Clinical Characteristics and Outcomes of Fungal Keratitis in the United Kingdom 2011–2020: A 10-Year Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Identification and Definition
2.2. Data Collection
3. Microbiological Culture, Diagnosis and Treatment
Statistical Analysis
4. Results
4.1. Overall Description
4.2. Causative Organisms and Risk Factors
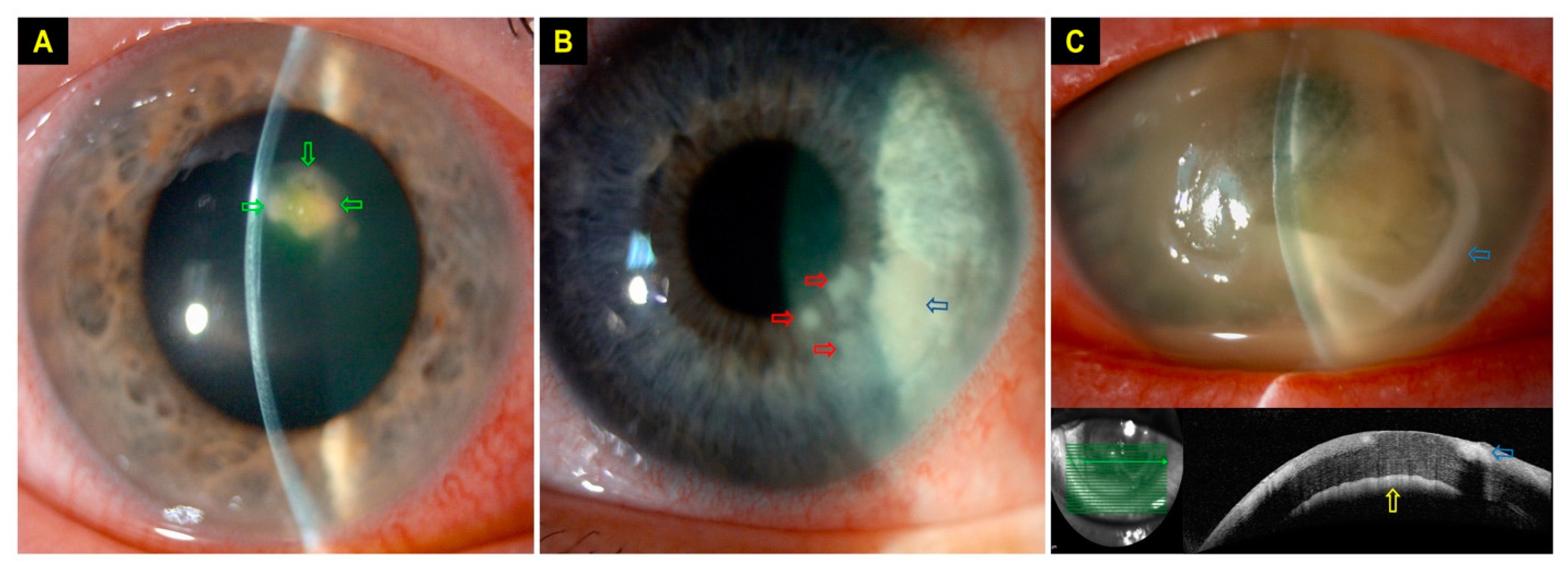
4.3. Clinical Characteristics
4.4. Medical and Surgical Treatment
4.5. Clinical Outcomes and Prognostic Factors
4.6. Complications
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ting, D.S.J.; Ho, C.S.; Deshmukh, R.; Said, D.G.; Dua, H.S. Infectious keratitis: An update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye 2021, 35, 1084–1101. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Carnt, N.; Apel, A.; Stapleton, F. Queensland Microbial Keratitis Database: 2005–2015. Br. J. Ophthalmol. 2019, 103, 1481–1486. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Ho, C.S.; Cairns, J.; Elsahn, A.; Al-Aqaba, M.; Boswell, T.; Said, D.G.; Dua, H.S. 12-year analysis of incidence, microbiological profiles and in vitro antimicrobial susceptibility of infectious keratitis: The Nottingham Infectious Keratitis Study. Br. J. Ophthalmol. 2021, 105, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Khor, W.-B.; Prajna, V.N.; Garg, P.; Mehta, J.S.; Xie, L.; Liu, Z.; Padilla, M.D.B.; Joo, C.-K.; Inoue, Y.; Goseyarakwong, P.; et al. The Asia Cornea Society Infectious Keratitis Study: A Prospective Multicenter Study of Infectious Keratitis in Asia. Am. J. Ophthalmol. 2018, 195, 161–170. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Ho, C.S.; Cairns, J.; Gopal, B.P.; Elsahn, A.; Al-Aqaba, M.; Boswell, T.; Said, D.G.; Dua, H.S. Seasonal patterns of incidence, demographic factors and microbiological profiles of infectious keratitis: The Nottingham Infectious Keratitis Study. Eye 2021, 35, 2543–2549. [Google Scholar] [CrossRef]
- Lin, L.; Duan, F.; Yang, Y.; Lou, B.; Liang, L.; Lin, X. Nine-year analysis of isolated pathogens and antibiotic susceptibilities of microbial keratitis from a large referral eye center in southern China. Infect. Drug Resist. 2019, 12, 1295–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arshad, S.; Petsoglou, C.; Lee, T.; Al-Tamimi, A.; Carnt, N.A. Twenty years since the Herpetic Eye Disease Study: Lessons, developments and applications to clinical practice. Clin. Exp. Optom. 2021, 104, 396–405. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Ghosh, N.; Ghosh, S. Herpes zoster ophthalmicus. BMJ 2019, 364, k5234. [Google Scholar] [CrossRef] [PubMed]
- Carnt, N.; Robaei, D.; Minassian, D.C.; Dart, J.K.G. Acanthamoeba keratitis in 194 patients: Risk factors for bad outcomes and severe inflammatory complications. Br. J. Ophthalmol. 2018, 102, 1431–1435. [Google Scholar] [CrossRef]
- Brown, L.; Leck, A.K.; Gichangi, M.; Burton, M.J.; Denning, D.W. The global incidence and diagnosis of fungal keratitis. Lancet Infect. Dis. 2021, 21, e49–e57. [Google Scholar] [CrossRef]
- Lin, C.C.; Lalitha, P.; Srinivasan, M.; Prajna, N.V.; McLeod, S.D.; Acharya, N.R.; Lietman, T.M.; Porco, T.C. Seasonal Trends of Microbial Keratitis in South India. Cornea 2012, 31, 1123–1127. [Google Scholar] [CrossRef] [Green Version]
- Cunha, A.M.; Loja, J.T.; Torrão, L.; Moreira, R.; Pinheiro, D.; Falcão-Reis, F.; Pinheiro-Costa, J. A 10-Year Retrospective Clinical Analysis of Fungal Keratitis in a Portuguese Tertiary Centre. Clin. Ophthalmol. 2020, 14, 3833–3839. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Bignardi, G.; Koerner, R.; Irion, L.D.; Johnson, E.; Morgan, S.J.; Ghosh, S. Polymicrobial Keratitis with Cryptococcus curvatus, Candida parapsilosis, and Stenotrophomonas maltophilia after Penetrating Keratoplasty: A Rare Case Report with Literature Review. Eye Contact Lens Sci. Clin. Pract. 2019, 45, e5–e10. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Mckenna, M.; Sadiq, S.N.; Martin, J.; Mudhar, H.S.; Meeney, A.; Patel, T. Arthrographis kalrae Keratitis Complicated by Endophthalmitis: A Case Report with Literature Review. Eye Contact Lens Sci. Clin. Pract. 2020, 46, e59–e65. [Google Scholar] [CrossRef] [PubMed]
- Prajna, N.V.; Krishnan, T.; Rajaraman, R.; Patel, S.; Shah, R.; Srinivasan, M.; Das, M.; Ray, K.J.; Oldenburg, C.E.; McLeod, S.D.; et al. Predictors of Corneal Perforation or Need for Therapeutic Keratoplasty in Severe Fungal Keratitis: A Secondary Analysis of the Mycotic Ulcer Treatment Trial II. JAMA Ophthalmol. 2017, 135, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Said, D.G.; Rallis, K.I.; Al-Aqaba, M.A.; Ting, D.S.; Dua, H.S. Surgical management of infectious keratitis. Ocul. Surf. 2021, S1542. [Google Scholar] [CrossRef]
- Lalitha, P.; Prajna, N.V.; Manoharan, G.; Srinivasan, M.; Mascarenhas, J.; Das, M.; D’Silva, S.S.; Porco, T.C.; Keenan, J.D. Trends in bacterial and fungal keratitis in South India, 2002–2012. Br. J. Ophthalmol. 2015, 99, 192–194. [Google Scholar] [CrossRef]
- Lalitha, P.; Prajna, N.V.; Oldenburg, C.E.; Srinivasan, M.; Krishnan, T.; Mascarenhas, J.; Vaitilingam, C.M.; McLeod, S.D.; Zegans, M.E.; Porco, T.C.; et al. Organism, Minimum Inhibitory Concentration, and Outcome in a Fungal Corneal Ulcer Clinical Trial. Cornea 2012, 31, 662–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dan, J.; Zhou, Q.; Zhai, H.; Cheng, J.; Wan, L.; Ge, C.; Xie, L. Clinical analysis of fungal keratitis in patients with and without diabetes. PLoS ONE 2018, 13, e0196741. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, M.; Vira, D.; Dey, M.; Tanzin, T.; Kumar, N.; Sharma, S. Comparison between Polymicrobial and Fungal Keratitis: Clinical Features, Risk Factors, and Outcome. Am. J. Ophthalmol. 2015, 160, 873–881.e2. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Salma, K.C.; Sharma, M.; Bastola, P.; Pradhan, R.; Kansakar, I. Pattern of fungal isolates in cases of corneal ulcer in the western periphery of Nepal. Nepal. J. Ophthalmol. 2011, 3, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Khurana, A.; Chanda, S.; Bhagat, P.; Aggarwal, S.; Sharma, M.; Chauhan, L. Clinical characteristics, predisposing factors, and treatment outcome of Curvularia keratitis. Indian J. Ophthalmol. 2020, 68, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, J.; Lalitha, P.; Prajna, N.V.; Srinivasan, M.; Das, M.; D’Silva, S.S.; Oldenburg, C.E.; Borkar, D.S.; Esterberg, E.J.; Lietman, T.M.; et al. Acanthamoeba, Fungal, and Bacterial Keratitis: A Comparison of Risk Factors and Clinical Features. Am. J. Ophthalmol. 2014, 157, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Ong, H.S.; Fung, S.S.; Macleod, D.; Dart, J.K.; Tuft, S.J.; Burton, M.J. Altered Patterns of Fungal Keratitis at a London Ophthalmic Referral Hospital: An Eight-Year Retrospective Observational Study. Am. J. Ophthalmol. 2016, 168, 227–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ting, D.S.J.; Cairns, J.; Gopal, B.P.; Ho, C.S.; Krstic, L.; Elsahn, A.; Lister, M.; Said, D.G.; Dua, H.S. Risk Factors, Clinical Outcomes, and Prognostic Factors of Bacterial Keratitis: The Nottingham Infectious Keratitis Study. Front. Med. 2021, 8, 715118. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Feltgen, N.; Junker, B.; Schulze-Bonsel, K.; Bach, M. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity Test (FrACT). Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Z.; Walkden, A.; Au, L.; Fullwood, C.; Hamilton, A.; Qamruddin, A.; Armstrong, M.; Brahma, A.K.; Carley, F. Twelve-year analysis of microbial keratitis trends at a UK tertiary hospital. Eye 2017, 31, 1229–1236. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Settle, C.; Morgan, S.J.; Baylis, O.; Ghosh, S. A 10-year analysis of microbiological profiles of microbial keratitis: The North East England Study. Eye 2018, 32, 1416–1417. [Google Scholar] [CrossRef] [Green Version]
- Tavassoli, S.; Nayar, G.; Darcy, K.; Grzeda, M.; Luck, J.; Williams, O.M.; Tole, D. An 11-year analysis of microbial keratitis in the South West of England using brain–heart infusion broth. Eye 2019, 33, 1619–1625. [Google Scholar] [CrossRef]
- Keay, L.; Gower, E.W.; Iovieno, A.; Oechsler, R.A.; Alfonso, E.C.; Matoba, A.; Colby, K.; Tuli, S.S.; Hammersmith, K.; Cavanagh, D.; et al. Clinical and Microbiological Characteristics of Fungal Keratitis in the United States, 2001–2007: A Multicenter Study. Ophthalmology 2011, 118, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galarreta, D.J.; Tuft, S.J.; Ramsay, A.; Dart, J.K. Fungal keratitis in London: Microbiological and clinical evaluation. Cornea 2007, 26, 1082–1086. [Google Scholar] [CrossRef]
- Khoo, P.; Cabrera-Aguas, M.; Nguyen, V.; Lahra, M.M.; Watson, S.L. Microbial keratitis in Sydney, Australia: Risk factors, patient outcomes, and seasonal variation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 1745–1755. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Han, L.; Yin, W. Study of Pathogens of Fungal Keratitis and the Sensitivity of Pathogenic Fungi to Therapeutic Agents with the Disk Diffusion Method. Curr. Eye Res. 2015, 40, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, P.; Abdel-Hadi, A.; Randhir Babu Singh, Y.; Revathi, R.; Anita, R.; Banawas, S.; Bin Dukhyil, A.A.; Alshehri, B.; Shobana, C.S.; Panneer Selvam, K.; et al. Fungal Keratitis: Epidemiology, Rapid Detection, and Antifungal Susceptibilities of Fusarium and Aspergillus Isolates from Corneal Scrapings. Biomed. Res. Int. 2019, 2019, 6395840. [Google Scholar] [CrossRef] [Green Version]
- Song, A.; Deshmukh, R.; Lin, H.; Ang, M.; Mehta, J.S.; Chodosh, J.; Said, D.G.; Dua, H.S.; Ting, D.S. Post-keratoplasty Infectious Keratitis: Epidemiology, Risk Factors, Management, and Outcomes. Front. Med. 2021, 8, 707242. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Said, D.G.; Dua, H.S. Interface Haze after Descemet Stripping Automated Endothelial Keratoplasty. JAMA Ophthalmol. 2019, 137, 1201. [Google Scholar] [CrossRef]
- Sharma, N.; Kaur, M.; Titiyal, J.S.; Aldave, A. Infectious keratitis after lamellar keratoplasty. Surv. Ophthalmol. 2021, 66, 623–643. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Henein, C.; Said, D.G.; Dua, H.S. Photoactivated chromophore for infectious keratitis—Corneal cross-linking (PACK-CXL): A systematic review and meta-analysis. Ocul. Surf. 2019, 17, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Prajna, N.V.; Radhakrishnan, N.; Lalitha, P.; Austin, A.; Ray, K.J.; Keenan, J.D.; Porco, T.C.; Lietman, T.M.; Rose-Nussbaumer, J. Cross-Linking-Assisted Infection Reduction: A Randomized Clinical Trial Evaluating the Effect of Adjuvant Cross-Linking on Outcomes in Fungal Keratitis. Ophthalmology 2020, 127, 159–166. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Henein, C.; Said, D.G.; Dua, H.S. Re: Prajna et al. Cross-Linking-Assisted Infection Reduction (CLAIR): A randomized clinical trial evaluating the effect of adjuvant cross-linking on outcomes in fungal keratitis. Ophthalmology 2020, 127, e55–e56. [Google Scholar] [CrossRef]
- Khoo, P.; Cabrera-Aguas, M.; Watson, S.L. Microbial Keratitis after Corneal Collagen Cross-Linking for Corneal Ectasia. Asia-Pac. J. Ophthalmol. 2021, 10, 355–359. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Bandyopadhyay, J.; Patel, T. Microbial keratitis complicated by acute hydrops following corneal collagen cross-linking for keratoconus. Clin. Exp. Optom. 2019, 102, 434–436. [Google Scholar] [CrossRef]
- Prajna, N.V.; Krishnan, T.; Mascarenhas, J.; Srinivasan, M.; Oldenburg, C.E.; Toutain-Kidd, C.M.; Sy, A.; McLeod, S.D.; Zegans, M.E.; Acharya, N.R.; et al. Predictors of outcome in fungal keratitis. Eye 2012, 26, 1226–1231. [Google Scholar] [CrossRef] [Green Version]
- Prajna, N.V.; Srinivasan, M.; Lalitha, P.; Krishnan, T.; Rajaraman, R.; Ravindran, M.; Mascarenhas, J.; Oldenburg, C.E.; Ray, K.J.; McLeod, S.D.; et al. Differences in Clinical Outcomes in Keratitis Due to Fungus and Bacteria. JAMA Ophthalmol. 2013, 131, 1088–1089. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Rathi, V.; Murthy, S.; Mitra, S.; Yamjala, B.; Mohamed, A. Masked comparison of trypan blue stain and potassium hydroxide with calcofluor white stain in the microscopic examination of corneal scrapings for the diagnosis of microbial keratitis. Indian J. Ophthalmol. 2021, 69, 2457. [Google Scholar] [CrossRef] [PubMed]
- Somerville, T.F.; Corless, C.E.; Sueke, H.; Neal, T.; Kaye, S.B. 16S Ribosomal RNA PCR versus Conventional Diagnostic Culture in the Investigation of Suspected Bacterial Keratitis. Transl. Vis. Sci. Technol. 2020, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Holmgaard, D.B.; Barnadas, C.; Mirbarati, S.H.; O’Brien, L.A.; Nielsen, H.V.; Stensvold, C.R. Detection and Identification of Acanthamoeba and Other Nonviral Causes of Infectious Keratitis in Corneal Scrapings by Real-Time PCR and Next-Generation Sequencing-Based 16S-18S Gene Analysis. J. Clin. Microbiol. 2021, 59, e02224-20. [Google Scholar] [CrossRef]
- Chidambaram, J.D.; Prajna, N.V.; Palepu, S.; Lanjewar, S.; Shah, M.; Elakkiya, S.; Macleod, D.; Lalitha, P.; Burton, M. In Vivo Confocal Microscopy Cellular Features of Host and Organism in Bacterial, Fungal, and A. canthamoeba Keratitis. Am. J. Ophthalmol. 2018, 190, 24–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ung, L.; Bispo, P.J.; Doan, T.; Van Gelder, R.N.; Gilmore, M.S.; Lietman, T.; Margolis, T.P.; Zegans, M.E.; Lee, C.S.; Chodosh, J. Clinical metagenomics for infectious corneal ulcers: Rags to riches? Ocul. Surf. 2019, 18, 1–12. [Google Scholar] [CrossRef]
- Lalitha, P.; Prajna, N.V.; Sikha, M.; Gunasekaran, R.; Hinterwirth, A.; Worden, L.; Chen, C.; Zhong, L.; Liu, Z.; Lietman, T.M.; et al. Evaluation of Metagenomic Deep Sequencing as a Diagnostic Test for Infectious Keratitis. Ophthalmology 2021, 128, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Gopal, B.P.; Deshmukh, R.; Seitzman, G.D.; Said, D.G.; Dua, H.S. Diagnostic armamentarium of infectious keratitis: A comprehensive review. Ocul. Surf. 2021, in press. [Google Scholar]
- Watson, S.; Cabrera-Aguas, M.; Khoo, P.; Pratama, R.; Gatus, B.J.; Gulholm, T.; El-Nasser, J.; Lahra, M.M. Keratitis antimicrobial resistance surveillance program, Sydney, Australia: 2016 Annual Report. Clin. Exp. Ophthalmol. 2019, 47, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Ting, D.S.J.; Foo, V.H.; Yang, L.W.Y.; Sia, J.T.; Ang, M.; Lin, H.; Chodosh, J.; Mehta, J.S.; Ting, D.S.W. Artificial intelligence for anterior segment diseases: Emerging applications in ophthalmology. Br. J. Ophthalmol. 2021, 105, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Piech, C.; Baitemirova, M.; Prajna, N.V.; Srinivasan, M.; Lalitha, P.; Villegas, N.; Balachandar, N.; Chua, J.T.; Redd, T.; et al. Differentiation of Active Corneal Infections from Healed Scars Using Deep Learning. Ophthalmology 2021, 0161. [Google Scholar] [CrossRef] [PubMed]
- Hung, N.; Shih, A.; Lin, C.; Kuo, M.-T.; Hwang, Y.-S.; Wu, W.-C.; Kuo, C.-F.; Kang, E.; Hsiao, C.-H. Using Slit-Lamp Images for Deep Learning-Based Identification of Bacterial and Fungal Keratitis: Model Development and Validation with Different Convolutional Neural Networks. Diagnostics 2021, 11, 1246. [Google Scholar] [CrossRef]
- Rampat, R.; Deshmukh, R.; Chen, X.; Ting, D.S.; Said, D.G.; Dua, H.S.; Ting, D.S. Artificial Intelligence in Cornea, Refractive Surgery, and Cataract. Asia-Pac. J. Ophthalmol. 2021, 10, 268–281. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Beuerman, R.W.; Dua, H.S.; Lakshminarayanan, R.; Mohammed, I. Strategies in Translating the Therapeutic Potentials of Host Defense Peptides. Front. Immunol. 2020, 11, 983. [Google Scholar] [CrossRef]
- Mayandi, V.; Xi, Q.; Goh, E.T.L.; Koh, S.K.; Toh, T.Y.J.; Barathi, V.A.; Fazil, M.H.U.T.; Chalasani, M.L.S.; Varadarajan, J.; Ting, D.S.J.; et al. Rational Substitution of ε-Lysine for α-Lysine Enhances the Cell and Membrane Selectivity of Pore-Forming Melittin. J. Med. Chem. 2020, 63, 3522–3537. [Google Scholar] [CrossRef]
- Mohammed, I.; Mohanty, D.; Said, D.G.; Barik, M.R.; Reddy, M.M.; Alsaadi, A.; Das, S.; Dua, H.S.; Mittal, R. Antimicrobial peptides in human corneal tissue of patients with fungal keratitis. Br. J. Ophthalmol. 2020, 105, 1172–1177. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Goh, E.T.L.; Mayandi, V.; Busoy, J.M.F.; Aung, T.T.; Periayah, M.H.; Nubile, M.; Mastropasqua, L.; Said, D.G.; Htoon, H.M.; et al. Hybrid derivative of cathelicidin and human beta defensin-2 against Gram-positive bacteria: A novel approach for the treatment of bacterial keratitis. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Górski, A.; Bollyky, P.L.; Przybylski, M.; Borysowski, J.; Międzybrodzki, R.; Jończyk-Matysiak, E.; Weber-Dąbrowska, B. Perspectives of Phage Therapy in Non-bacterial Infections. Front. Microbiol. 2019, 9, 3306. [Google Scholar] [CrossRef] [PubMed]

| Parameters | All Cases | Culture-Proven | Culture-Negative | p-Value # |
|---|---|---|---|---|
| Total N = 117; N (%) | Total N = 52; N (%) | Total N = 65; N (%) | ||
| Hospital | 0.12 | |||
| QVH | 87 (74.4) | 35 (67.3) | 52 (80.0) | |
| QMC | 30 (25.6) | 17 (32.7) | 13 (20.0) | |
| Age, years | 59.0 ± 19.6 | 56.5 ± 20.8 | 61.1 ± 18.5 | 0.21 |
| Gender | 0.11 | |||
| Female | 60 (51.3) | 31 (59.6) | 29 (44.6) | |
| Male | 57 (48.7) | 21 (40.4) | 36 (55.4) | |
| Laterality | 0.23 | |||
| Left | 50 (42.7) | 19 (36.5) | 31 (47.7) | |
| Right | 67 (57.3) | 33 (63.5) | 34 (52.3) | |
| Risk factors $ | 0.37 | |||
| OSD * | 60 (51.3) | 30 (57.7) | 30 (46.2) | |
| Prior corneal surgery | 44 (37.6) | 18 (34.6) | 26 (40.0) | |
| Immunosuppression ** | 42 (35.9) | 21 (40.4) | 21 (32.3) | |
| Contact lens wears | 28 (23.9) | 17 (32.7) | 11 (16.9) | |
| Topical corticosteroids | 19 (16.2) | 10 (19.2) | 9 (13.8) | |
| Lid diseases *** | 16 (13.7) | 4 (7.7) | 12 (18.5) | |
| Trauma | 7 (6.0) | 3 (5.8) | 4 (6.2) | |
| Presenting CDVA, in logMAR | 0.038 | |||
| 0.0–0.3 | 16 (13.7) | 10 (19.3) | 6 (9.2) | |
| <0.3–0.6 | 7 (6.0) | 4 (7.7) | 3 (4.6) | |
| <0.6–1.0 | 11 (9.4) | 8 (15.4) | 3 (4.6) | |
| <1.0 | 83 (70.9) | 30 (57.7) | 53 (81.5) | |
| Size of epithelial defect | 0.22 | |||
| Very small (<1 mm) | 6 (5.1) | 2 (3.8) | 4 (6.2) | |
| Small (1–3 mm) | 38 (32.5) | 19 (36.5) | 19 (29.2) | |
| Moderate (3.1–6 mm) | 45 (38.5) | 23 (44.2) | 22 (33.8) | |
| Large (>6 mm) | 28 (23.9) | 8 (15.4) | 20 (30.8) | |
| Size of infiltrate | 0.52 | |||
| Very small (<1 mm) | 10 (8.5) | 5 (9.6) | 5 (7.7) | |
| Small (1–3 mm) | 45 (38.5) | 21 (40.4) | 24 (36.9) | |
| Moderate (3.1–6 mm) | 47 (40.2) | 22 (42.3) | 25 (38.5) | |
| Large (>6 mm) | 15 (12.8) | 4 (7.7) | 11 (16.9) | |
| Location | 0.71 | |||
| Central | 70 (59.8) | 29 (55.8) | 41 (63.1) | |
| Paracentral | 34 (29.0) | 17 (32.7) | 17 (26.2) | |
| Peripheral | 13 (11.1) | 6 (11.5) | 7 (10.8) | |
| Hypopyon | 0.76 | |||
| Yes | 40 (34.2) | 17 (32.7) | 23 (35.4) | |
| No | 77 (65.8) | 35 (67.3) | 42 (64.6) | |
| Hospitalisation required | 0.29 | |||
| Yes | 95 (81.2) | 40 (76.9) | 55 (84.6) | |
| No | 22 (18.8) | 12 (33.1) | 10 (15.4) | |
| Duration of hospitalisation, days | 18.9 ± 16.3 | 17.5 ± 15.0 | 19.8 ± 17.2 | 0.5 |
| Co-infection with bacteria | 0.18 | |||
| Yes | 32 (27.3) | 11 (21.2) | 21 (32.3) | |
| No | 85 (72.7) | 41 (78.8) | 44 (67.7) | |
| Need for surgical intervention (s) | 0.62 | |||
| Yes | 66 (56.4) | 28 (53.8) | 38 (58.5) | |
| No | 51 (43.6) | 24 (46.2) | 27 (41.5) |
| Organisms | N (%) |
|---|---|
| Fungi | |
| Total | 53 (100) |
| Yeast | 33 (62.3) |
| Candida spp. | 33 (62.3) |
| Filamentous fungi | 19 (35.8) |
| Fusarium spp. | 9 (17.0) |
| Aspergillus spp. | 5 (9.4) |
| Peniophora spp. | 2 (3.8) |
| Acremonium spp. | 1 (1.9) |
| Scedosporium spp. | 1 (1.9) |
| Mixed yeast and filamentous infection | 1 (1.9) |
| Rhodotorula spp. + Alternaria spp. | 1 (1.9) |
| Bacteria (co-infection with fungal keratitis) | |
| Total * | 32 (27.4) |
| Gram-positive | 22 (18.8) |
| Staphylococci spp. | 16 (13.7) |
| Streptococcus pneumonia | 3 (2.6) |
| Bacillus spp. | 2 (1.7) |
| Enterococcus faecalis | 1 (0.9) |
| Gram-negative | 10 (8.) |
| Moraxella spp. | 3 (2.6) |
| Serratia marcescens | 3 (2.6) |
| Pseudomonas spp. | 2 (1.7) |
| Haemophilus influenza | 1 (0.9) |
| Acinetobacter lwoffii | 1 (0.9) |
| Parameters | Yeast FK | Filamentous FK | p-Value # |
|---|---|---|---|
| Total N = 33; | Total N = 18; | ||
| N (%) | N (%) | ||
| Age | 60.2 ± 18.9 | 51.3 ± 22.9 | 0.14 |
| Gender | 0.34 | ||
| Female | 21 (63.6) | 9 (50.0) | |
| Male | 12 (36.4) | 9 (50.0) | |
| Hospital | 0.57 | ||
| QVH | 21 (63.6) | 10 (55.6) | |
| QMC | 12 (36.4) | 8 (44.4) | |
| Risk factors $ | 0.31 | ||
| OSD | 14 (42.4) | 11 (61.1) | 0.2 |
| Prior corneal surgery | 7 (21.2) | 2 (11.1) | 0.37 |
| Immunosuppression | 8 (24.2) | 7 (38.9) | 0.27 |
| Contact lens wear | 6 (18.2) | 9 (50.0) | 0.017 |
| Topical corticosteroids | 6 (18.2) | 1 (5.6) | 0.21 |
| Trauma | 2 (6.1) | 1 (5.6) | 0.94 |
| Clinical Features | All Cases | Culture-Positive | Culture-Negative | p-Value |
|---|---|---|---|---|
| Total N = 117; | Total N = 52; | Total N = 65; | ||
| N (%) | N (%) | N (%) | ||
| Typical clinical signs | 0.8 | |||
| Feathery border | 52 (44.4) | 25 (48.1) | 27 (41.5) | |
| Satellite lesions | 39 (33.3) | 16 (30.8) | 23 (35.4) | |
| Deep stromal/endothelial plaque | 39 (33.3) | 14 (26.9) | 25 (38.5) | |
| Multifocal lesion | 32 (27.4) | 15 (28.8) | 17 (26.2) | |
| Ring infiltrate | 29 (24.8) | 13 (25.0) | 16 (24.6) | |
| Number of typical clinical signs | 0.74 | |||
| None | 24 (20.5) | 12 (23.1) | 12 (18.5) | |
| 1 | 31 (26.5) | 15 (28.8) | 16 (24.6) | |
| 2 | 35 (29.9) | 13 (25.0) | 22 (33.8) | |
| 3 or more | 27 (23.1) | 12 (23.1) | 15 (23.1) |
| Poor Visual Outcome | Poor Corneal Healing | |||
|---|---|---|---|---|
| Parameters | Odd Ratio (95% CI) | p-Value * | Odd Ratio (95% CI) | p-Value * |
| Age > 50 years | 4.72 (1.40–15.89) | 0.012 | 5.81 (1.83–18.37) | 0.003 |
| Male gender | 0.99 (0.33–3.00) | 0.99 | 0.93 (0.33–2.72) | 0.91 |
| Right eye | 1.13 (0.37–3.43) | 0.83 | 2.86 (0.95–8.61) | 0.06 |
| Presenting CDVA < 1.0 | 14.92 (4.19–53.18) | <0.001 | 3.91 (1.19–12.82) | 0.025 |
| Infiltrate size > 3 mm | 3.61 (1.11–11.81) | 0.034 | 3.91 (1.18–12.88) | 0.025 |
| Central ulcer | 1.51 (0.50–4.53) | 0.47 | 1.58 (0.55–4.58) | 0.4 |
| Presence of hypopyon | 2.87 (0.81–10.18) | 0.1 | 2.78 (0.78–9.86) | 0.12 |
| Culture results | 0.88 | 0.44 | ||
| Negative | Reference | - | Reference | - |
| Yeast | 1.36 (0.37–4.97) | 0.64 | 1.67 (0.49–5.76) | 0.42 |
| Filamentous | 0.95 (0.19–4.71) | 0.95 | 2.74 (0.52–14.41) | 0.23 |
| Co-infection with bacteria | 1.70 (0.49–5.93) | 0.4 | 0.71 (0.23–2.26) | 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ting, D.S.J.; Galal, M.; Kulkarni, B.; Elalfy, M.S.; Lake, D.; Hamada, S.; Said, D.G.; Dua, H.S. Clinical Characteristics and Outcomes of Fungal Keratitis in the United Kingdom 2011–2020: A 10-Year Study. J. Fungi 2021, 7, 966. https://doi.org/10.3390/jof7110966
Ting DSJ, Galal M, Kulkarni B, Elalfy MS, Lake D, Hamada S, Said DG, Dua HS. Clinical Characteristics and Outcomes of Fungal Keratitis in the United Kingdom 2011–2020: A 10-Year Study. Journal of Fungi. 2021; 7(11):966. https://doi.org/10.3390/jof7110966
Chicago/Turabian StyleTing, Darren Shu Jeng, Mohamed Galal, Bina Kulkarni, Mohamed S. Elalfy, Damian Lake, Samer Hamada, Dalia G. Said, and Harminder S. Dua. 2021. "Clinical Characteristics and Outcomes of Fungal Keratitis in the United Kingdom 2011–2020: A 10-Year Study" Journal of Fungi 7, no. 11: 966. https://doi.org/10.3390/jof7110966
APA StyleTing, D. S. J., Galal, M., Kulkarni, B., Elalfy, M. S., Lake, D., Hamada, S., Said, D. G., & Dua, H. S. (2021). Clinical Characteristics and Outcomes of Fungal Keratitis in the United Kingdom 2011–2020: A 10-Year Study. Journal of Fungi, 7(11), 966. https://doi.org/10.3390/jof7110966






