The Bicarbonate Transporter (MoAE4) Localized on Both Cytomembrane and Tonoplast Promotes Pathogenesis in Magnaporthe oryzae
Abstract
1. Introduction
2. Materials and Methods
2.1. Sequence Alignment Assays
2.2. Fungal Strains and Culture Conditions
2.3. Assays for the Subcellular Localization of MoAE4
2.4. Targeted Gene Deletion and Complementation
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. Assays for Conidial Production, Growth, and Development
2.7. Rice Sheath Penetration and Plant Infection Assays
2.8. Assays for NaHCO3 Treatment
2.9. Assays for HCO3− Transport and Intracellular pH Measurements
2.10. H2O2 Treatment and Endogenous H2O2 Measurements
2.11. Statistical Analysis
3. Results
3.1. The Bicarbonate Transporter AE4 Homologue in M. oryzae
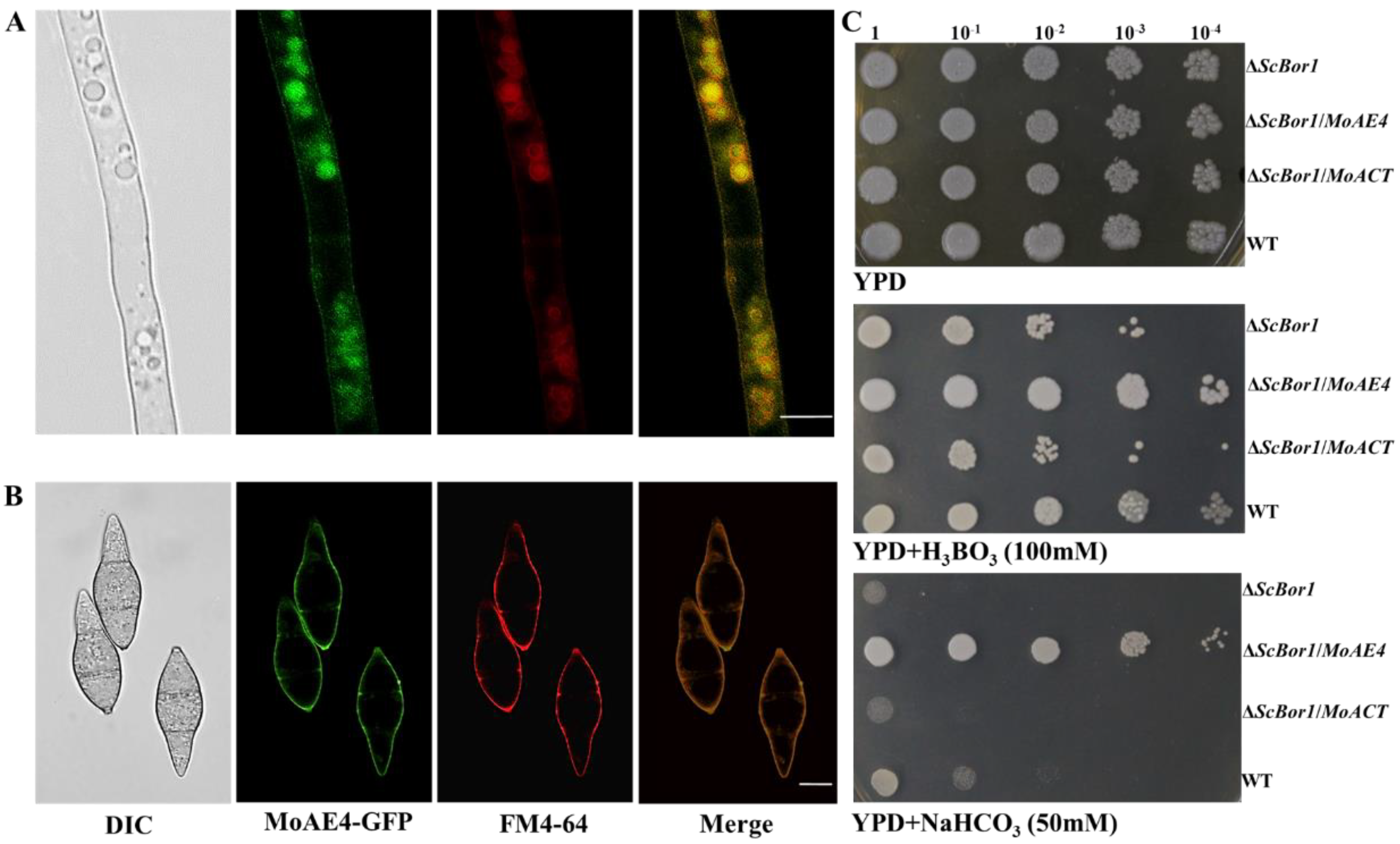
3.2. MoAE4 Localizes on Cytomembrane and Tonoplast and Functions in Yeast
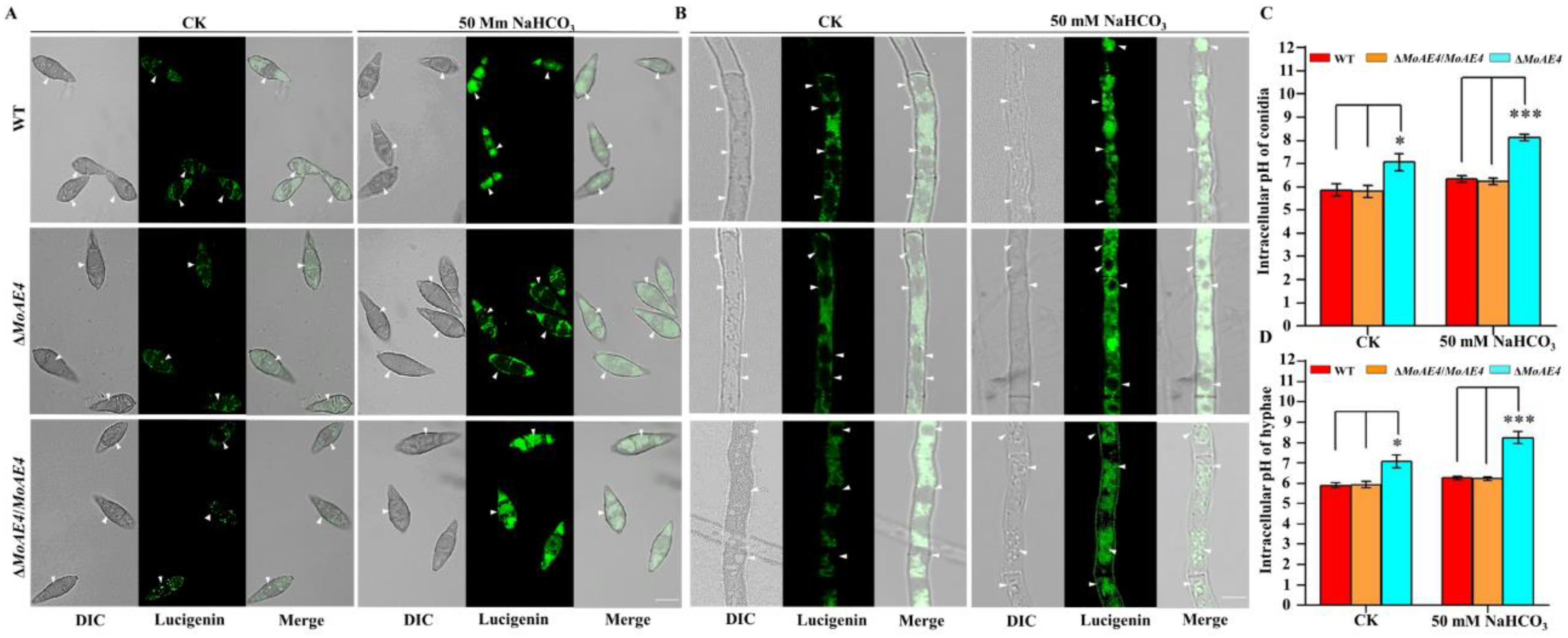
3.3. MoAE4 Transports Cytosolic HCO3− to Vacuole and Cell outside
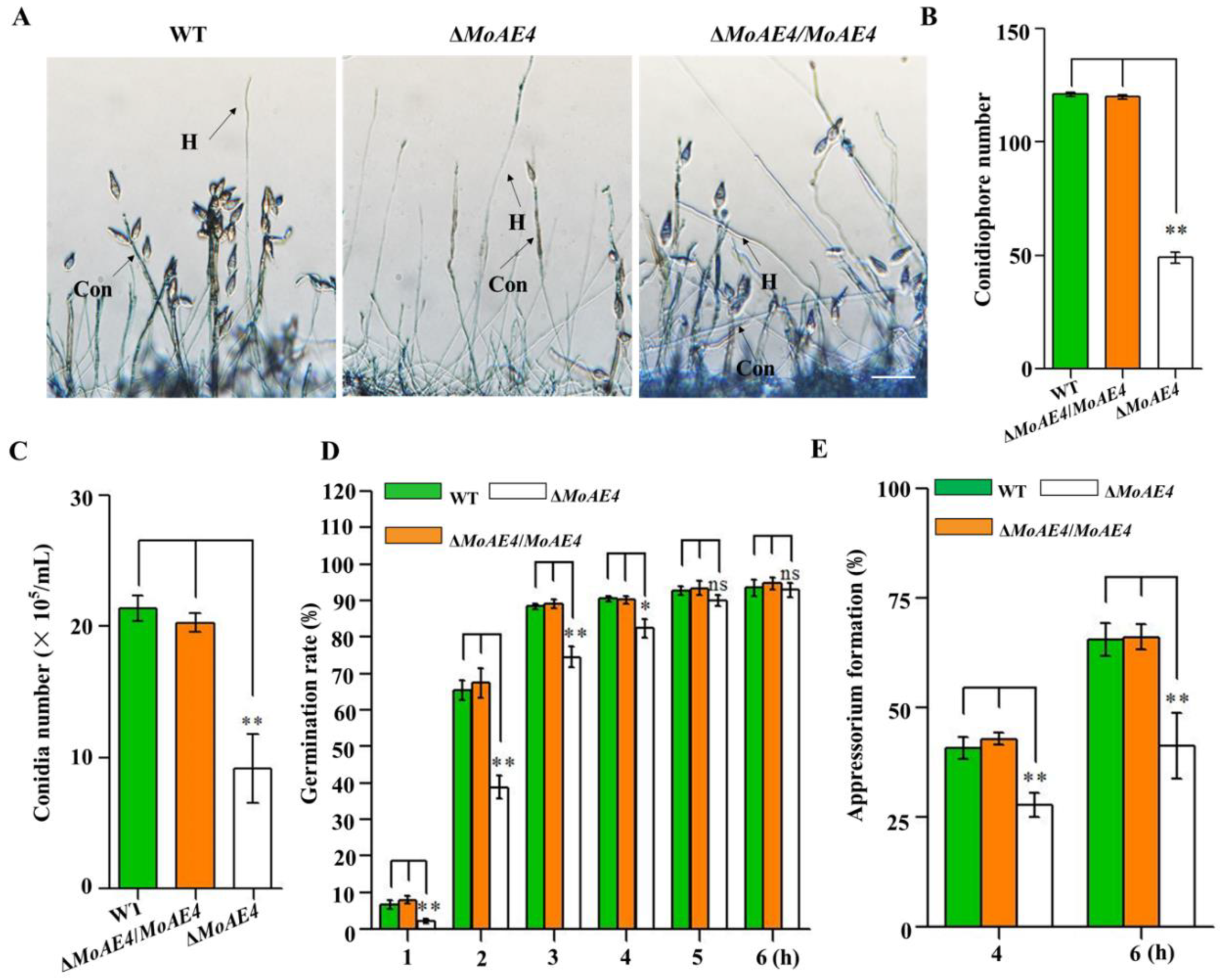
3.4. MoAE4 Is Important for Conidiation and Appressorium Development
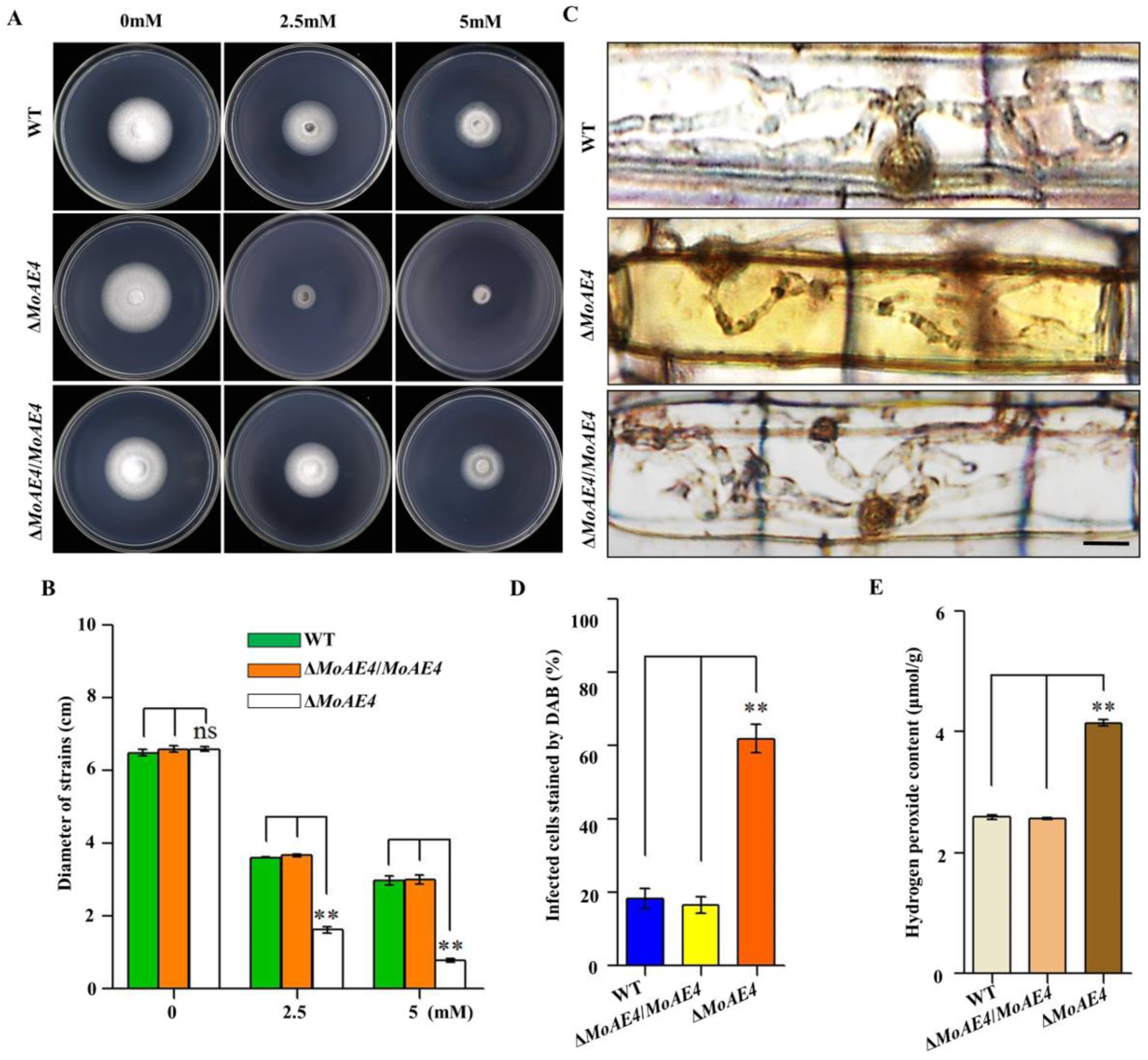
3.5. Requirement of MoAE4 for Pathogenicity in M. oryzae
3.6. MoAE4 Is Important for H2O2 Tolerance and Clearance Inside or Outside Cells
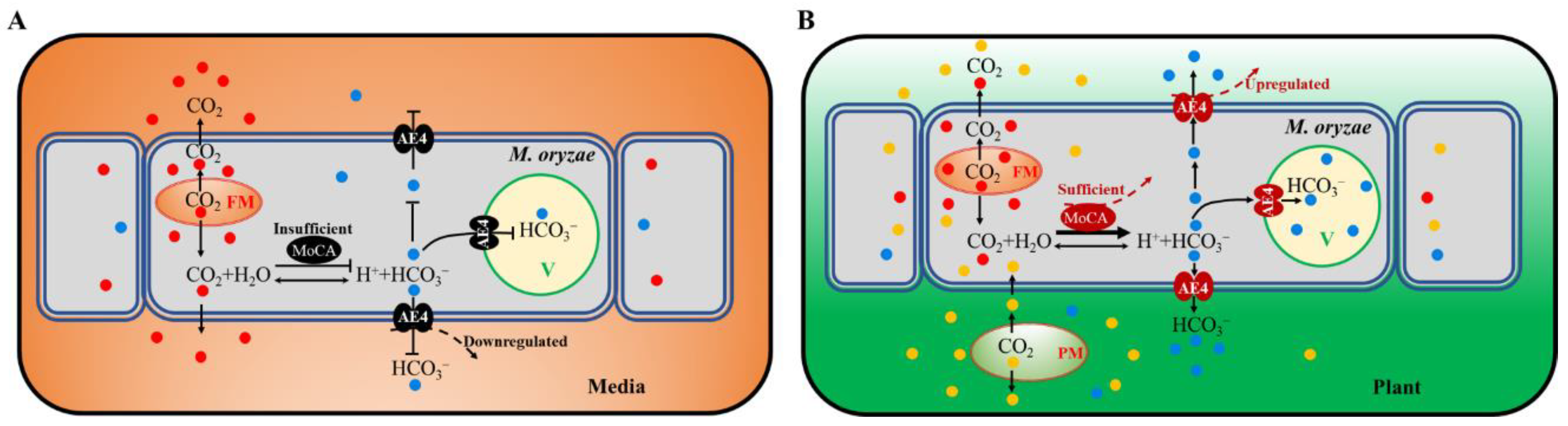
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cordat, E.; Casey, J.R. Bicarbonate Transport in Cell Physiology and Disease. Biochem. J. 2009, 417, 423–439. [Google Scholar] [CrossRef]
- Romero, M.F.; Chen, A.P.; Parker, M.D.; Boron, W.F. The SLC4 Family of Bicarbonate (HCO3−) Transporters. Mol. Asp. Med. 2013, 34, 159–182. [Google Scholar] [CrossRef]
- Shieh, Y.W.; Minguez, P.; Bork, P.; Auburger, J.J.; Guilbride, D.L.; Kramer, G.; Bukau, B. Operon Structure and Cotranslational Subunit Association Direct Protein Assembly in Bacteria. Science 2015, 350, 678–680. [Google Scholar] [CrossRef]
- Hatae, H.; Inaka, K.; Okamura, R.; Furubayashi, N.; Kamo, M.; Kobayashi, T.; Abe, Y.; Iwata, S.; Hamasaki, N. Crystallization of Human Erythrocyte Band 3, the Anion Exchanger, at the International Space Station “KIBO”. Anal. Biochem. 2018, 559, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Tsuganezawa, H.; Kobayashi, K.; Iyori, M.; Araki, T.; Koizumi, A.; Watanabe, S.I.; Kaneko, A.; Fukao, T.; Monkawa, T.; Yoshida, T.; et al. A New Member of the HCO3− Transporter Superfamily Is an Apical Anion Exchanger of β-Intercalated Cells in the Kidney. J. Biol. Chem. 2001, 276, 8180–8189. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.B.H.; Luo, X.; Hager, H.; Rojek, A.; Choi, J.Y.; Licht, C.; Suzuki, M.; Muallem, S.; Nielsen, S.; Ishibashi, K. AE4 Is a DIDS-Sensitive Cl−/HCO3− Exchanger in the Basolateral Membrane of the Renal CCD and the SMG Duct. Am. J. Physiol. Cell Physiol. 2002, 283, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Peña-Münzenmayer, G.; Catalán, M.A.; Kondo, Y.; Jaramillo, Y.; Liu, F.; Shull, G.E.; Melvin, J.E. Ae4 (Slc4a9) Anion Exchanger Drives Cl- Uptake-Dependent Fluid Secretion by Mouse Submandibular Gland Acinar Cells. J. Biol. Chem. 2015, 290, 10677–10688. [Google Scholar] [CrossRef]
- Peña-Münzenmayer, G.; George, A.T.; Shull, G.E.; Melvin, J.E.; Catalán, M.A. Ae4 (Slc4a9) Is an Electroneutral Monovalent Cation-Dependent Cl−/HCO3− Exchanger. J. Gen. Physiol. 2016, 147, 423–436. [Google Scholar] [CrossRef]
- Vera-Sigüenza, E.; Catalán, M.A.; Peña-Münzenmayer, G.; Melvin, J.E.; Sneyd, J. A Mathematical Model Supports a Key Role for Ae4 (Slc4a9) in Salivary Gland Secretion. Bull. Math. Biol. 2018, 80, 255–282. [Google Scholar] [CrossRef]
- Coudray, N.; Seyler, S.L.; Lasala, R.; Zhang, Z.; Clark, K.M.; Dumont, M.E.; Rohou, A.; Beckstein, O.; Stokes, D.L. Structure of the SLC4 Transporter Bor1p in an Inward-Facing Conformation. Protein Sci. 2017, 26, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Reithmeier, R.A.F. Expression and Characterization of the Anion Transporter Homologue YNL275w in Saccharomyces cerevisiae. Am. J. Physiol. Cell Physiol. 2001, 281, 33–45. [Google Scholar] [CrossRef]
- Nozawa, A.; Takano, J.; Kobayashi, M.; Von Wirén, N.; Fujiwara, T. Roles of BOR1, DUR3, and FPS1 in Boron Transport and Tolerance in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2006, 262, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Takano, J.; Kobayashi, M.; Noda, Y.; Fujiwara, T. Saccharomyces Cerevisiae Bor1p Is a Boron Exporter and a Key Determinant of Boron Tolerance. FEMS Microbiol. Lett. 2007, 267, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.L.; Howren, T.R.; Cui, J.; Winters, M.; Hannigan, R. Transport and Regulatory Characteristics of the Yeast Bicarbonate Transporter Homolog Bor1p. Am. J. Physiol. Cell Physiol. 2007, 293, 468–476. [Google Scholar] [CrossRef]
- Takano, J.; Noguchi, K.; Yasumori, M.; Kobayashi, M.; Gajdos, Z.; Miwa, K.; Hayashi, H.; Yoneyama, T.; Fujiwara, T. Arabidopsis Boron Transporter for Xylem Loading. Nature 2002, 420, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Villarino, M.; Etxebeste, O.; Mendizabal, G.; Garzia, A.; Ugalde, U.; Espeso, E.A. Boron Tolerance in Aspergillus nidulans Is Sustained by the SltA Pathway through the SLC-Family Transporters SbtA and SbtB. Genes 2017, 8, 188. [Google Scholar] [CrossRef]
- Howard, R.J.; Ferrari, M.A.; Roach, D.H.; Money, N.P. Penetration of Hard Substrates by a Fungus Employing Enormous Turgor Pressures. Proc. Natl. Acad. Sci. USA 1991, 88, 11281–11284. [Google Scholar] [CrossRef]
- Talbot, N.J. On the Trail of a Cereal Killer: Exploring the Biology of Magnaporthe grisea. Annu. Rev. Microbiol. 2003, 57, 177–202. [Google Scholar] [CrossRef]
- Heath, M.C.; Valent, B.; Howard, R.J.; Chumley, F.G. Interactions of Two Strains of Magnaporthe grisea with Rice, Goosegrass, and Weeping Lovegrass. Can. J. Bot. 1990, 68, 1627–1637. [Google Scholar] [CrossRef]
- Kankanala, P.; Czymmek, K.; Valent, B. Roles for Rice Membrane Dynamics and Plasmodesmata during Biotrophic Invasion by the Blast Fungus. Plant Cell 2007, 19, 706–724. [Google Scholar] [CrossRef]
- Hammond-Kosack, K.E.; Parker, J.E. Deciphering Plant-Pathogen Communication: Fresh Perspectives for Molecular Resistance Breeding. Curr. Opin. Biotechnol. 2003, 14, 177–193. [Google Scholar] [CrossRef]
- Nürnberger, T.; Brunner, F.; Kemmerling, B.; Piater, L. Innate Immunity in Plants and Animals: Striking Similarities and Obvious Differences. Immunol. Rev. 2004, 198, 249–266. [Google Scholar] [CrossRef]
- Egan, M.J.; Wang, Z.Y.; Jones, M.A.; Smirnoff, N.; Talbot, N.J. Generation of Reactive Oxygen Species by Fungal NADPH Oxidases Is Required for Rice Blast Disease. Proc. Natl. Acad. Sci. USA 2007, 104, 11772–11777. [Google Scholar] [CrossRef]
- Grahl, N.; Puttikamonkul, S.; Macdonald, J.M.; Gamcsik, M.P.; Ngo, L.Y.; Hohl, T.M.; Cramer, R.A. In Vivo Hypoxia and a Fungal Alcohol Dehydrogenase Influence the Pathogenesis of Invasive Pulmonary Aspergillosis. PLoS Pathog. 2011, 7, e1002145. [Google Scholar] [CrossRef]
- Choi, J.; Chung, H.; Lee, G.W.; Koh, S.K.; Chae, S.K.; Lee, Y.H. Genome-Wide Analysis of Hypoxia-Responsive Genes in the Rice Blast Fungus, Magnaporthe oryzae. PLoS ONE 2015, 10, e0134939. [Google Scholar] [CrossRef]
- Fernandez, J.; Marroquin-Guzman, M.; Wilson, R.A. Mechanisms of Nutrient Acquisition and Utilization during Fungal Infections of Leaves. Annu. Rev. Phytopathol. 2014, 52, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wei, Y.; Wang, Y.H.; Li, J.; Wong, F.L.; Zheng, Y.J.; Yan, H.; Liu, S.S.; Liu, J.L.; Jia, B.L.; et al. Proteins Interacting with Mitochondrial ATP-Dependent Lon Protease (MAP1) in Magnaporthe oryzae Are Involved in Rice Blast Disease. Mol. Plant Pathol. 2015, 16, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Bertram, G.; Swoboda, R.K.; Gooday, G.W.; Gow, N.A.R.; Brown, A.J.P. Structure and Regulation of the Candida Albicans ADH1 Gene Encoding an Immunogenic Alcohol Dehydrogenase. Yeast 1996, 12, 115–127. [Google Scholar] [CrossRef]
- Li, Z.; Pei, X.; Zhang, Z.; Wei, Y.; Song, Y.; Chen, L.; Liu, S.; Zhang, S.H. The Unique GH5 Cellulase Member in the Extreme Halotolerant Fungus Aspergillus glaucus CCHA Is an Endoglucanase with Multiple Tolerance to Salt, Alkali and Heat: Prospects for Straw Degradation Applications. Extremophiles 2018, 22, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhou, J.; He, Y.; Xie, Q.; Chen, A.; Zheng, H.; Shi, L.; Zhao, X.; Zhang, C.; Huang, Q.; et al. Retromer Is Essential for Autophagy-Dependent Plant Infection by the Rice Blast Fungus. PLoS Genet. 2015, 11, e1005704. [Google Scholar] [CrossRef]
- Dang, Y.; Wei, Y.; Wang, Y.; Liu, S.; Julia, C.; Zhang, S.H. Cleavage of PrePL by Lon Promotes Growth and Pathogenesis in Magnaporthe oryzae. Environ. Microbiol. 2020, 23, 4881–4895. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, G.; Lin, C.; He, C. Conidiophore Stalk-Less1 Encodes a Putative Zinc-Finger Protein Involved in the Early Stage of Conidiation and Mycelial Infection in Magnaporthe oryzae. Mol. Plant-Microbe Interact. 2009, 22, 402–410. [Google Scholar] [CrossRef]
- Qi, D.; Wang, D.; Zhang, C.; Tang, X.; He, J.; Zhao, Y.; Deng, W.; Deng, X. Vaspin Protects against LPS-Induced ARDS by Inhibiting Inflammation, Apoptosis and Reactive Oxygen Species Generation in Pulmonary Endothelial Cells via the Akt/GSK-3β Pathway. Int. J. Mol. Med. 2017, 40, 1803–1817. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oosthuizen, M.M.J.; Greyling, D. Hydroxyl Radical Generation: The Effect of Bicarbonate, Dioxygen and Buffer Concentration on PH-Dependent Chemiluminescence. Redox Rep. 2001, 6, 105–116. [Google Scholar] [CrossRef]
- Schwiening, C.J.; Boron, W.F. Regulation of Intracellular PH in Pyramidal Neurones from the Rat Hippocampus by Na(+)-dependent Cl(-)-HCO3− Exchange. J. Physiol. 1994, 475, 59–67. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, L.; Xu, Y.; Zhang, H.; Ye, W.; Mao, J.; Chen, L.; Wang, L. Uncoupling of K+ and Cl− Transport across the Cell Membrane in the Process of Regulatory Volume Decrease. Biochem. Pharmacol. 2012, 84, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.; Frenkel, C. Involvement of Hydrogen Peroxide in the Regulation of Senescence in Pear. Plant Physiol. 2020, 59, 411–416. [Google Scholar] [CrossRef]
- Cui, X.; Wei, Y.; Xie, X.L.; Chen, L.N.; Zhang, S.H. Mitochondrial and Peroxisomal Lon Proteases Play Opposing Roles in Reproduction and Growth but Co-Function in the Normal Development, Stress Resistance and Longevity of Thermomyces lanuginosus. Fungal Genet. Biol. 2017, 103, 42–54. [Google Scholar] [CrossRef]
- Liu, X.; Qian, B.; Gao, C.; Huang, S.; Cai, Y.; Zhang, H.; Zheng, X.; Wang, P.; Zhang, Z. The Putative Protein Phosphatase MoYvh1 Functions Upstream of MoPdeH to Regulate the Development and Pathogenicity in Magnaporthe oryzae. Mol. Plant-Microbe Interact. 2016, 29, 496–507. [Google Scholar] [CrossRef]
- Rakotonirainy, M.S.; Héraud, C.; Lavédrine, B. Detection of Viable Fungal Spores Contaminant on Documents and Rapid Control of the Effectiveness of an Ethylene Oxide Disinfection Using ATP Assay. Luminescence 2003, 18, 113–121. [Google Scholar] [CrossRef]
- van den Akker, E.; Satchwell, T.J.; Williamson, R.C.; Toye, A.M. Band 3 Multiprotein Complexes in the Red Cell Membrane; of Mice and Men. Blood Cells Mol. Dis. 2010, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Carignan, C.C.; Punshon, T.; Karagas, M.R. HHS Public Access. Physiol. Behav. 2016, 176, 100–106. [Google Scholar] [CrossRef]
- Alka, K.; Casey, J.R. Bicarbonate Transport in Health and Disease. IUBMB Life 2014, 66, 596–615. [Google Scholar] [CrossRef]
- Rodríguez-Urra, A.B.; Jimenez, C.; Dueñas, M.; Ugalde, U. Bicarbonate Gradients Modulate Growth and Colony Morphology in Aspergillus nidulans. FEMS Microbiol. Lett. 2009, 300, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Decker, B.L.; Wickner, W.T. Enolase Activates Homotypic Vacuole Fusion and Protein Transport to the Vacuole in Yeast. J. Biol. Chem. 2006, 281, 14523–14528. [Google Scholar] [CrossRef]
- Sarder, H.A.M.; Li, X.; Funaya, C.; Cordat, E.; Schmitt, M.J.; Becker, B. Saccharomyces Cerevisiae: First Steps to a Suitable Model System To Study the Function and Intracellular Transport of Human Kidney Anion Exchanger 1. mSphere 2020, 5, e00802-19. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.A.; de Ávila Rodrigues, F.; Debona, D.; Silva, L.C. Photosynthetic Gas Exchange in Leaves of Wheat Plants Supplied with Silicon and Infected with Pyricularia oryzae. Acta Physiol. Plant. 2014, 36, 371–379. [Google Scholar] [CrossRef]
- Domiciano, G.P.; Cacique, I.S.; Freitas, C.C.; Filippi, M.C.C.; DaMatta, F.M.; Do Vale, F.X.R.; Rodrigues, F.Á. Alterations in Gas Exchange and Oxidative Metabolism in Rice Leaves Infected by Pyricularia oryzae Are Attenuated by Silicon. Phytopathology 2015, 105, 738–747. [Google Scholar] [CrossRef]
- Torres, M.A.; Dangl, J.L. Functions of the Respiratory Burst Oxidase in Biotic Interactions, Abiotic Stress and Development. Curr. Opin. Plant Biol. 2005, 8, 397–403. [Google Scholar] [CrossRef]
- Massey, M.K.; Reiterman, M.J.; Mourad, J.; Luckie, D.B. Is CFTR an Exchanger? Regulation of HCO3− Transport and Extracellular PH by CFTR. Biochem. Biophys. Rep. 2021, 25, 100863. [Google Scholar] [CrossRef]
- Chen, Y.; Cann, M.J.; Litvin, T.N.; Iourgenko, V.; Sinclair, M.L.; Levin, L.R.; Buck, J. Soluble Adenylyl Cyclase as an Evolutionarily Conserved Bicarbonate Sensor. Science 2000, 289, 625–628. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, Y.; Wei, Y.; Zhang, P.; Liu, X.; Li, X.; Wang, S.; Liang, H.; Zhang, S.-H. The Bicarbonate Transporter (MoAE4) Localized on Both Cytomembrane and Tonoplast Promotes Pathogenesis in Magnaporthe oryzae. J. Fungi 2021, 7, 955. https://doi.org/10.3390/jof7110955
Dang Y, Wei Y, Zhang P, Liu X, Li X, Wang S, Liang H, Zhang S-H. The Bicarbonate Transporter (MoAE4) Localized on Both Cytomembrane and Tonoplast Promotes Pathogenesis in Magnaporthe oryzae. Journal of Fungi. 2021; 7(11):955. https://doi.org/10.3390/jof7110955
Chicago/Turabian StyleDang, Yuejia, Yi Wei, Penghui Zhang, Xinchun Liu, Xinrui Li, Shaowei Wang, Hao Liang, and Shi-Hong Zhang. 2021. "The Bicarbonate Transporter (MoAE4) Localized on Both Cytomembrane and Tonoplast Promotes Pathogenesis in Magnaporthe oryzae" Journal of Fungi 7, no. 11: 955. https://doi.org/10.3390/jof7110955
APA StyleDang, Y., Wei, Y., Zhang, P., Liu, X., Li, X., Wang, S., Liang, H., & Zhang, S.-H. (2021). The Bicarbonate Transporter (MoAE4) Localized on Both Cytomembrane and Tonoplast Promotes Pathogenesis in Magnaporthe oryzae. Journal of Fungi, 7(11), 955. https://doi.org/10.3390/jof7110955





