Abstract
Microbial endolithic communities are the main and most widespread life forms in the coldest and hyper-arid desert of the McMurdo Dry Valleys and other ice-free areas across Victoria Land, Antarctica. There, the lichen-dominated communities are complex and self-supporting assemblages of phototrophic and heterotrophic microorganisms, including bacteria, chlorophytes, and both free-living and lichen-forming fungi living at the edge of their physiological adaptability. In particular, among the free-living fungi, microcolonial, melanized, and anamorphic species are highly recurrent, while a few species were sometimes found to be associated with algae. One of these fungi is of paramount importance for its peculiar traits, i.e., a yeast-like habitus, co-growing with algae and being difficult to propagate in pure culture. In the present study, this taxon is herein described as the new genus Antarctolichenia and its type species is A. onofrii, which represents a transitional group between the free-living and symbiotic lifestyle in Arthoniomycetes. The phylogenetic placement of Antarctolichenia was studied using three rDNA molecular markers and morphological characters were described. In this study, we also reappraise the evolution and the connections linking the lichen-forming and rock-inhabiting lifestyles in the basal lineages of Arthoniomycetes (i.e., Lichenostigmatales) and Dothideomycetes.
1. Introduction
The ice-free zones of continental Antarctica, which include the peaks of the Transantarctic Mountains emerging from the Polar Plateau and the widest area of the McMurdo Dry Valleys, are the driest, coldest, and most remote environment on Earth. In the McMurdo Dry Valleys in particular, annual snowfalls range from 3 to 50 mm (water equivalent only) [1], which decreased by 1 mm through 2017 [2]; water rarely reaches the ground as a few drops, while mostly sublimes, or is blown away [3]. In certain locations, precipitations have been lacking for nearly two million years (Ma). In these regions, accounted as the Mars analogue on our planet, the environmental conditions reach the limits for supporting life in terms of low temperature, oligotrophy, and aridity. They have been considered to be devoid of life for a long time, until microbial communities were discovered as dwelling inside rocks, finding an ultimate refuge in the more buffered conditions offered by the endolithic niche [4,5]. In this environment, endolithic microbes represent one of the most widespread life forms and the main standing biomass [6,7,8]. The ability to exploit the endolithic niche is, therefore, a key adaptation for microbes to successfully reproduce and spread in the Antarctic desert.
Among the Antarctic endolithic communities described, the most studied and widespread are those dominated by lichens occurring both cryptoendolithically, mainly in sedimentary rocks (i.e., sandstones [9,10]), and chasmoendolithically, mainly in granite systems [11]. These are complex and self-supporting assemblages of phototrophic and heterotrophic microorganisms, including bacteria, chlorophytes, and both free-living and lichen-forming fungi. Chlorophycean (lichenized and free-living algae) and cyanobacteria are the only primary producers in these niches [3]. Lichens, in particular, show a peculiar morphological adaptation, whereby they give up their typical thallus organization and grow simply as filamentous forms to more efficiently colonize the thinnest airspaces of porous rocks [3,4]. In the endolithic niche, the lichen-forming fungi still clearly develop haustoria and appressoria to assure the specific relationship with their compatible algal partner. The adaptation to a loose morphological fungal–algal relationship may have promoted the key transition between the free-living and the fully lichenized lifestyles. The most frequent lichenized fungi in the Antarctic cryptoendolithic communities belong to the orders Lecanorales and Lecideales and, to a lesser extent, to Acarosporales and Caliciales [12,13]. This is not surprising since Lecanora fuscobrunnea and Lecidea cancriformis (Lecanorales and Lecideales, respectively) are the two most widespread endemic species in continental Antarctica [14,15]. These species are especially dominant in the dry Antarctic areas, in which the presence of other lichens, such as Buellia grisea, B. pallida, and Carbonea capsulata has been reported. Lichenized fungi, mainly in the genus Acarospora (e.g., Acarospora gwynii), are also frequently retrieved from these communities [12,16] and, recently, L. fuscobrunnea was obtained in pure culture (data unpublished).
A fungus with peculiar characteristics has been recurrently isolated (first isolate MNA-CCFEE5176, [17]) in over approximately 25 years of culture-based studies, undertaken on a wide selection of colonized rocks collected during numerous sampling campaigns in Antarctica. Its nuclear internal transcribed spacer (nucITS) sequence showed an identity of 87–88% in GenBank, with sequences barely related to the lichenized order Arthoniales, while, in a multilocus phylogeny, this fungus was placed close to the lichenicolous genus Lichenostigma [18]. This fungus appeared new also for morphological and cultural characteristics, having a yeast-like habitus and was difficult to propagate in pure culture, possibly suggesting that it represented a transitional group between a free-living and symbiotic lifestyle in the Arthoniomycetes. To support this hypothesis, we obtained morphological and sequence data from additional isolates and compared them with molecular data retrieved from public databases.
In the present study, we delineated (i) the phylogenetic placement of the new taxon analyzing three molecular markers; (ii) the morphological characteristics to explain the degree of the relationship with neighboring phylogenetic lineages in an evolutionary context; and (iii) the ecology of the fungus while considering the connections established with algae, to clarify their degree of interaction/interdependence. Along with the novelty of this phylogenetic fungal group and its description, we also studied the taxonomy of the associated algae and presented our considerations on the evolution of the different lifestyles and the potentiality of lichenization in the basal lineages of Arthoniomycetes.
2. Materials and Methods
2.1. Study Area and Rock Sampling
Endolithically colonized rocks were collected in eleven different localities in Victoria Land (continental Antarctica), during the Italian Antarctic Expeditions (December 2004 and December 2010–January 2011) and the Ganovex XI Expedition (the Dutch Antarctic Expedition of the German Antarctic North Victoria Land Expedition project, December 2015–January 2016) (Figure 1, Table 1).
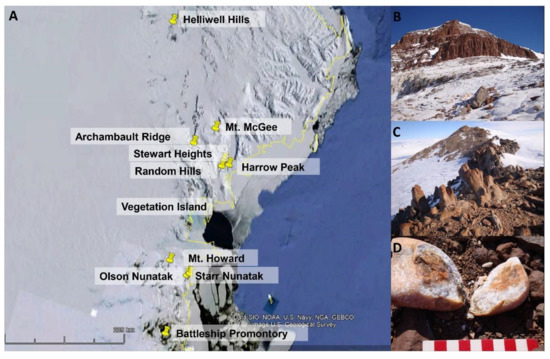
Figure 1.
(A) Map of Victoria Land in continental Antarctica and the localities from where Antarctolichenia onofrii strains have been isolated; (B,C) a typical environment characterizing the sampling localities: the Archambault Ridge (B) and Random Hills (C); and (D) the rock colonized by cryptoendolithic communities on Mt. Howard.

Table 1.
Details of the geographic origin and NCBI accession numbers of Antarctolichenia onofrii analyzed in this study. The type strain is highlighted in bold. SVL = southern Victoria Land and NVL = northern Victoria Land. * T = type strain.
The direct observation, in situ, using magnifying lenses assessed the presence of endolithic colonization. Rock samples were aseptically excised using a geological hammer, collected in plastic sterile bags, transported, and then stored at −20 °C at the University of Tuscia (Italy), until downstream analyses were performed.
2.2. Fungal Isolation and Molecular Identification
Fungal isolation from rocks were performed by grinding the samples and fragments were inoculated (in duplicate) in Petri dishes filled with 2% malt extract agar (MEA, AppliChem, GmbH, Darmstadt, Germany). Growth media were supplemented with chloramphenicol (100 ppm) to prevent bacterial growth. Plates were incubated at 15 °C and inspected on a weekly basis until no new fungal colonies appeared. The colonies were then transferred to MEA slant tubes and incubated at 15 °C, from weeks to a few months, to obtain a sufficient biomass for DNA extraction.
DNA was extracted using the Nucleospin Plant kit (Macherey-Nagel, Düren, Germany). The nucITS was amplified using the primers ITS5 and ITS4 [19]. The nuclear ribosomal large subunit (nucLSU) was amplified using the primers LR0R and LR7, while the nuclear ribosomal small subunit (nucSSU) was amplified uisng the primers SR1R and NS8 ([20], http://www.biology.duke.edu/fungi/mycolab/primers.htm, accessed on February 2021). Polymerase chain reactions (PCRs) were performed using BioMix (BioLine GmbH, Luckenwalde, Germany). The PCR mixtures were prepared with 5 pmol of each primer and 20 ng of template DNA; Milli-Q sterile water was added to a final volume of 25 µL. Amplification was carried out using a MyCycler™ Thermal Cycler (Bio-Rad Laboratories, GmbH, Munich, Germany).
The PCR protocol was as follows: 3 min at 95 °C for the first denaturation step; then 35 cycles of a denaturation step at 95 °C for 30 s; followed by an annealing step at 55 °C for 30 s for the ITS region; an annealing step at 52 °C for 30 s for the nucLSU–nucSSU regions; and an extension step at 72 °C for 30 s. The last extension was at 72 °C for 5 and 7 min for the nucITS and the nucLSU–nucSSU regions, respectively. All the amplicons were checked for their quality and size using 1.5% of agarose gel electrophoresis stained with GelRed™ (Biotium, Fremont, CA, USA), and purified using a Mag-Bind® Normalizer Kit (Omega Bio-Tek Inc, Norcross, GA, USA). Purified amplicons were sequenced with the same PCR primers using Macrogen Inc. (Seoul, Korea). The sequence assembly was performed using the ChromasPro v.1.32 software (Technelysium, Southport, Queensland, Australia) and sequences were compared in the GenBank (NCBI) database using BLASTn [21].
All the fungal strains obtained were both cultivated on slant agar (MEA) in glass tubes (15 × 2 cm) and cryopreserved in a metabolically inactive state at −150 °C in the the Italian National Antarctic Museum—Culture Collection of Fungi From Extreme Environments (MNA-CCFEE).
2.3. Algal Isolation and Molecular Identification
Algal colonies were noted to develop and co-grow within the fungal biomasses of two isolates, MNA-CCFEE6564 and MNA-CCFEE6583, and were isolated axenically. Algal cells were transferred and further subcultured axenically on new agar plates containing Bold’s Basal medium (BBM, [22]), a malt yeast extract medium (MY, [23]), and the Trebouxia medium (TM, [22]). Algal DNA was extracted following the cetyltrimethyl ammonium bromide (CTAB) protocol by Cubero et al. [24] and amplified using the universal primers LR7 and LR0R [20] for the nucLSU, and rbcL320 and rbcL803 primers [25] for the plastidial locus coding the ribulose-1,5-bisphosphate carboxylase large subunit (rbcL) using the PCR condition as in Muggia et al. [26].
2.4. Phylogenetic Analyses
After checking the identity of the fungal sequences of the three sequenced loci (nuclear ITS, LSU, and SSU; Table 1) by blast search in GenBank, the phylogenetic position of the strains was initially studied using a comprehensive taxon sampling, including representative taxa of Eurotiomycetes, Dothideomycetes, and Arthoniomycetes for each individual locus of nucITS, nucLSU, and nucSSU (Supporting Table S1). In this selection, we considered both lichenized and non-lichenized fungi, as well as basal lineages in Arthoniomycetes and Dothideomycetes, which represent the borderline lichens. The fungal taxa have been selected according to previous phylogenetic studies [18,27,28,29,30,31,32,33,34] and their sequences were downloaded from the NCBI GenBank (Supporting Table S1). Due to missing sequence data in GenBank for many taxa, which caused inconsistency among the nucITS and the other two loci, we decided to follow first a consensus phylogenetic approach in which the three sequenced loci were analyzed individually. We kept the nucITS locus dataset alone, as its taxon sampling (due to availability of sequence data in GenBank) substantially differed from the nucLSU and nucSSU datasets. In particular, nucITS sequence data are lacking for taxa belonging to Lichenostigmatales. Having verified the topological consistency between the nucLSU and the nucSSU, we decided to combine these two datasets in a concatenated analysis. Having confirmed the placement of the new sequences within Arthoniomycetes, we used representatives of Dothideomycetes as outgroups and proceeded with the concatenation approach for the nucLSU and nucSSU datasets. The combined nucLSU-nucSSU dataset was implemented to include all representative species and relative type species within Arthoniomycetes to improve the resolution of the new taxon within this class.
A total of 157 taxa were included in the combined nucLSU-nucSSU dataset, which were representatives of Lichenostigmatales (with the single family Phaeococcomycetaceae), Arthoniales (with the families Arthoniaceae, Chrysothricaceae, Lecanographaceae, Opegraphaceae, Roccellaceae and Roccellographaceae), and Dothideomyceta (with the order Collemopsidiales); additional eight species of Dothideomycetes were selected as outgroup (Supporting Table S1).
Similarly, the identity of the algal strains was checked by blast search [21] in GenBank, and the closest hits were selected for the phylogenetic analyses. Other algal sequences were selected from previously published phylogenies [35,36] and downloaded from GenBank. The two sequenced loci nucLSU and plastidial rbcL were analyzed individually due to the lack of sequence data for both loci for each algal taxon. The nucLSU algal sequence dataset included a broad spectrum of taxa belonging to the classes Trebouxiophyceae (with the orders Chlorellales, Prasiolales and Trebouxiales) and Chlorophyceae, while two species of Chlorodendrophyceae, i.e., Tetranselmis striata and T. suecica represented the outgroup. The rbcL dataset was restricted to the class Trebouxiophyceae and three species of Prasiolales were selected as outgroup, i.e., Prasiola furfuracea, P. linearis and P. meridionalis (Supporting Table S2).
The preparation of the multiple sequence alignments (MSA) for each individual locus of either the fungal and algal datasets was performed with clustalW using BioEdit v.7.2 [37], while the combined fungal dataset (nucLSU-nucSSU) was prepared with SequenceMatrix v.1.7.8 [38]; introns and ambiguous regions were not included in the analyses. The phylogenetic analyses were performed for the fungal and the algal datasets using the maximum likelihood (ML) approach [39,40] as implemented in RAxML v.8.2.10 [41], applying the GTRGAMMA model and running 1000 bootstrap replicates. For the fungal dataset, we also run a Bayesian analysis with the program MrBayes v3.2.5 [42]. Two runs of four simultaneous Markov chains were run for 2,000,000 generations and trees were sampled every 100th generation. The distribution of log-likelihood scores was examined using the program Tracer v.1.5 [43] to determine the stationary phase for each search was reached and chains had achieved convergence. The first 25% of the sampled topologies were discarded as part of a burn-in procedure, while the remaining trees were used for calculating the posterior probabilities in the majority rule consensus tree. The convergence of the chains was also confirmed by the convergent diagnostic of the Potential Scale Reduction Factor (PSRF), which approached 1 [42]. The phylogenetic trees were visualized in TreeView v1.6.6 [44].
2.5. Morphological Analyses
The analyses of morphological and anatomical characters have been performed on five fungal strains that represent the newly recognized lineage, i.e., MNA-CCFEE5176, MUT 6405 (=MNA-CCFEE6102), MNA-CCFEE6163, MNA-CCFEE6574 and MUT 6552 (=MNA-CCFEE6564) which was selected as holotype. The strains were analyzed using standard microscopic techniques and documented with digital photographs. Analyses and photographs were performed on subcultures that were approximately one year old, and the following characteristics were considered: the form of growth (filamentous vs. yeast-like); the melanization of the hyphae; the form and size of the hyphal cells; the branching of the hyphae; the development of conidiogenous cells; and the formation of conidia. Small fragments of mycelia were removed, and squashed sections were mounted in water and studied using light microscopy. Morphological analyses were performed on the two algal strains that were axenically isolated from the fungi MNA-CCFEE6564 and MNA-CCFEE6583 and considered both the form and size (length/width) of the cells.
All images were acquired with a ZeissAxioCam MRc5 digital camera that was fitted to the microscope, digitally processed, and slightly refined in sharpness and color tone using Adobe Photoshop 7.0. The figures were prepared using CorelDRAW X4.
3. Results
3.1. Fungal Isolation and Molecular Identification
We obtained a total of 14 fungal isolates for which we generated 14 new nucLSU, 12 nucSSU and 11 nucITS sequences (Table 1). Over the previous years, seven strains were continuously propagated in culture and stored as cryostocks in the MNA-CCFEE. Two of the strains analyzed here were also deposited in the Mycotheca Universitatis Taurinensis (MUT) collection (MUT 6405 = MNA-CCFEE6102; MUT 6552, holotype = MNA-CCFEE6564).
The ITS single locus inference congruently resolved the new taxon as a new lineage basal in Arthoniomycetes (Supplementary Figure S1), placing the new sequences together with four samples of Phaeococcomycetaceae within the Lichenostigmatales. Lichenostigmatales is a monophyletic and fully supported sister clade of Arthoniales. The ML and Bayesian analyses of the combined nucLSU–nucSSU dataset are topologically concordant (Figure 2). Furthermore, this tree topology is concordant with previous phylogenies, including Dothideomyceta [18,29,32], and consistently supports the phylogenetic placement of the new taxon as the sister lineage of Etayoa (Figure 2). Dothideomycetes clearly segregates from Arthoniomycetes and the order Collemopsidiales is recovered basal to Dothideomycetes. Within Arthoniomycetes, all orders and families are monophyletic and fully supported (both by bootstrap values and Bayesian posterior probabilities), and their topology is congruent with the phylogenetic inferences previously published by Ertz and coauthors [27,30,31]. Moreover, the genus-based lineages within Phaeococcomycetaceae/Lichenostigmatales, i.e., Lichenostigma, Phaeococcomyces, Etayoa, and the new taxon are fully supported and individually monophyletic. The phylogenetic analysis places the two Mediterranean rock-inhabiting fungi (TRN213 and TRN529) on individual branches basal to the clades of Etayoa and of the new taxon, respectively.
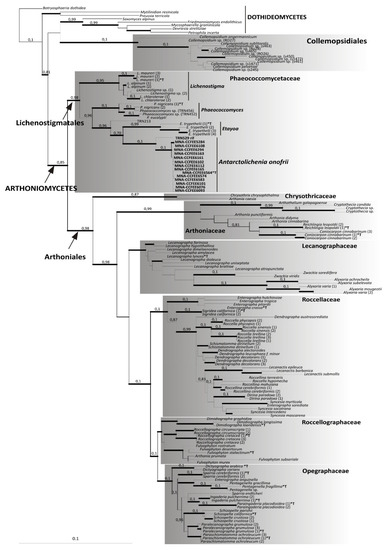
Figure 2.
Phylogenetic analysis based on the combined nucLSU and nucSSU sequences of Arthoniomycetes, using Dothideomycetes as an outgroup. The data for taxa retrieved from GenBank are reported in the Supplementary Table S1 and the different samples of the same species are identified by a numeration in parenthesis after the genus/species name. The maximum likelihood and Bayesian topology are fully consistent. The newly identified lineage of Antarctolichenia is highlighted by reporting the strain numbers in bold. The type species included in the analyses are marked by an asterisk and a T (*T). Bayesian posterior probabilities (PP) are reported above the branches and the thickness of the branches highlights those receiving a full ML bootstrap support of 96–100%. The scale bar is proportional to the substitution rate.
3.2. Morphological Analyses of Isolated Fungal Strains
The new taxon shows a meristematic growth, producing cerebriform colonies (Figure 3A,B) and developing both as filamentous or yeast-like forms (Figure 4). The two mycelium types have been observed in different strains and seem to neither depend on the type of growth medium on which they are propagated/subcultured, nor on the presence of the co-growing algae (Figure 3C). Isodiametric, yeast-like cells present a thick cell wall (Figure 4A–G) which is heavily melanized in more mature cells, while cells are almost hyaline in their young stages (Figure 4B,C,G). The filamentous thallus presents branching hyphae with rectangular-to-oblong cells with a melanized cell wall (Figure 4H–P). Many hyphae also present isodiametric cells, sometimes at the branchings (Figure 4K,M), while others are composed entirely of isodiametric cells (Figure 4O,P). When growing together with the algae, both yeast-like cells and filamentous hyphae developed among the algal cells, but no haustoria-like structures or a more organized mycelium/thallus were observed.
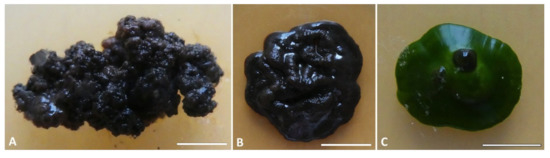
Figure 3.
Colony appearance in the culture of Antarctolichenia onofrii: (A) the isolated strains of MNA-CCFEE6163 and (B) MUT 6552 alone, and (C) the strain MNA-CCFEE6574 grown together with the Stichococcus-like algae. Strains were grown on MEA (17 °C, 20 μmol fot·m−2·s−1, with a light/dark cycle of 14/10 h). Scale bars: (A) 3 mm; (B) 8 mm; and (C) 6 mm.
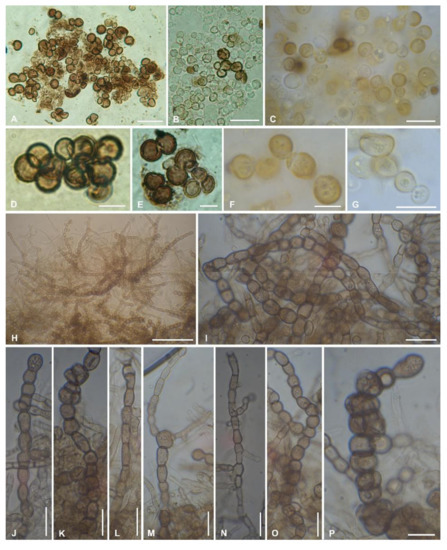
Figure 4.
Morphological characters of Antarctolichenia onofrii analyzed in the isolated strains MNA-CCFEE5176 (A,B,D,E); MUT 6552 (type strain) (C); MNA-CCFEE6163 (F,G); and MUT 6495 (H–P). Both isodiametric, yeast-like cells with a thick, melanized cell wall (A–G) and filamentous branching hyphae with a thinner cell wall (H–P) are observed. The filamentous hyphae are composed of isodiametric-to-rectangular cells (H–P). Scale bars: (H) 50 μm; (A,B) 20 μm; (C,G,I–O) 10 μm; and (D,E,F,P) 5 μm.
3.3. Algal Isolation and Molecular Identification
The sequence analyses of the isolated algae revealed a great similarity of both the nucLSU and rbcL sequences with Stichococcus bacillaris (Supplementary Figure S2). Furthermore, the rbcL dataset strengthened the identity of the isolated algae with its geographic origin by presenting its close phylogenetic relationship with S. antarcticus. In the rbcL phylogeny, two sequences of Diplosphaera retrieved from GenBank, which were the closest blast hits of the isolated algae together with other Stichococcus sequences, are nested with Stichococcus sequences, suggesting their incorrect identity assignment in GenBank.
Morphological inspections of the isolated algae confirmed Stichococcus-like cells, i.e., elongated cells of about 5–8 μm × 2–3 um wide with one chloroplast, either parietal or placed at one edge of the cell (Supplementary Figure S2C).
4. Taxonomy
Etymology: the genus name refers to the geographic origin of the fungus, as it was collected in the Antarctic continent, and to its lifestyle, growing in association with algae, resembling that of a lichen-forming fungus, within endolithic lichen communities.
Monotypic genus in the family Phaeococcomycetaceae, order Lichenostigmatales, Arthoniomycetes, and Ascomycota. Endolithic, anamorphic fungus, for which the sexual morph is unknown. Colonies growing rather slowly in vitro and heavily melanized. Thallus composed of yeast-like cells and filamentous hyphae; yeast-like cells with a thick cell wall, heavily melanized, and slightly verrucose in more mature cells, almost hyaline in young stages; filamentous thallus with rectangular cells and more isodiametric cells at the branchings.
Type species: Antarctolichenia onofrii Selbmann and Muggia sp. nov.—MycoBank: MB839456 (Figure 3 and Figure 4).
Holotype: MUT 6552 = MNA-CCFEE 6564 cultured strain, isolated from cryptoendolithically colonized sandstone collected in Helliwell Hills, Antarctica. The culture is preserved in a metabolically inactive state at −150 °C.
Antarctolichenia onofrii MUT 6552 is the unique identifier of the holotype sheet in the Mycotheca Universitatis Taurinensis (MUT), Department of Life Sciences and Systems Biology, University of Turin. Etymology: the species is named after the Italian mycologist Silvano Onofri, who collected the rock sample from which the fungus was isolated for the very first time (Antarctic Expedition PNRA 1996/97).
Diagnosis: Strictly rock-inhabiting, endolithic, and asexual fungus. Colonies growing extremely slowly in vitro (reaching about 1 cm in a year), black. A yeast-like and filamentous thallus. Yeast-like isodiametric cells, 5–10 um, with a thick, slightly verrucose cell wall (Figure 4A–G), heavily melanized in more mature cells, and almost hyaline in their young stages (Figure 4B,C,G). Filamentous thallus with rectangular-to-oblong cells, 4–5 × 5–6 um, with a melanized and slightly verrucose cell wall (Figure 4H–P), branching hyphae, isodiametric cells sometimes present at the branchings (Figure 4K,M), and rarely building an entire hyphae (Figure 4O,P). Occasionally growing together with Stichococcus-like algae (Supplementary Figure S2C) but not forming haustoria-like or more organized mycelium or lichen-like thallus structures.
Distribution: Continental Antarctica, isolated from endolithic lichen-dominated communities in Victoria Land.
Material examined: the examined strains and metadata are reported in Table 1.
Notes: as reported for other species in the family Phaeococcomycetaceae, Antarctolichenia onofrii displays both yeast-like and mycelial organization, conversely, Phaeococcomyces spp., reproduced by budding. Unlike Lichenostigma, conidiomata and ascomata were not observed in Antarctolichenia. Antarctolichenia onofrii, is also peculiar for its distribution and ecology, occurring exclusively in lichen-dominated endolithic communities in continental Antarctica.
5. Discussion
5.1. The Lichen–RIF Connections
The newly described lineage of Antarctolichenia represents an evolutionary connection between non-lichenized and lichen-forming fungi and, interestingly, it emerges within the order Lichenostigmatales in the class Arthoniomycetes, one of the widest classes of lichen-forming and non-lichenized fungi in Pezizomycotina [45,46,47,48,49]. Within Arthoniomycetes, Arthoniales was the first, well recognized order; it is known to host the highest diversity of mainly corticolous (epiphytic) taxa, forming lichen symbioses with trentepohlioid algae from the tropics [27,50,51,52] and lichenicolous fungi, mostly highly host-specific and commensal on lichens [53]. Furthermore, the existence of Lichenostigmatales was first suggested by the phylogenetic studies of Ruibal et al. [54]. The authors, while studying rock-inhabiting, microcolonial fungi (RIF) from the Mediterranean region, identified a group of several rock isolates as Phaeococcomyces spp. or Phaeococcomyces-like species, but left this lineage unnamed. Regarding this unnamed group, which at that time was placed at the base of Dothideomycetes, the authors suggested that it could represent an example of an early diverging lineage of that class [54]. At the same time, several multilocus phylogenetic studies, which also included diverse fungal classes, supplied a strong support for the sister relationship between Arthoniomycetes and Dothideomycetes [46,47,55]. Thus, the clade grouping of these two big classes (that does not include the unnamed group of Ruibal et al.) was defined as the rankless taxon ‘Dothideomyceta’ [47,48,56]. These intriguing findings increased the interest of researchers concerning the evolution of Arthoniomycetes. Indeed, this class was already presented as an intermediate group (“Zwischengruppe”, [57]) due to the ontological development of the ascomata and a multiplicity of morphological traits owned by its representatives. Due to this, several analyses highlighted the importance of including lichen-forming fungi in the Dothideomycetes phylogenies [49,58]. In fact, phylogenetic inferences showed that Arthoniomycetes was the result of a single, independent lichenization event, and that non-lichenized and lichenicolous species within the class would represent reversions to the non-lichenized state [27,58]. Some years later, Ertz et al. [18] revised the taxonomic relationships between anamorphic and teleomorphic states of lichenicolous species in the genera Lichenostigma and Etayoa, and recovered these genera next to the Phaeococcomyces spp. and Phaeococcomyces-like RIF strains/species of Ruibal et al. [54]. The so formed clade was supported as the sister lineage of Arthoniales and was formally described as the order Lichenstigmatales [18]. Given the position of Lichenostigmatales in Arthoniomycetes, Ertz et al. [18] stated that the entire order may represent a possible transitional group between Arthoniomycetes and Dothideomycetes, showing clear affinities with both the lichen-forming fungi in Arthoniales and the rock-inhabiting, lichenicolous, and not-lichenized fungi recovered in Dothideomycetes. Therefore, the phylogenetic placement of Antarctolichenia in Lichenostigmatales additionally strengthens the recognition of this order as a key evolutionary connection between the lichen-forming and rock-inhabiting lifestyles in this group of fungi.
In the present study, the connection of Antarctolichenia and the entire order Lichenostigmatales with the genus Lichenothelia is especially crucial to complete the link recurrently hypothesized between rock-inhabiting, meristematic fungi, and lichenized fungi. Indeed, Lichenothelia was originally hypothesized to represent “the” link between RIF and lichenized fungi in Dothideomycetes [59,60]. However, due to the unclear morphological separation from the strictly lichenicolous genus Lichenostigma, some species of either genera were placed together in the family Lichenotheliaceae [61]. When Ertz et al. [18] clarified the phylogenetic placement of Lichenostigma, including the generic type and other species, they also performed detailed morphological analyses and stated that Lichenostigma is distinguishable from the Lichenothelia species, by the way the cells divide. The cells in Lichenostigma are spherical and multiply by “budding”, instead of by the division through the formation of septa, as in Lichenothelia, whose hyphae are clearly filamentous [18,28]. In addition, the phylogenetic inference of Ertz et al. [18] recovered samples of Lichenothelia (i.e., corresponding to the formally recognized Lichenotheliaceae, Lichenotheliales [62]) at the base of Arthoniomycetes and as the most basal lineage of the several Dothideomycetes orders. The position of the order Collemopsidiales, introduced by Pérez-Ortega et al. [32] a few years later, and the description of Lichenostigmatales to accommodate the species of the ascomycetous genus Collemopsidium (Xanthopyreniaceae), also confirmed the evolution of primitive lichen-like associations at the base of Dothideomycetes. Simultaneously, this further suggests that the multiple basal lineages in Dothideomyceta, which bear the potential to generate borderline forms of lichenization, still lack that particular fungal–algal interdependence characteristic of the lichen symbioses. Collemopsidiales was first described as a sister lineage of Arthoniales within Dothideomyceta [32], while in our analysis it is recovered nested with the Dothideomycetes outgroups. Most Collemopsidium species, such as Lichenostigma, Lichenothelia, and Antartctolichenia, associate with photosynthetic algae. Collemopsidium comprises saxicolous taxa, many of which are rocky seashore dwelling species [63]. Those which associate with cyanobacteria form simple inconspicuous thalli interpreted as borderline lichens [64], one species grows on lichen cephalodia (C. cephalodiorum) and another on brown seaweed (C. pelvetiae).
Considering the potential of Antarctolichenia to grow and interact with algae (see below), and its phylogenetic position in Lichenostigmatales, we may infer two different hypothesis: (i) this lineage may represent a very primitive form of lichenization that may have arisen from an original rock-inhabitant ancestor, as in Phaeococcomyces, but has neither evolved further into lichens (as best exemplified by Arthoniales) nor into specific parasitic interactions, as in the cases of Lichenostigma and Etayoa; and (ii) Antarctolichenia may represent a half way transition from a lichenized to a free-living lifestyle, still not completely exempt from the relation with algae, since, apparently, they cannot subsist in axenic cultures for a long time. The rise of loose relations with symbiont algae, promoted to adapt and exploit the endolithic niche, may have boosted the transition.
5.2. Morphological Traits and the Fungal–Algal Association in Lichenostigmatales
The mycelium structure and lifestyles/ecologies in fungi are strictly correlated, as the mycelium is the feeding and interaction interface of the fungus, modulating the way individuals explore the substrate and the surrounding environment [65]. Only a few fungal species have the capacity to switch between two morphologies, i.e., building either a filamentous or a yeast-like mycelium, and are therefore recognized as dimorphic fungi. Some dimorphic fungi are melanized, microcolonial fungi (so-called “black yeast”), presenting thick cell walls; additionally, they may shift to meristematic growth, traits that confer to them polyextremotolerance [66]. In these fungi, the switch between the two growth forms is triggered by environmental stress factors (i.e., temperature variation, radiation, drought, and anaerobic conditions) or by the capacity to develop virulence and pathogenicity towards their hosts, either plants or animals [67,68]. For species living under permanent stress, meristematic growth may become a stable characteristic. The transition across different growth forms is often a species-specific process in dimorphic fungi, and involves changes in the composition of the cell walls coupled with the thermotolerance of the fungus, sexual reproduction (present in the mycelial phase), and dissemination of conidiospore [67].
The formation of mycelial and yeast-like stages is a phenomenon also documented for representatives of Arthoniomycetes. Indeed, species of Arthoniales exist in the mycelial stage (i.e., the apical growth of the hyphae and formation of septa between cells) and hyphae are seldom melanized. On the contrary, in Lichenostigmatales, in particular in the Lichenostigma species, the cells forming the conidiomata and ascomata are melanized, spherical, and multiply by budding, resembling a yeast-like stage, while only asci, ascospores, and conidiogenous cells are not spherical [18]. The ascospores are the only cells possessing real septa but the formation of a mycelial stage was rarely observed; alternatively, conidia were observed to germinate exclusively by budding [18]. Furthermore, the dense and organized agglomeration of yeast cells that form ascomata and conidiomata were reported as a unique characteristic amongst fungi for both the genera Lichenostigma [18,69] and Lichenothelia [29,70]. Instead, in Phaeococcomyces, the sister genus of Lichenostigma comprising black yeasts, reproduction is exclusively enacted by budding and no agglomerations are reported [18]. In this study, we observed that also Antarctolichenia presents either a filamentous or a yeast-like growth and that this is independent from the culture medium and the isolated strains (Figure 4). Unfortunately, we could not ascertain which growth morphology is developed inside rocks under natural conditions, as the fungus is strictly endolithic and outgrew from rock fragments deposited on an agar medium. While in culture, the yeast-like stage with heavily melanized budding cells is well observable in Lichenostigmatales and Lichenothelia spp. [29,33,71]; however, ascomata were never observed in neither taxa, so far.
Melanization is known to make fungal cell walls more rigid and less flexible, and coupled with the yeast-like growth, it seems to hinder the formation of haustorium-like hyphae with which the fungus could enwrap or build tight contact with algal cells. However, because melanized fungi, in general, share traits of stress tolerance that allow them to survive in oligotrophic and extremely dry environments, their association with microscopic algae was suggested to potentially improve the meager carbon supplies present in the environment [66]. A borderline lichen symbioses was already observed from some divergent lineages of black fungi in Dothideomycetes [29,47,48,49,59,71]; it is also observed in with Antarctolichenia, being often isolated together with algal cells. Indeed, two species of melanized Dothideomycetes (i.e., Cystocoleus ebeneus and Racodium rupestre, Capnodiales) are the only representatives of well-established lichen symbioses. Their rather primitive thallus, relatively simple in structure, is made by a tight fungal coat of one cell layer around the filamentous photobiont, a thread of Trentepohlia, which does not differ much from the free-living forms of the individual symbionts [72]. Other melanized, filamentous, and yeast-like fungi discovered to co-grow in nature in a tight association with algae were used to attempt in vitro co-culture experiments to study their interaction [71,73,74]. The two RIF genera, Lichenothelia and Saxomyces, are usually found on rocks together with coccoid Trebouxiophyceae algae and can be co-grown with them in culture [71]. However, with their phenotypically more plastic mycelia (which develop either filamentous or short yeast-like cells), they are unable to form any clear thallus structures [33,71]. The same behavior was also observed in the halophilic black yeast Hortaea werneckii, which was found to co-grow with the algae Dunaliella atacamensis on spiderwebs [75]; nevertheless, attempts to co-grow and study this association in vitro culture failed [74]. A certain degree of interaction was also observed by co-culturing some Coniosporium or Knufia RIF species, where a slight increase in hyphal branching near algal cells was reported [76].
Antarctolichenia grows in nature within the rocks together with the algae, as the two bionts were isolated together from the same cell clumps. Though similar to other melanized fungi co-growing with algae, the photosynthetic partners for Antarctolichenia seem to be supplementary and not essential for its survival, at least temporarily. Indeed, strains isolated axenically present the same growth development as those in co-culture with the algae, but the axenic culture can be propagated for a limited time only and invariably extinguished if repeatedly subcultured. The perpetuation of pure cultures can be guaranteed by cryopreservation. The type of fungal-algal co-growth observed for Antarctolichenia, Hortaea, Lichenothelia, and Saxomyces recalls forms of mycophycobioses, in which fungi are immersed in unchanged colonies of algae without any sign of structural integration. In all these fungi, the development of either the filamentous or the yeast-like mycelium is not concerted with the growth of the algal colony and hyphae or budding cells, respectively, do not form any clear texture in which algal cells are hosted. Nevertheless, these fungal–algal associations represent pivotal springboards for the more specific, stepwise transition that was suggested to have led to the typical lichen symbioses that are now understood as self-sustaining ecosystems [77], in which an ex-habitant fungal partner (the mycobiont) forms a covering structure of hyphal cells embedding the photosynthetic partner (the photobiont) in an extracellular matrix of polysaccharides [78,79].
Sequence analyses and morphological inspections identified the algae associated with Antarctolichenia as a Stichococcus species, highly similar, genetically, to S. bacillaris and closely related to S. antarcticus. These results, still preliminary and based on two algal isolates, let us exclude the fact that these algae may introduce contamination during the isolation protocol. Stichococcus-like algae are very common and can be found in almost all types of habitats, either aquatic or terrestrial ([36] and references therein). S. bacillaris has also been recovered as a secondary photobiont in lichen thalli [80], but, to date, no lichen symbioses are known to share it as a principal photosynthetic partner. This allows us to hypothesize that the species may not be a suitable algal partner for lichenized fungi, and it would be worthwhile to search for other algae as potential photobionts in Antarctic communities. As a detailed analysis of this “potential photobiont” goes beyond the scope of this study, due to the small number of samples available to date, we refrain from further discussing its identity and its capacity to build more or less specific relationships with Antarctolichenia. Forthcoming analyses will shed more light on this new fungal–algal partnership.
6. Conclusions
Antarctic endolithic communities represent a unique model to address general questions concerning evolutionary ecology given their very simple and stable organization, the strong genetic and geographic isolation, the severe biological constraints imposed by the polar environment that promote adaptive radiation, and the absence of interaction with higher organisms. One of the primary concerns in mycology is to uncover the evolution of lichen symbiotic lifestyles and their role in the diversification of ascomycetes. The new fungal genus and species described in this study further contributes to on the understanding of this evolutionary process. In fact, Antarctolichenia onofrii, albeit clearly free-living, still maintains a loose relation to and interdependence with algae, being unable to propagate in axenic culture for a long time; furthermore, it belongs to Lichenostigmatales, whose representatives have affinities both with the lichen-forming fungi in Arthoniales and the rock-inhabiting, lichenicolous, and not-lichenized fungi in Dothideomycetes. Thus, it additionally strengthens its key evolutionary and connective role between the lichen-forming and rock-inhabiting lifestyles.
Supplementary Materials
The following materials are available online at https://www.mdpi.com/article/10.3390/jof7110935/s1. Supplementary Table S1. Fungal taxa selected for the phylogenetic analyses of Figure 2 and Figure S1 are reported with their species name (if present), their ID number, and the corresponding NCBI accessions for the sequences of the ribosomal nuclear large (nucLSU) and small (nucSSU) subunits. Supplementary Table S2. Algal taxa selected for the phylogenetic analyses in Figure S2 are reported with their species name (if present), their ID number, and the corresponding NCBI accessions for the ribosomal nuclear large subunit (nucLSU) and the ribulose 1,5-biphosphate carboxylase large subunit (rbcL) sequences. Supplementary Figure S1. Maximum likelihood phylogenetic analysis based on the nucLSU (A) and plastidial rbcL (B) sequences of the algae Stichococcus sp. co-growing with Antarctolichenia onofrii isolated strains. (C) Morphology of the algae in light microscopy mounted in water. Supplementary Figure S2. Maximum likelihood phylogenetic analysis based on the nucLSU (A) and plastidial rbcL (B) sequences of the algae Stichococcus sp. co-growing with Antarctolichenia onofrii isolated strains. (C) Morphology of the algae in light microscopy mounted in water.
Author Contributions
Conceptualization, L.M. and L.S.; methodology, L.M. and L.S.; formal analysis, L.M., C.C., A.C. and R.D.C.; resources, L.M. and L.S.; data curation, L.M., C.C. and L.S.; writing—original draft preparation, L.M., C.C. and L.S.; writing—review and editing, L.M., C.C. and L.S.; visualization, L.M., C.C. and L.S.; supervision, L.M. and L.S.; project administration, L.M. and L.S.; funding acquisition, L.M. and L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian National Program for Antarctic Research (PNRA).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets supporting the conclusions of this article are available in the NCBI GenBank and the accession numbers are listed in Table 1.
Acknowledgments
L.M., L.S. and C.C. wish to thank the PNRA for funding the sampling campaigns and research activities in Italy in the frame of PNRA projects. The Italian Antarctic National Museum (MNA) is kindly acknowledged for the financial support given to the Culture Collection of Fungi from Extreme Environments (MNA-CCFEE, University of Tuscia, Italy), Mycological Section of the MNA, where the rocks and cultures analyzed in this study are stored. JP De Vera is kindly acknowledged for supplying rock samples from Helliwell Hills, from which some strains were also isolated.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ITS | Internal Transcribed Spacer |
| MEA | Malt Extract Agar |
| ML | Maximum Likelihood |
| MNA-CCFEE | National Antarctic Museum-Culture Collection of Fungi from Extreme Environments |
| MUT | Mycotheca Universitatis Taurinenesis |
| NCBI | National Center for Biotechnology Information |
| nucITS | nuclear Internal Transcribed Spacers |
| nucLSU | nuclear ribosomal Large SubUnit |
| nucSSU | nuclear ribosomal Small SubUnit |
| PCR | Polymerase Chain Reaction |
| PSFR | Potential Scale Reduction Factor |
| RIF | Rock Inhabitant Fungi |
| TM | Trebouxia Medium |
References
- Fountain, A.G.; Nylen, T.H.; Monaghan, A.; Basagic, H.J.; Bromwich, D. Snow in the McMurdo dry valleys, Antarctica. Int. J. Climatol. A J. R. Meteorol. Soc. 2010, 30, 633–642. [Google Scholar] [CrossRef]
- Myers, M.E.; Doran, P.T.; Myers, K.F. Summer valley-floor snowfall in Taylor Valley, Antarctica from 1995–2017. Cryosphere Discuss. 2020, 1–24. [Google Scholar] [CrossRef]
- Nienow, J.A.; Friedmann, E.I. Terrestrial lithophytic (rock) communities. In Antarctic Microbiology; Friedmann, E.I., Ed.; Wiley-Liss: New York, NY, USA, 1993; pp. 343–412. [Google Scholar]
- Friedmann, E.I. Endolithic microorganisms in the Antarctic cold desert. Science 1982, 215, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, E.I.; Ocampo, R. Endolithic blue-green algae in the dry valleys: Primary producers in the Antarctic desert ecosystem. Science 1976, 193, 1247–1249. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.D.; de los Ríos, A.; Lee, K.C.; Niederberger, T.S.; Cary, S.C.; Coyne, K.J.; Douglas, S.; Lacap-Bugler, D.C.; Pointing, S.B. Endolithic microbial diversity in sandstone and granite from the McMurdo Dry Valleys, Antarctica. Polar Biol. 2017, 40, 997–1006. [Google Scholar] [CrossRef]
- Gonçalves, V.N.; Alves, I.M.S.; de Oliveira, F.S.; Schaefer, C.E.G.R.; Turbay, C.V.G.; Rosa, C.A.; Rosa, L.H. Rock-inhabiting fungi in Antarctica: New frontiers of the edge of life. In Fungi of Antarctica; Springer: Cham, Switzerlands, 2019; pp. 99–126. [Google Scholar]
- Coleine, C.; Stajich, J.E.; de los Ríos, A.; Selbmann, L. Beyond the extremes: Rocks as ultimate refuge for fungi in drylands. Mycologia 2021, 113, 108–133. [Google Scholar] [CrossRef]
- Zucconi, L.; Onofri, S.; Cecchini, C.; Isola, D.; Ripa, C.; Fenice, M.; Madonna, S.; Reboleiro-Rivas, P.; Selbmann, L. Mapping the lithic colonization at the boundaries of life in Northern Victoria Land, Antarctica. Polar Biol. 2016, 39, 91–102. [Google Scholar] [CrossRef]
- Selbmann, L.; Onofri, S.; Coleine, C.; Buzzini, P.; Canini, F.; Zucconi, L. Effect of environmental parameters on biodiversity of the fungal component in lithic Antarctic communities. Extremophiles 2017, 21, 1069–1080. [Google Scholar] [CrossRef]
- Yung, C.C.; Chan, Y.; Lacap, D.C.; Pérez-Ortega, S.; de Los Rios-Murillo, A.; Lee, C.K.; Cary, S.C.; Pointing, S.B. Characterization of chasmoendolithic community in Miers valley, McMurdo dry valleys, Antarctica. Microb. Ecol. 2014, 68, 351–359. [Google Scholar] [CrossRef]
- Coleine, C.; Stajich, J.E.; Zucconi, L.; Onofri, S.; Pombubpa, N.; Egidi, E.; Franks, A.; Buzzini, P.; Selbmann, L. Antarctic cryptoendolithic fungal communities are highly adapted and dominated by Lecanoromycetes and Dothideomycetes. Front. Microbiol. 2018, 9, 1392. [Google Scholar] [CrossRef] [Green Version]
- Coleine, C.; Biagioli, F.; de Vera, J.P.; Onofri, S.; Selbmann, L. Endolithic microbial composition in Helliwell Hills, a newly investigated Mars-like area in Antarctica. Environ. Microbiol. 2021, 23, 4002–4016. [Google Scholar] [CrossRef]
- Castello, M. Lichens of Terra Nova Bay area, northern Victoria Land (continental Antarctica). Studia Geobot. 2003, 22, 3–54. [Google Scholar]
- Hertel, L.H. Notes on and records of Southern Hemisphere lecideoid lichens. Bibl. Lichenol. 2007, 95, 267–296. [Google Scholar]
- Scalzi, G.; Selbmann, L.; Zucconi, L.; Rabbow, E.; Horneck, G.; Albertano, P.; Onofri, S. LIFE experiment: Isolation of cryptoendolithic organisms from Antarctic colonized sandstone exposed to space and simulated Mars conditions on the International Space Station. Orig. Life Evol. Biosph. 2012, 42, 253–262. [Google Scholar] [CrossRef]
- Selbmann, L.; De Hoog, G.S.; Mazzaglia, A.; Friedmann, E.I.; Onofri, S. Fungi at the edge of life: Cryptoendolithic black fungi from Antarctic desert. Stud. Mycol. 2005, 51, 1–32. [Google Scholar]
- Ertz, D.; Lawrey, J.D.; Common, R.S.; Diederich, P. Molecular data resolve a new order of Arthoniomycetes sister to the primarily lichenized Arthoniales and composed of black yeasts, lichenicolous and rock-inhabiting species. Fungal Divers. 2014, 66, 113–137. [Google Scholar] [CrossRef]
- White, T.J.; Burns, T.D.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal DNA genes for phylogenies. In PCR Protocols, a Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Snisky, J.J., White, T.J., Eds.; Academic: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bold, H.C. The morphology of Chlamydomonas chlamydogama sp. nov. Bull. Torrey Bot. Club 1949, 76, 101–108. [Google Scholar] [CrossRef]
- Ahmadjian, V. The Lichen Symbiosis; Blaisdell Publishing Company: Waltham, MA, USA, 1967. [Google Scholar]
- Cubero, O.F.; Crespo, A.; Fatehi, J.; Bridge, P.D. DNA extraction and PCR amplification method suitable for fresh, herbarium stored and lichenized fungi. Plant Syst. Evol. 1999, 217, 243–249. [Google Scholar] [CrossRef]
- Nozaki, H.; Ito, M.; Sano, R.; Uchida, H.; Watanabe, M.M.; Kuroiwa, T. Phylogenetic relationships within the colonial Volvocales (Chlorophyta) inferred from rbcL gene sequence data. J. Phycol. 1995, 31, 970–979. [Google Scholar] [CrossRef]
- Muggia, L.; Pérez-Ortega, S.; Kopun, T.; Zellnig, G.; Grube, M. Photobiont selectivity leads to ecological tolerance and evolutionary divergence in a polymorphic complex of lichenized fungi. Ann. Bot. 2014, 114, 463–475. [Google Scholar] [CrossRef] [Green Version]
- Ertz, D.; Tehler, A. The phylogeny of Arthoniales (Pezizomycotina) inferred from nucLSU and RPB2 sequences. Fungal Divers. 2011, 49, 47–71. [Google Scholar] [CrossRef]
- Gueidan, C.; Aptroot, A.; Silvia Caceres, M.E.; Badali, H.; Stenroos, S. A reappraisal of orders and families within the subclass Chaetothyriomycetidae (Eurotiomycetes, Ascomycota). Mycol. Prog. 2014, 13, 1027–1039. [Google Scholar] [CrossRef]
- Muggia, L.; Kocourkova, J.; Knudsen, K. Disentangling the complex of Lichenothelia species from rock communities in the desert. Mycologia 2015, 107, 1233–1253. [Google Scholar] [CrossRef] [Green Version]
- Ertz, D.; Tehler, A.; Irestedt, M.; Frisch, A.; Thor, G.; van den Boom, P. A large-scale phylogenetic revision of Roccellaceae (Arthoniales) reveals eight new genera. Fungal Divers. 2015, 70, 31–53. [Google Scholar] [CrossRef]
- Ertz, D.; Sanderson, N.; Lubek, A.; Kukwa, M. Two new species of Arthoniaceae from old-growth European forests, Arthonia thoriana and Inoderma sorediatum, and a new genus for Schismatomma niveum. Lichenologist 2018, 50, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Ortega, S.; Garrido-Benavent, I.; Grube, M.; de los Rios, A. Hidden diversity of marine borderline lichens and a new order of fungi: Collemopsidiales (Dothideomyceta). Fungal Divers. 2016, 80, 285–300. [Google Scholar] [CrossRef]
- Ametrano, C.G.; Knudsen, K.; Kocourkova, J.; Grube, M.; Selbmann, L.; Muggia, L. Phylogenetic relationships of rock-inhabiting black fungi belonging to the widespread genera Lichenothelia and Saxomyces. Mycologia 2019, 111, 127–160. [Google Scholar] [CrossRef]
- Quan, Y.; Muggia, L.; Moreno, L.F.; Wang, M.; Al-Hatmi, A.M.S.; Menezes da Silva, N.; Shi, D.; Deng, S.; Ahmed, S.; Hyde, K.D.; et al. A re-evaluation of the Chaetothyriales using criteria of comparative biology. Fungal Divers. 2020, 103, 47–85. [Google Scholar] [CrossRef]
- Hodač, L.; Hallmann, C.; Spitzer, K.; Elster, J.; Faßhauer, F.; Brinkmann, N.; Lepka, D.; Diwan, V.; Friedl, T. Widespread green algae Chlorella and Stichococcus exhibit polar-temperate and tropical-temperate biogeography. FEMS Microbiol. Ecol. 2016, 92, fiw122. [Google Scholar] [CrossRef] [Green Version]
- Pröschold, T.; Darienko, T. The green puzzle Stichococcus (Trebouxiophyceae, Chlorophyta): New generic and species concept among this widely distributed genus. Phytotaxa 2020, 441, 113–142. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic Acids Symposium Series; c1979–c2000; Information Retrieval Ltd.: London, UK, 1999; Volume 41, pp. 95–98. [Google Scholar]
- Vaidya, G.; Lohman, D.J.G.; Meier, R. SequenceMatrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 2011, 27, 171–180. [Google Scholar] [CrossRef]
- Mason-Gamer, R.; Kellogg, E. Testing for phylogenetic conflict among molecular dataset in the tribe Triticeae (Gramiae). Syst. Biol. 1996, 45, 524–545. [Google Scholar] [CrossRef]
- Reeb, V.; Lutzoni, F.; Roux, C. Contribution of RPB2 to multilocus phylogenetic studies of the euascomycetes (Pezizomycotina, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Mol. Phylogenet. Evol. 2004, 32, 1036–1060. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P.; Teslenko, M. MrBayes version 3.2 Manual: Tutorials and Model Summaries. 2011. Available online: mrbayes.sourceforge.net/mb3.2_manual.pdf (accessed on 19 October 2018).
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018. [Google Scholar] [CrossRef] [Green Version]
- Page, R.D.M. TREEVIEW: An application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 1996, 12, 357–358. [Google Scholar]
- Hibbett, D.S.; Binder, M.; Bischoff, J.F.; Blackwell, M.; Cannon, P.F.; Eriksson, O.; Huhndorf, S.; James, T.; Kirk, P.M.; Lucking, R.; et al. A higher-level phylogenetic classification of the Fungi. Mycol. Res. 2007, 111, 509–547. [Google Scholar] [CrossRef]
- Spatafora, J.; Sung, G.-H.; Johnson, D.; Hesse, C.; O’Rourke, B.; Serdani, M.; Spotts, R.; Lutzoni, F.; Hofstetter, V.; Miadlikowska, J.; et al. A five gene phylogeny of Pezizomycotina. Mycologia 2006, 98, 1018–1028. [Google Scholar] [CrossRef]
- Schoch, C.L.; Sung, G.H.; López-Giráldez, F.; Townsend, J.P.; Miadlikowska, J.; Hofstetter, V.; Robbertse, B.; Matheny, P.B.; Kauff, F.; Wang, Z.; et al. The Ascomycota Tree of Life: A Phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Syst. Biol. 2009, 58, 224–239. [Google Scholar] [CrossRef]
- Schoch, C.L.; Wang, Z.; Townsend, J.P.; Spatafora, J.W. Geoglossomycetes cl. nov., Geoglossales ord. nov. and taxa above class rank in the Ascomycota Tree Life. Persoonia 2009, 22, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.L.; Crous, P.W.; Groenewald, J.Z.; Boehm, E.W.A.; Burgess, T.I.; De Gruyter, J.; de Hoog, G.S.; Dixon, L.J.; Grube, M.; Gueidan, C.; et al. A class-wide phylogenetic assessment of Dothideomycetes. Study Mycol. 2009, 64, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Grube, M. Classification and phylogeny in the Arthoniales (lichenized Ascomycetes). Bryologist 1998, 101, 377–391. [Google Scholar] [CrossRef]
- Ertz, D.; Sérusiaux, E. A new species of Lecanactis (Arthoniales, Roccellaceae) from Madagascar. Lichenologist 2009, 41, 147–150. [Google Scholar] [CrossRef]
- Caceres, M.; Aptroot, A.; Ertz, D. New species and interesting records of Arthoniales from the Amazon, Rondonia, Brazil. Lichenologist 2014, 46, 573–588. [Google Scholar] [CrossRef]
- Lawrey, J.D.; Diederich, P. Lichenicolous fungi: Interactions, evolution, and biodiversity. Bryologist 2003, 106, 80–120. [Google Scholar] [CrossRef]
- Ruibal, C.; Gueidan, C.; Selbmann, L.; Gorbushina, A.A.; Crous, P.W.; Groenewald, J.Z.; Muggia, L.; Grube, M.; Isola, D.; Schoch, C.L.; et al. Phylogeny of rock-inhabiting fungi related to Dothideomycetes. Stud. Mycol. 2009, 64, 123–133. [Google Scholar] [CrossRef]
- Lumbsch, H.T.; Schmitt, I.; Lindemuth, R.; Miller, A.; Mangold, A.; Fernandez, F.; Huhndorf, S. Performance of four ribosomal DNA regions to infer higher-level phylogenetic relationships of inoperculate euascomycetes (Leotiomyceta). Mol. Phylogenet. Evol. 2005, 34, 512–524. [Google Scholar] [CrossRef]
- Schoch, C.L.; Shoemaker, R.A.; Seifert, K.A.; Hambleton, S.; Spatafora, J.W.; Crous, P.W. A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 2006, 98, 1041–1052. [Google Scholar] [CrossRef]
- Henssen, A.; Thor, G. Developmental morphology of the “Zwischengruppe” between Ascohymeniales and Ascoloculares. In Ascomycete Systematics; Springer: Boston, MA, USA, 1994; pp. 43–56. [Google Scholar]
- Nelsen, M.P.; Lücking, R.; Grube, M.; Mbatchou, J.S.; Muggia, L.; Rivas Plata, E.; Lumbsch, H.T. Unravelling the phylogenetic relationships of lichenized fungi in Dothideomyceta. Study Mycol. 2009, 64, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Muggia, L.; Gueidan, C.; Knudsen, K.; Perlmutter, G.; Grube, M. The lichen connections of black fungi. Mycopathologia 2013, 175, 523–535. [Google Scholar] [CrossRef]
- Hawksworth, D.L. Lichenothelia, a new genus for the Microthelia aterrima group. Lichenologist 1981, 13, 141–153. [Google Scholar] [CrossRef]
- Henssen, A. Lichenothelia, a genus of microfungi on rocks. Bibl. Lichenol. 1987, 25, 257–293. [Google Scholar]
- Hyde, K.D.; Jones, E.G.; Liu, J.K.; Ariyawansa, H.; Boehm, E.; Boonmee, S.; Braun, U.; Chomnunti, P.; Crous, P.W.; Dai, D.Q.; et al. Families of Dothideomycetes. Fungal Divers. 2013, 63, 1–313. [Google Scholar] [CrossRef]
- Mohr, F.; Ekman, S.; Heegaard, E. Evolution and taxonomy of the marine Collemopsidium species (lichenized Ascomycota) in northwest Europe. Mycol. Res. 2004, 108, 515–532. [Google Scholar] [CrossRef]
- Kohlmeyer, J.; Hawksworth, D.; Volkmann-Kohlmeyer, B. Observations on two marine and maritime “borderline” lichens: Mastodia tessellata and Collemopsidium pelvetiae. Mycol Prog. 2004, 3, 51–56. [Google Scholar] [CrossRef]
- Lehmann, A.; Zheng, W.; Soutschek, K.; Roy, J.; Yurkov, A.M.; Rillig, M.C. Tradeoffs in hyphal traits determine mycelium architecture in saprobic fungi. Sci. Rep. 2019, 9, 14152. [Google Scholar] [CrossRef] [Green Version]
- Gostinčar, C.; Muggia, L.; Grube, M. Polyextremotolerant black fungi: Oligotrophism, adaptive potential, and a link to lichen symbioses. Front. Microbiol. 2012, 3, 390. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, G.M. Dimorphism in fungal pathogens of Mammals, Plants, and Insects. PLoS Pathog. 2015, 11, e1004608. [Google Scholar] [CrossRef]
- Gostinčar, C.; Zajc, J.; Lenassi, M.; Plemenitaš, A.; de Hoog, S.; Al-Hatmi, A.M.S.; Gunde-Cimerman, N. Fungi between extremotolerance and opportunistic pathogenicity on humans. Fungal Divers. 2018, 93, 195–213. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, K.; Kocourková, J. A new Lichenostigma species (Genus incertae sedis) from southern California. Bryologist 2010, 113, 229–234. [Google Scholar] [CrossRef]
- Kocourková, J.; Knudsen, K. Lichenological notes 2: Lichenothelia convexa, a poorly known rock inhabiting and lichenicolous fungus. Mycotaxon 2011, 115, 345–351. [Google Scholar] [CrossRef]
- Ametrano, C.G.; Selbmann, L.; Muggia, L. A standardized approach for co-culturing Dothidealean rock-inhabiting fungi and lichen photobionts in vitro. Symbiosis 2017, 73, 35–44. [Google Scholar] [CrossRef]
- Muggia, L.; Hafellner, J.; Wirtz, N.; Hawksworth, D.L.; Grube, M. The sterile microfilamentous lichenized fungi Cystocoleus ebeneus and Racodium rupestre are relatives of plant pathogens and clinically important dothidealean fungi. Mycol. Res. 2008, 112, 50–56. [Google Scholar] [CrossRef]
- Muggia, L.; Kraker, S.; Gößler, T.; Grube, M. Enforced fungal-algal symbioses in alginate spheres. FEMS Microbiol. Lett. 2018, 365, fny115. [Google Scholar] [CrossRef]
- Muggia, L.; Zalar, P.; Azua-Bustos, A.; González-Silva, C.; Grube, M.; Gunde-Cimerman, N. The beauty and the yeast: Can the microalgae Dunaliella form a borderline lichen with Hortaea werneckii? Symbiosis 2020, 82, 123–131. [Google Scholar] [CrossRef]
- Azúa-Bustos, A.; González-Silva, C.; Salas, L.; Palma, R.E.; Vicuña, R. A novel subaerial Dunaliella species growing on cave spiderwebs in the Atacama Desert. Extremophiles 2010, 14, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Gorbushina, A.A.; Beck, A.; Schulte, A. Microcolonial rock inhabiting fungi and lichen photobionts: Evidence for mutualistic interactions. Mycol. Res. 2005, 109, 1288–1296. [Google Scholar] [CrossRef]
- Hawksworth, D.; Grube, M. Lichens redefined as complex ecosystems. New Phytol. 2020, 227, 1281–1283. [Google Scholar] [CrossRef]
- Grube, M.; Wedin, M. Lichenized fungi and the evolution of symbiotic organization. Fungal Kingd. 2016, 4, 749–765. [Google Scholar]
- Spribille, T.; Tagirdzhanova, G.; Goyette, S.; Tuovinen, V.; Case, R.; Zandberg, W.F. 3D biofilms: In search of the polysaccharides holding together lichen symbioses. FEMS Microbiol. Lett. 2020, 367, fnaa023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voytsekhovich, A.; Beck, A. Lichen photobionts of the rocky outcrops of Karadag massif (Crimean Peninsula). Symbiosis 2016, 68, 9–24. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).