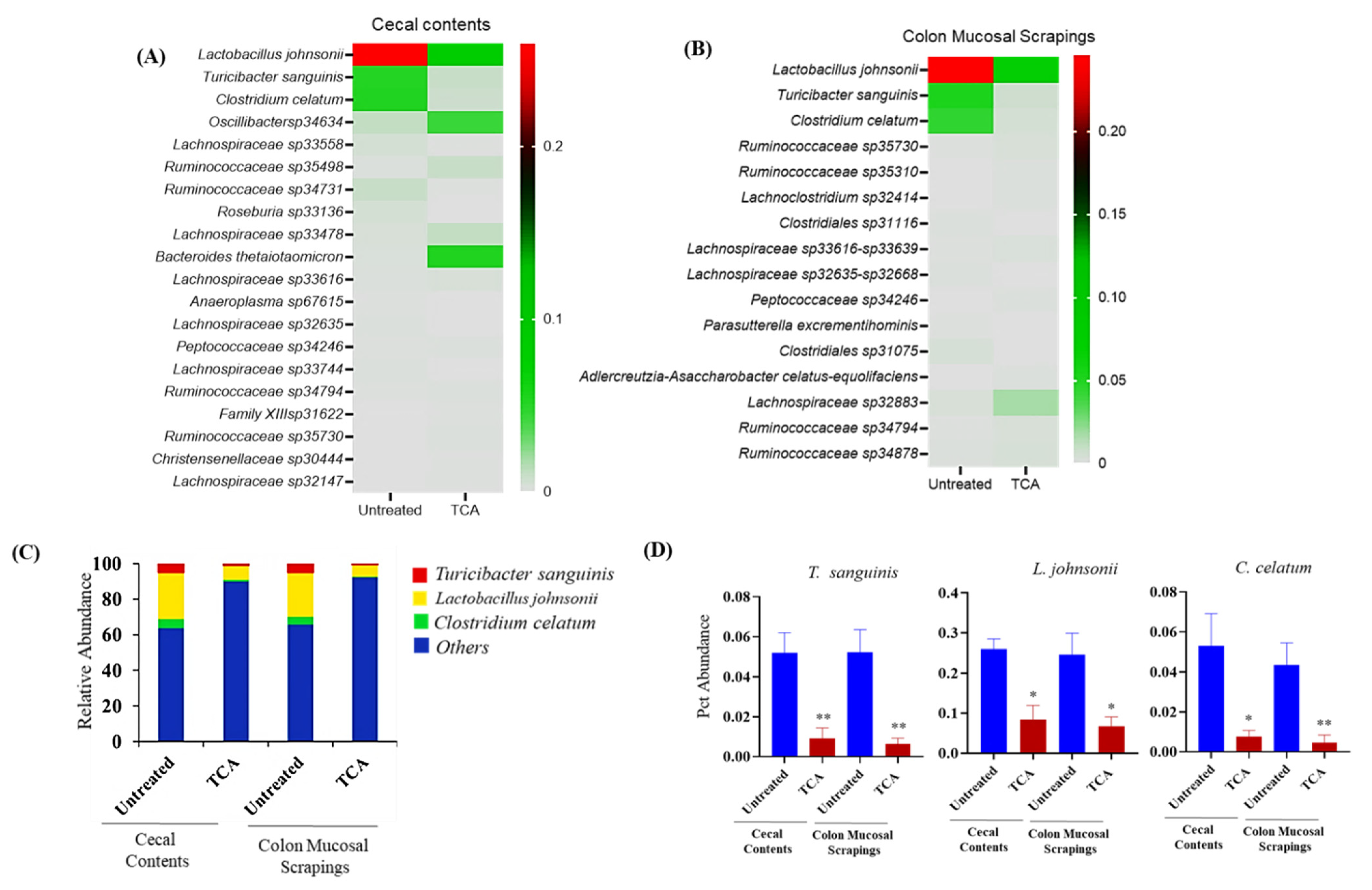
3.6. TCA Alters Microbial Composition in Both Luminal and Mucosal Parts of the GI Tract
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angarone, M. Fungal infections in cancer patients. Cancer Treat. Res. 2014, 161, 129–155. [Google Scholar]
- Low, C.-Y.; Rotstein, C. Emerging fungal infections in immunocompromised patients. F1000 Med. Rep. 2011, 3, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perfect, J.R.; Hachem, R.; Wingard, J.R. Update on epidemiology of and preventive strategies for invasive fungal infections in cancer patients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 59 (Suppl. S5), S352–S355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, B.; Ola, M.; Rolling, T.; Tosini, N.L.; Joshowitz, S.; Littmann, E.R.; Amoretti, L.A.; Fontana, E.; Wright, R.J.; Miranda, E.; et al. High-resolution mycobiota analysis reveals dynamic intestinal translocation preceding invasive candidiasis. Nat. Med. 2020, 26, 59–64. [Google Scholar] [CrossRef]
- Neville, B.A.; d’Enfert, C.; Bougnoux, M.E. Candida albicans commensalism in the gastrointestinal tract. FEMS Yeast Res. 2015, 15, fov081. [Google Scholar] [CrossRef] [Green Version]
- Fan, D.; Coughlin, L.A.; Neubauer, M.M.; Kim, J.; Kim, M.S.; Zhan, X.; Simms-Waldrip, T.R.; Xie, Y.; Hooper, L.V.; Koh, A.Y. Activation of HIF-1α and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat. Med. 2015, 21, 808–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huppert, M.; Cazin, J., Jr. Pathogenesis of Candida albicans infection following antibiotic therapy. II. Further studies of the effect of antibiotics on the in vitro growth of Candida albicans. J. Bacteriol. 1955, 70, 435–439. [Google Scholar] [CrossRef] [Green Version]
- Krause, R.; Krejs, G.J.; Wenisch, C.; Reisinger, E.C. Elevated Fecal Candida Counts in Patients with Antibiotic-Associated Diarrhea: Role of Soluble Fecal Substances. Clin. Vaccine Immunol. 2003, 10, 167–168. [Google Scholar] [CrossRef] [Green Version]
- Krause, R.; Schwab, E.; Bachhiesl, D.; Daxböck, F.; Wenisch, C.; Krejs, G.J.; Reisinger, E.C. Role of Candida in Antibiotic-Associated Diarrhea. J. Infect. Dis. 2001, 184, 1065–1069. [Google Scholar] [CrossRef] [Green Version]
- Schulte, D.M.; Sethi, A.K.; Gangnon, R.E.; Duster, M.; Maki, D.G.; Safdar, N. Risk factors for Candida colonization and Co-colonization with multi-drug resistant organisms at admission. Antimicrob. Resist. Infect. Control 2015, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Guiot, H.F.; Fibbe, W.E.; van ’t Wout, J.W. Risk factors for fungal infection in patients with malignant hematologic disorders: Implications for empirical therapy and prophylaxis. Clin. Infect. Dis. 1994, 18, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Guastalegname, M.; Russo, A.; Falcone, M.; Giuliano, S.; Venditti, M. Candidemia subsequent to severe infection due to Clos-tridium difficile: Is there a link? Clin. Infect. Dis. 2013, 57, 772–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nerandzic, M.M.; Mullane, K.; Miller, M.A.; Babakhani, F.; Donskey, C.J. Reduced acquisition and overgrowth of vancomycin-resistant enterococci and Candida species in patients treated with fidaxomicin versus vancomycin for Clostridium difficile infection. Clin. Infect. Dis. 2012, 55 (Suppl. S2), S121–S126. [Google Scholar] [CrossRef]
- Samonis, G.; Gikas, A.; Anaissie, E.J.; Vrenzos, G.; Maraki, S.; Tselentis, Y.; Bodey, G.P. Prospective evaluation of effects of broad-spectrum antibiotics on gastrointestinal yeast colonization of humans. Antimicrob. Agents Chemother. 1993, 37, 51–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiesner, S.M.; Jechorek, R.P.; Garni, R.M.; Bendel, C.; Wells, C.L. Gastrointestinal Colonization by Candida albicans Mutant Strains in Antibiotic-Treated Mice. Clin. Diagn. Lab. Immunol. 2001, 8, 192–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekenna, O.; Sherertz, R.J. Factors affecting colonization and dissemination of Candida albicans from the gastrointestinal tract of mice. Infect. Immun. 1987, 55, 1558–1563. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.Y. Murine Models of Candida Gastrointestinal Colonization and Dissemination. Eukaryot. Cell 2013, 12, 1416–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto, D.; Pla, J. Distinct stages during colonization of the mouse gastrointestinal tract by Candida albicans. Front. Microbiol. 2015, 6, 792. [Google Scholar] [CrossRef]
- Vautier, S.; Drummond, R.A.; Chen, K.; Murray, G.I.; Kadosh, D.; Brown, A.J.; Gow, N.A.; MacCallum, D.M.; Kolls, J.K.; Brown, G.D. Candida albicans colonization and dissemination from the murine gastrointestinal tract: The influence of morphology and Th17 immunity. Cell. Microbiol. 2015, 17, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Conti, H.R.; Huppler, A.R.; Whibley, N.; Gaffen, S.L. Animal Models for Candidiasis. Curr. Protoc. Immunol. 2014, 105, 19.6.1–19.6.17. [Google Scholar] [CrossRef] [Green Version]
- Mason, K.L.; Erb Downward, J.R.; Mason, K.D.; Falkowski, N.R.; Eaton, K.A.; Kao, J.Y.; Young, V.B.; Huffnagle, G.B. Candida albicans and bacterial microbiota interactions in the cecum during recolonization following broad-spectrum antibiotic therapy. Infect. Immun. 2012, 80, 3371–3380. [Google Scholar] [CrossRef] [Green Version]
- Erb Downward, J.R.; Falkowski, N.R.; Mason, K.L.; Muraglia, R.; Huffnagle, G.B. Modulation of post-antibiotic bacterial community reassembly and host response by Candida albicans. Sci. Rep. 2013, 3, 2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suez, J.; Elinav, E. The path towards microbiome-based metabolite treatment. Nat. Microbiol. 2017, 2, 17075. [Google Scholar] [CrossRef] [PubMed]
- Buffie, C.G.; Bucci, V.; Stein, R.R.; McKenney, P.T.; Ling, L.; Gobourne, A.; No, D.; Liu, H.; Kinnebrew, M.; Viale, A.; et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 2015, 517, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Kohli, N.; Crisp, Z.; Riordan, R.; Li, M.; Alaniz, R.C.; Jayaraman, A. The microbiota metabolite indole inhibits Salmonella virulence: Involvement of the PhoPQ two-component system. PLoS ONE 2018, 13, e0190613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, D.; Weinstock, A.; Antharam, V.C.; Gu, H.; Jasbi, P.; Shi, X.; Dirks, B.; Krajmalnik-Brown, R.; Maldonado, J.; Guinan, J.; et al. Antibiotic-induced gut metabolome and microbiome alterations increase the susceptibility to Candida albicans colonization in the gastrointestinal tract. FEMS Microbiol. Ecol. 2020, 96, fiz187. [Google Scholar] [CrossRef] [Green Version]
- Chiang, J.Y.L. Bile Acid Metabolism and Signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [CrossRef] [Green Version]
- Staels, B.; Fonseca, V.A. Bile Acids and Metabolic Regulation: Mechanisms and clinical responses to bile acid sequestration. Diabetes Care 2009, 32, S237–S245. [Google Scholar] [CrossRef] [Green Version]
- Sayin, S.I.; Wahlstrom, A.; Felin, J.; Jantti, S.; Marschall, H.U.; Bamberg, K.; Angelin, B.; Hyotylainen, T.; Oresic, M.; Backhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell. Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Song, W.-S.; Park, H.-M.; Ha, J.M.; Shin, S.G.; Park, H.-G.; Kim, J.; Zhang, T.; Ahn, D.-H.; Kim, S.-M.; Yang, Y.-H.; et al. Discovery of glycocholic acid and taurochenodeoxycholic acid as phenotypic biomarkers in cholangiocarcinoma. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Manieri, E.; Folgueira, C.; Rodríguez, M.E.; Leiva-Vega, L.; Esteban-Lafuente, L.; Chen, C.; Cubero, F.J.; Barrett, T.; Cavanagh-Kyros, J.; Seruggia, D.; et al. JNK-mediated disruption of bile acid homeostasis promotes intrahepatic cholangiocarcinoma. Proc. Natl. Acad. Sci. USA 2020, 117, 16492–16499. [Google Scholar] [CrossRef]
- Kühn, T.; Stepien, M.; López-Nogueroles, M.; Damms-Machado, A.; Sookthai, D.; Johnson, T.; Roca, M.; Hüsing, A.; Maldonado, S.G.; Cross, A.J.; et al. Prediagnostic Plasma Bile Acid Levels and Colon Cancer Risk: A Prospective Study. J. Natl. Cancer Inst. 2020, 112, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Yang, R.; Wang, Y.; Liu, J.; Wee, A.; Saxena, R.; Wang, L.; Li, M.; Liu, L.; Shan, S.; et al. A High Serum Level of Taurocholic Acid Is Correlated with the Severity and Resolution of Drug-induced Liver Injury. Clin. Gastroenterol. Hepatol. 2021, 19, 1009–1019.e11. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Aubrecht, J.; Li, D.; Warner, R.L.; Johnson, K.J.; Kenny, J.; Colangelo, J.L. Assessment of serum bile acid profiles as biomarkers of liver injury and liver disease in humans. PLoS ONE 2018, 13, e0193824. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Wolf, P.G.; Gaskins, H.R. Taurocholic acid metabolism by gut microbes and colon cancer. Gut Microbes 2016, 7, 201–215. [Google Scholar] [CrossRef] [Green Version]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhang, Z.; Huang, M.; Sun, X.; Liu, B.; Guo, Q.; Chang, Q.; Duan, Z. Taurocholic acid is an active promoting factor, not just a biomarker of progression of liver cirrhosis: Evidence from a human metabolomic study and in vitro experiments. BMC Gastroenterol. 2018, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Liu, E.J.; Kheradman, R.; Fagan, A.; Heuman, D.M.; White, M.; Gavis, E.A.; Hylemon, P.; Sikaroodi, M.; Gillevet, P.M. Fungal dysbiosis in cirrhosis. Gut 2017, 67, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Maraolo, A.E.; Scotto, R.; Zappulo, E.; Pinchera, B.; Moriello, N.S.; Nappa, S.; Buonomo, A.R.; Gentile, I. Novel strategies for the management of bacterial and fungal infections in patients with liver cirrhosis: Focus on new antimicrobials. Expert Rev. Anti-Infect. Ther. 2020, 18, 191–202. [Google Scholar] [CrossRef]
- Li, B.; Yang, C.; Qian, Z.; Huang, Y.; Wang, X.; Zhong, G.; Chen, J. Spontaneous Fungal Ascites Infection in Patients with Cirrhosis: An Analysis of 10 Cases. Infect. Dis. Ther. 2021, 10, 1033–1043. [Google Scholar] [CrossRef]
- Fiore, M.; Leone, S. Spontaneous fungal peritonitis: Epidemiology, current evidence and future prospective. World J. Gastroenterol. 2016, 22, 7742–7747. [Google Scholar] [CrossRef] [PubMed]
- Ferrarese, A.; Cattelan, A.; Cillo, U.; Gringeri, E.; Russo, F.P.; Germani, G.; Gambato, M.; Burra, P.; Senzolo, M. Invasive fungal infec-tion before and after liver transplantation. World J. Gastroenterol. 2020, 26, 7485–7496. [Google Scholar] [CrossRef]
- Ethanic, M.; Stanimirov, B.; Pavlovic, N.; Golocorbin-Kon, S.; Al-Salami, H.; Stankov, K.; Mikov, M. Pharmacological Applications of Bile Acids and Their Derivatives in the Treatment of Metabolic Syndrome. Front. Pharmacol. 2018, 9, 1382. [Google Scholar]
- Li, T.; Chiang, J.Y.L. Bile acid-based therapies for non-alcoholic steatohepatitis and alcoholic liver disease. Hepatobiliary Surg. Nutr. 2020, 9, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.; Ferrell, J.M. Bile Acid Biology, Pathophysiology, and Therapeutics. Clin. Liver Dis. 2020, 15, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Limaye, P.B.; Renaud, H.J.; Klaassen, C.D. Effect of various antibiotics on modulation of intestinal microbiota and bile acid profile in mice. Toxicol. Appl. Pharmacol. 2014, 277, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culpepper, T.; Rowe, C.C.; Rusch, C.T.; Burns, A.M.; Federico, A.P.; Girard, S.A.; Tompkins, T.A.; Nieves, C.; Dennis-Wall, J.C., Jr.; Christman, M.C.; et al. Three probiotic strains exert different effects on plasma bile acid profiles in healthy obese adults: Randomised, double-blind placebo-controlled crossover study. Benef. Microbes 2019, 10, 497–509. [Google Scholar] [CrossRef]
- Begley, M.; Hill, C.; Gahan, C.G.M. Bile Salt Hydrolase Activity in Probiotics. Appl. Environ. Microbiol. 2006, 72, 1729–1738. [Google Scholar] [CrossRef] [Green Version]
- Vrieze, A.; Out, C.; Fuentes, S.; Jonker, L.; Reuling, I.; Kootte, R.S.; van Nood, E.; Holleman, F.; Knaapen, M.; Romijn, J.A.; et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J. Hepatol. 2014, 60, 824–831. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Chaix, A.; Xu, Z.Z.; Chang, M.W.; Marotz, C.A.; Saghatelian, A.; Knight, R.; Panda, S. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat. Commun. 2018, 9, 2872. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Zamani, N.; Mikov, M.; Goločorbin-Kon, S.; Stojanovic, G.; Arfuso, F.; Al-Salami, H. Stability and biological testing of taurine-conjugated bile acid antioxidant microcapsules for diabetes treatment. Ther. Deliv. 2019, 10, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Khatun, Z.; Nurunnabi, M.; Reeck, G.R.; Cho, K.J.; Lee, Y.-K. Oral delivery of taurocholic acid linked heparin–docetaxel conjugates for cancer therapy. J. Control Release 2013, 170, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.Y.; Kohler, J.R.; Coggshall, K.T.; Van Rooijen, N.; Pier, G.B. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog. 2008, 4, e35. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; Joosten, L.A.; van der Meer, J.W.; Kullberg, B.J.; van de Veerdonk, F.L. Immune defence against Candida fungal infec-tions. Nat. Rev. Immunol. 2015, 15, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, I.; Li, X.; Semon, A.; Li, D.; Doron, I.; Putzel, G.; Bar, A.; Prieto, D.; Rescigno, M.; McGovern, D.P.B.; et al. CX3CR1(+) mononuclear phagocytes control immunity to intestinal fungi. Science 2018, 359, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Drummond, R.; Gaffen, S.L.; Hise, A.G.; Brown, G. Innate Defense against Fungal Pathogens. Cold Spring Harb. Perspect. Med. 2015, 5, a019620. [Google Scholar] [CrossRef] [Green Version]
- Wahlström, A.; Sayin, S.I.; Marschall, H.-U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Dekaney, C.M.; von Allmen, D.C.; Garrison, A.P.; Rigby, R.; Lund, P.K.; Henning, S.J.; Helmrath, M.A. Bacterial-dependent up-regulation of intestinal bile acid binding protein and transport is FXR-mediated following ileocecal resection. Surgery 2008, 144, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Fiorucci, S.; Biagioli, M.; Zampella, A.; Distrutti, E. Bile Acids Activated Receptors Regulate Innate Immunity. Front. Immunol. 2018, 9, 1853. [Google Scholar] [CrossRef] [Green Version]
- Vavassori, P.; Mencarelli, A.; Renga, B.; Distrutti, E.; Fiorucci, S. The Bile Acid Receptor FXR Is a Modulator of Intestinal Innate Immunity. J. Immunol. 2009, 183, 6251–6261. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, Q.; Peng, J.; Jiang, C.; Zhang, Y.; Shen, L.; Dong, J.; Wang, Y.; Jiang, Y. Activation of farnesoid X receptor downregulates monocyte chemoattractant protein-1 in murine macrophage. Biochem. Biophys. Res. Commun. 2015, 467, 841–846. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. Available online: https://pubmed.ncbi.nlm.nih.gov/24625896 (accessed on 18 November 2021). [CrossRef] [Green Version]
- Ramirez-Perez, O.; Cruz-Ramon, V.; Chinchilla-Lopez, P.; Mendez-Sanchez, N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann. Hepatol. 2017, 16 (Suppl. S1), S15–S20. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridlon, J.M.; Hylemon, P.B. Identification and characterization of two bile acid coenzyme A transferases from Clostridium scindens, a bile acid 7α-dehydroxylating intestinal bacterium. J. Lipid Res. 2012, 53, 66–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. Available online: https://pubmed.ncbi.nlm.nih.gov/16299351/ (accessed on 26 December 2020). [CrossRef] [Green Version]
- O’Flaherty, S.; Crawley, A.B.; Theriot, C.M.; Barrangou, R. The Lactobacillus Bile Salt Hydrolase Repertoire Reveals Niche-Specific Adaptation. mSphere 2018, 3, e00140-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooper, L.V.; Stappenbeck, T.S.; Hong, C.V.; Gordon, J.I. Angiogenins: A new class of microbicidal proteins involved in innate immunity. Nat. Immunol. 2003, 4, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Forman, R.A.; Deschoolmeester, M.L.; Hurst, R.J.M.; Wright, S.H.; Pemberton, A.D.; Else, K.J. The Goblet Cell Is the Cellular Source of the Anti-Microbial Angiogenin 4 in the Large Intestine Post Trichuris muris Infection. PLoS ONE 2012, 7, e42248. [Google Scholar] [CrossRef]
- Walker, C.R.; Hautefort, I.; Dalton, J.; Overweg, K.; Egan, C.E.; Bongaerts, R.J.; Newton, D.J.; Cruickshank, S.; Andrew, E.M.; Carding, S.R. Intestinal Intraepithelial Lymphocyte-Enterocyte Crosstalk Regulates Production of Bactericidal Angiogenin 4 by Paneth Cells upon Microbial Challenge. PLoS ONE 2013, 8, e84553. [Google Scholar] [CrossRef]
- Doron, I.; Leonardi, I.; Li, X.V.; Fiers, W.D.; Semon, A.; Bialt-DeCelie, M.; Migaud, M.; Gao, I.H.; Lin, W.Y.; Kusakabe, T.; et al. Human gut mycobiota tune immunity via CARD9-dependent induction of anti-fungal IgG antibodies. Cell 2021, 184, 1017–1031.e14. [Google Scholar] [CrossRef] [PubMed]
- Medina-Contreras, O.; Geem, D.; Laur, O.; Williams, I.R.; Lira, S.A.; Nusrat, A.; Parkos, C.A.; Denning, T. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J. Clin. Investig. 2011, 121, 4787–4795. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Roos, S.; Jonsson, H.; Ahl, D.; Dicksved, J.; Lindberg, J.E.; Lundh, T. Effects of Lactobacillus johnsonii and Lactobacillus reuteri on gut barrier function and heat shock proteins in intestinal porcine epithelial cells. Physiol. Rep. 2015, 3, e12355. [Google Scholar] [CrossRef] [PubMed]
- Antonini, M.; Lo Conte, M.; Sorini, C.; Falcone, M. How the Interplay Between the Commensal Microbiota, Gut Barrier Integrity, and Mucosal Immunity Regulates Brain Autoimmunity. Front. Immunol. 2019, 10, 1937. [Google Scholar] [CrossRef]
- Guo, W.; Zhou, X.; Li, X.; Zhu, Q.; Peng, J.; Zhu, B.; Zheng, X.; Lu, Y.; Yang, D.; Wang, B.; et al. Depletion of Gut Microbiota Impairs Gut Barrier Function and Antiviral Immune Defense in the Liver. Front. Immunol. 2021, 12, 897. [Google Scholar] [CrossRef]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef] [Green Version]
- Charlet, R.; Bortolus, C.; Sendid, B.; Jawhara, S. Bacteroides thetaiotaomicron and Lactobacillus johnsonii modulate intestinal inflammation and eliminate fungi via enzymatic hydrolysis of the fungal cell wall. Sci. Rep. 2020, 10, 11510. [Google Scholar] [CrossRef]
- Honda, M.; Surewaard, B.G.J.; Watanabe, M.; Hedrick, C.C.; Lee, W.-Y.; Brown, K.; McCoy, K.D.; Kubes, P. Perivascular localization of macrophages in the intestinal mucosa is regulated by Nr4a1 and the microbiome. Nat. Commun. 2020, 11, 1329. [Google Scholar] [CrossRef]
- Out, C.; Patankar, J.V.; Doktorova, M.; Boesjes, M.; Bos, T.; de Boer, S.; Havinga, R.; Wolters, H.; Boverhof, R.; van Dijk, T.H.; et al. Gut microbiota inhibit Asbt-dependent intestinal bile acid reabsorption via Gata4. J. Hepatol. 2015, 63, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Islam, K.B.; Fukiya, S.; Hagio, M.; Fujii, N.; Ishizuka, S.; Ooka, T.; Ogura, Y.; Hayashi, T.; Yokota, A. Bile acid is a host factor that regu-lates the composition of the cecal microbiota in rats. Gastroenterology 2011, 141, 1773–1781. [Google Scholar] [CrossRef]
- Ruiz, L.; Margolles, A.; Sánchez, B. Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front. Microbiol. 2013, 4, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masson, D.; Lagrost, L.; Athias, A.; Gambert, P.; Brimer-Cline, C.; Lan, L.; Schuetz, J.D.; Schuetz, E.G.; Assem, M. Expression of the Pregnane X Receptor in Mice Antagonizes the Cholic Acid–Mediated Changes in Plasma Lipoprotein Profile. Arter. Thromb. Vasc. Biol. 2005, 25, 2164–2169. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.L.; Chen, P.; Zhou, Y.Y.; Jiang, Y.M.; Gao, Y.; Zhang, H.Z.; Guan, L.H.; Wang, C.H.; Liu, J.L.; Huang, M.; et al. Hepatic Vps33b deficiency aggravates cholic acid-induced cholestatic liver injury in male mice. Acta Pharmacol. Sin. 2021. [Google Scholar] [CrossRef]
- Nguyen, J.T.; Riessen, R.R.; Zhang, T.; Kieffer, C.; Anakk, S. Deletion of Intestinal SHP Impairs Short-term Response to Cholic Acid Challenge in Male Mice. Endocrinology 2021, 162, bqab063. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.A.; Srivastava, N.; Averna, M. Dietary cholic acid lowers plasma levels of mouse and human apolipoprotein A-I primarily via a transcriptional mechanism. Eur. J. Biochem. 2000, 267, 4272–4280. [Google Scholar] [CrossRef] [PubMed]
- Fickert, P.; Zollner, G.; Fuchsbichler, A.; Stumptner, C.; Pojer, C.; Zenz, R.; Lammert, F.; Stieger, B.; Meier, P.J.; Zatloukal, K.; et al. Effects of Ursodeoxycholic and Cholic Acid Feeding on Hepatocellular Transporter Expression in Mouse Liver. Gastroenterology 2001, 121, 170–183. [Google Scholar] [CrossRef]
- Robinson, J.I.; Weir, W.H.; Crowley, J.R.; Hink, T.; Reske, K.A.; Kwon, J.H.; Burnham, C.D.; Dubberke, E.R.; Mucha, P.J.; Henderson, J.P. Metabolomic networks connect host-microbiome processes to human Clostridioides difficile infections. J. Clin. Investig. 2019, 129, 3792–3806. [Google Scholar] [CrossRef]
- Patton, L.; Li, N.; Garrett, T.; Ruoss, J.; Russell, J.; De La Cruz, D.; Bazacliu, C.; Polin, R.; Triplett, E.; Neu, J. Antibiotics Effects on the Fecal Metabolome in Preterm Infants. Metabolites 2020, 10, 331. [Google Scholar] [CrossRef]
- Reikvam, H.; Grønningsæter, I.-S.; Mosevoll, K.A.; Lindås, R.; Hatfield, K.; Bruserud, Ø. Patients with Treatment-Requiring Chronic Graft versus Host Disease after Allogeneic Stem Cell Transplantation Have Altered Metabolic Profiles due to the Disease and Immunosuppressive Therapy: Potential Implication for Biomarkers. Front. Immunol. 2018, 8, 1979. [Google Scholar] [CrossRef] [Green Version]
- Guinan, J.; Villa, P.; Thangamani, S. Secondary bile acids inhibit Candida albicans growth and morphogenesis. Pathog. Dis. 2018, 76, fty038. [Google Scholar] [CrossRef] [Green Version]
- Guinan, J.; Thangamani, S. Antibiotic-induced alterations in taurocholic acid levels promote gastrointestinal colonization of Candida albicans. FEMS Microbiol. Lett. 2018, 365, fny196. [Google Scholar] [CrossRef]
- Meadows, V.; Kennedy, L.; Kundu, D.; Alpini, G.; Francis, H. Bile Acid Receptor Therapeutics Effects on Chronic Liver Diseases. Front. Med. 2020, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Dayan, S.H.; Humphrey, S.; Jones, D.H.; Lizzul, P.F.; Gross, T.M.; Stauffer, K.; Beddingfield, F.C., 3rd. Overview of ATX-101 (Deoxycholic Acid Injection): A Nonsurgical Approach for Reduction of Submental Fat. Dermatol. Surg. 2016, 42 (Suppl. S1), S263–S270. [Google Scholar] [CrossRef] [PubMed]
- Beer, K.; Weinkle, S.H.; Cox, S.E.; Rubin, M.G.; Shamban, A.; Somogyif, C. ATX-101 (Deoxycholic Acid Injection) for Reduction of Submental Fat: Results From a 12-Month Open-Label Study. J. Drugs Dermatol. 2019, 18, 870–877. [Google Scholar] [PubMed]
- Daruich, A.; Picard, E.; Boatright, J.H.; Behar-Cohen, F. Review: The bile acids urso- and tauroursodeoxycholic acid as neuroprotective therapies in retinal disease. Mol. Vis. 2019, 25, 610–624. [Google Scholar]
- Gordon, S.C.; Wu, K.H.; Lindor, K.; Bowlus, C.L.; Rodriguez, C.V.; Anderson, H.; Boscarino, J.A.; Trudeau, S.; Rupp, L.B.; Haller, I.V.; et al. Ursodeoxycholic Acid Treatment Preferentially Improves Overall Survival Among African Americans With Primary Biliary Cholangitis. Am. J. Gastroenterol. 2020, 115, 262–270. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, K.; Lu, Y.; Yan, W.; Cai, W.; Wang, Y. Administration of antibiotics contributes to cholestasis in pediatric patients with intestinal failure via the alteration of FXR signaling. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Singla, P.; Dalal, P.; Kaur, M.; Arya, G.; Nimesh, S.; Singh, R.; Salunke, D.B. Bile Acid Oligomers and Their Combination with Antibiotics to Combat Bacterial Infections. J. Med. Chem. 2018, 61, 10265–10275. [Google Scholar] [CrossRef]
- Winston, J.A.; Theriot, C.M. Diversification of host bile acids by members of the gut microbiota. Gut Microbes 2019, 11, 158–171. [Google Scholar] [CrossRef]
- Sinha, S.R.; Haileselassie, Y.; Nguyen, L.P.; Tropini, C.; Wang, M.; Becker, L.S.; Sim, D.; Jarr, K.; Spear, E.T.; Singh, G.; et al. Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation. Cell Host Microbe 2020, 27, 659–670.e655. [Google Scholar] [CrossRef] [PubMed]
- Trojanowska, D.; Zwolinska-Wcislo, M.; Tokarczyk, M.; Kosowski, K.; Mach, T.; Budak, A. The role of Candida in inflammatory bowel disease. Estimation of transmission of C. albicans fungi in gastrointestinal tract based on genetic affinity between strains. Med. Sci. Monit. 2010, 16, 451–457. [Google Scholar]
- Gerard, R.; Sendid, B.; Colombel, J.F.; Poulain, D.; Jouault, T. An immunological link between Candida albicans colonization and Crohn’s disease. Crit. Rev. Microbiol. 2015, 41, 135–139. [Google Scholar] [CrossRef]
- Iliev, I.D.; Funari, V.A.; Taylor, K.D.; Nguyen, Q.; Reyes, C.N.; Strom, S.P.; Brown, J.; Becker, C.A.; Fleshner, P.R.; Dubinsky, M.; et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science 2012, 336, 1314–1317. [Google Scholar] [CrossRef] [Green Version]
- Leonardi, I.; Paramsothy, S.; Doron, I.; Semon, A.; Kaakoush, N.O.; Clemente, J.C.; Faith, J.J.; Borody, T.J.; Mitchell, H.M.; Colombel, J.F.; et al. Fungal Trans-kingdom Dynamics Linked to Responsiveness to Fecal Microbiota Transplantation (FMT) Therapy in Ul-cerative Colitis. Cell Host Microbe 2020, 27, 823–829.e823. [Google Scholar] [CrossRef] [PubMed]
- Wajszczuk, C.P.; Dummer, J.S.; Ho, M.; Van Thiel, D.H.; Starzl, T.E.; Iwatsuki, S.; Shaw, B., Jr. Fungal infections in liver transplant recipients. Transplantation 1985, 40, 347–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brumble, L.; Keaveny, A.P. Editorial: The Risky Business of Fungal Infections in Patients with Cirrhosis. Am. J. Gastroenterol. 2018, 113, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.; Yarlagadda, S.; Carreon, T.A.; Schader, L.M.; Hsu, C.-H. Fungal Infection in Acutely Decompensated Cirrhosis Patients: Value of Model for End-Stage Liver Disease Score. Gastroenterol. Res. 2020, 13, 199–207. [Google Scholar]
- Hassan, E.A.; El-Rehim, A.S.A.; Hassany, S.M.; Ahmed, A.O.; Elsherbiny, N.M.; Mohammed, M.H. Fungal infection in patients with end-stage liver disease: Low frequency or low index of suspicion. Int. J. Infect. Dis. 2014, 23, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pramar, Y.V.; Mandal, T.K.; Bostanian, L.A.; Nguyen, A.T.; Miller, V.; Morris, T.C.; Graves, R.A. Stability of Compounded Ursodiol Suspensions in PCCA Base, SuspendIt. Int. J. Pharm. Compd. 2019, 23, 70–76. [Google Scholar]
- Simental-Mendía, M.; Sánchez-García, A.; Simental-Mendía, L.E. Effect of ursodeoxycholic acid on liver markers: A systematic review and meta-analysis of randomized placebo-controlled clinical trials. Br. J. Clin. Pharmacol. 2020, 86, 1476–1488. [Google Scholar] [CrossRef]
- Phaw, N.A.; Dyson, J.K.; Jones, D. Emerging drugs for the treatment of primary biliary cholangitis. Expert Opin. Emerg. Drugs 2020, 25, 101–112. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Kandasamy, S.; Chattha, K.S.; Rajashekara, G.; Saif, L.J. Comparison of probiotic lactobacilli and bifidobacteria effects, immune responses and rotavirus vaccines and infection in different host species. Vet. Immunol. Immunopathol. 2016, 172, 72–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciorba, M.A. A gastroenterologist’s guide to probiotics. Clin. Gastroenterol. Hepatol. 2012, 10, 960–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, Y.; Jiang, M.Z.; Xu, B.; Wang, W.J.; Chen, D.; Li, X.W.; Zhang, Y.J.; Liang, J. Specific changes of enteric mycobiota and virome in inflammatory bowel disease. J. Dig. Dis. 2018, 19, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Stamatiades, G.A.; Ioannou, P.; Petrikkos, G.; Tsioutis, C. Fungal infections in patients with inflammatory bowel disease: A systematic review. Mycoses 2018, 61, 366–376. [Google Scholar] [CrossRef]
- Enaud, R.; Vandenborght, L.-E.; Coron, N.; Bazin, T.; Prevel, R.; Schaeverbeke, T.; Berger, P.; Fayon, M.; Lamireau, T.; Delhaes, L. The Mycobiome: A Neglected Component in the Microbiota-Gut-Brain Axis. Microorganisms 2018, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Kantarcioglu, A.S.; Kiraz, N.; Aydin, A. Microbiota–Gut–Brain Axis: Yeast Species Isolated from Stool Samples of Children with Suspected or Diagnosed Autism Spectrum Disorders and In Vitro Susceptibility Against Nystatin and Fluconazole. Mycopathologia 2015, 181, 1–7. [Google Scholar] [CrossRef]
- Zuo, T.; Wong, S.H.; Cheung, C.P.; Lam, K.; Lui, R.; Cheung, K.; Zhang, F.; Tang, W.; Ching, J.Y.L.; Wu, J.C.Y.; et al. Gut fungal dysbiosis correlates with reduced efficacy of fecal microbiota transplantation in Clostridium difficile infection. Nat. Commun. 2018, 9, 3663. [Google Scholar] [CrossRef]
- Tso, G.H.W.; Reales-Calderon, J.A.; Tan, A.S.M.; Sem, X.; Le, G.T.T.; Tan, T.G.; Lai, G.C.; Srinivasan, K.G.; Yurieva, M.; Liao, W.; et al. Experimental evolution of a fungal pathogen into a gut symbiont. Science 2018, 362, 589–595. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangamani, S.; Monasky, R.; Lee, J.K.; Antharam, V.; HogenEsch, H.; Hazbun, T.R.; Jin, Y.; Gu, H.; Guo, G.L. Bile Acid Regulates the Colonization and Dissemination of Candida albicans from the Gastrointestinal Tract by Controlling Host Defense System and Microbiota. J. Fungi 2021, 7, 1030. https://doi.org/10.3390/jof7121030
Thangamani S, Monasky R, Lee JK, Antharam V, HogenEsch H, Hazbun TR, Jin Y, Gu H, Guo GL. Bile Acid Regulates the Colonization and Dissemination of Candida albicans from the Gastrointestinal Tract by Controlling Host Defense System and Microbiota. Journal of Fungi. 2021; 7(12):1030. https://doi.org/10.3390/jof7121030
Chicago/Turabian StyleThangamani, Shankar, Ross Monasky, Jung Keun Lee, Vijay Antharam, Harm HogenEsch, Tony R. Hazbun, Yan Jin, Haiwei Gu, and Grace L. Guo. 2021. "Bile Acid Regulates the Colonization and Dissemination of Candida albicans from the Gastrointestinal Tract by Controlling Host Defense System and Microbiota" Journal of Fungi 7, no. 12: 1030. https://doi.org/10.3390/jof7121030






