Elucidation of the Natural Function of Sophorolipids Produced by Starmerella bombicola
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Antimicrobial Assay
2.2. Strains, Media, and Culture Conditions
2.3. Preparation of (Partly) Purified Sophorolipids
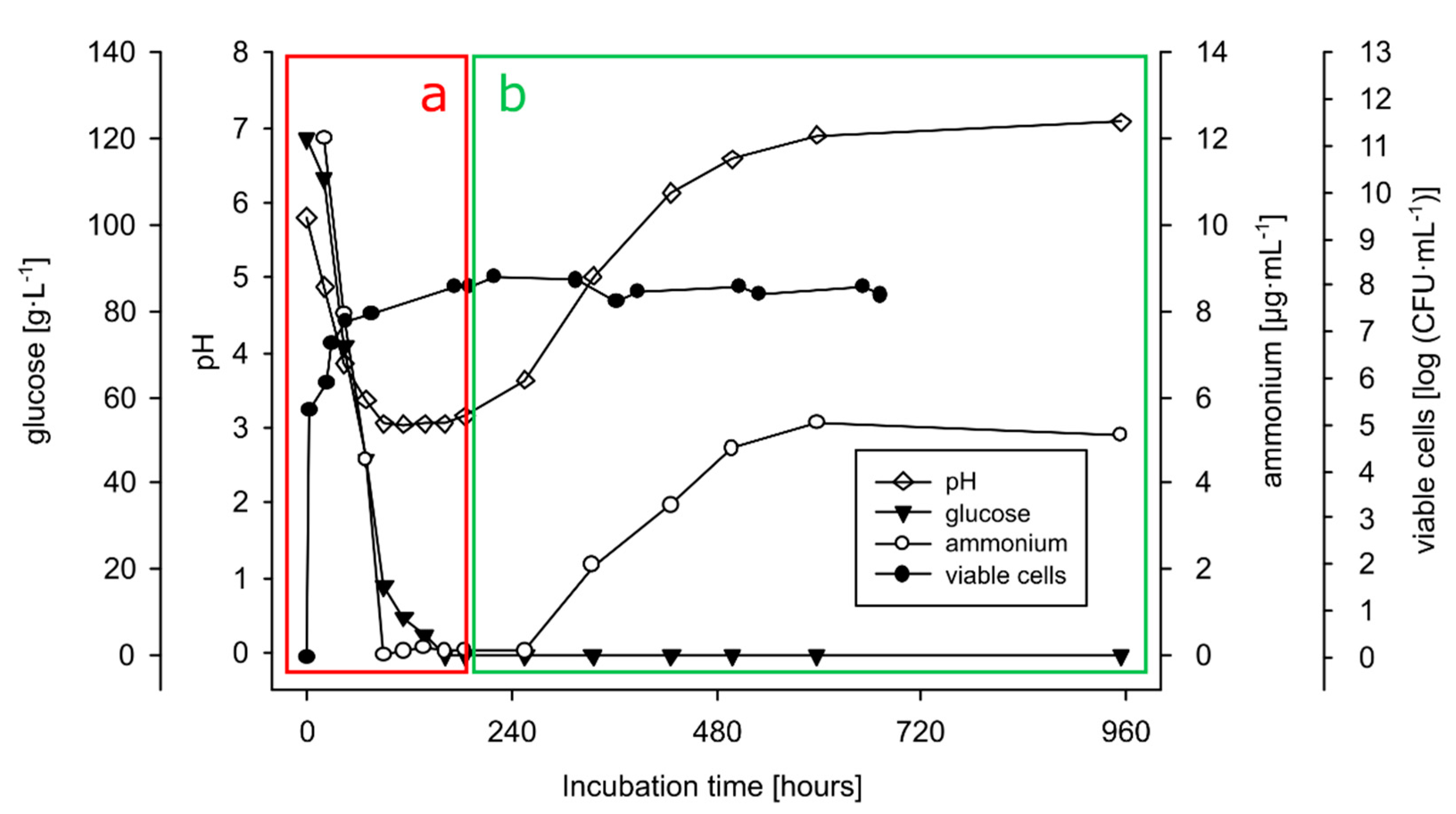
2.4. Sampling and Determination of Growth Parameters
2.5. Assays with Extracellular Secretomes
2.6. Assays with Intracellular Cell Lysates
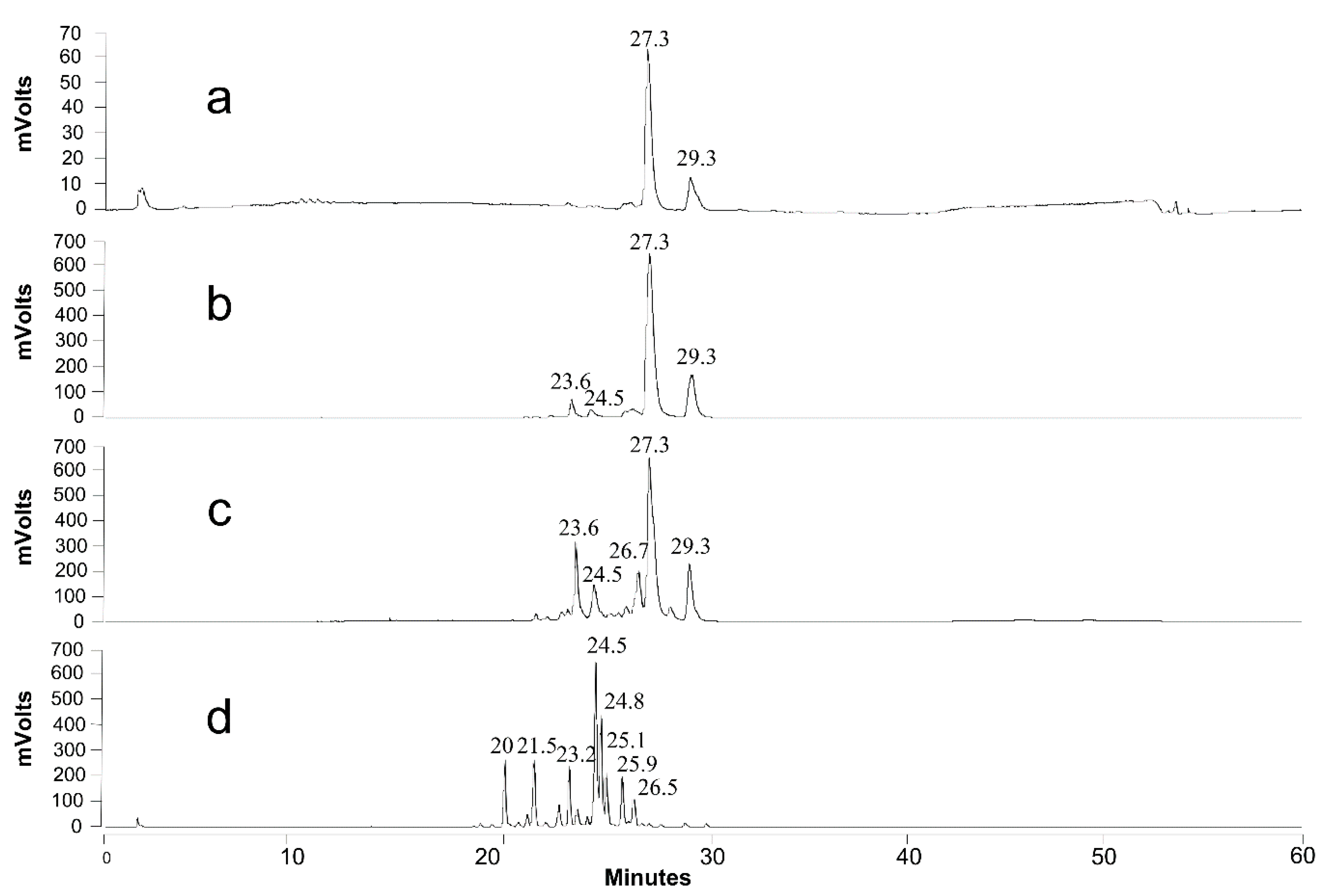
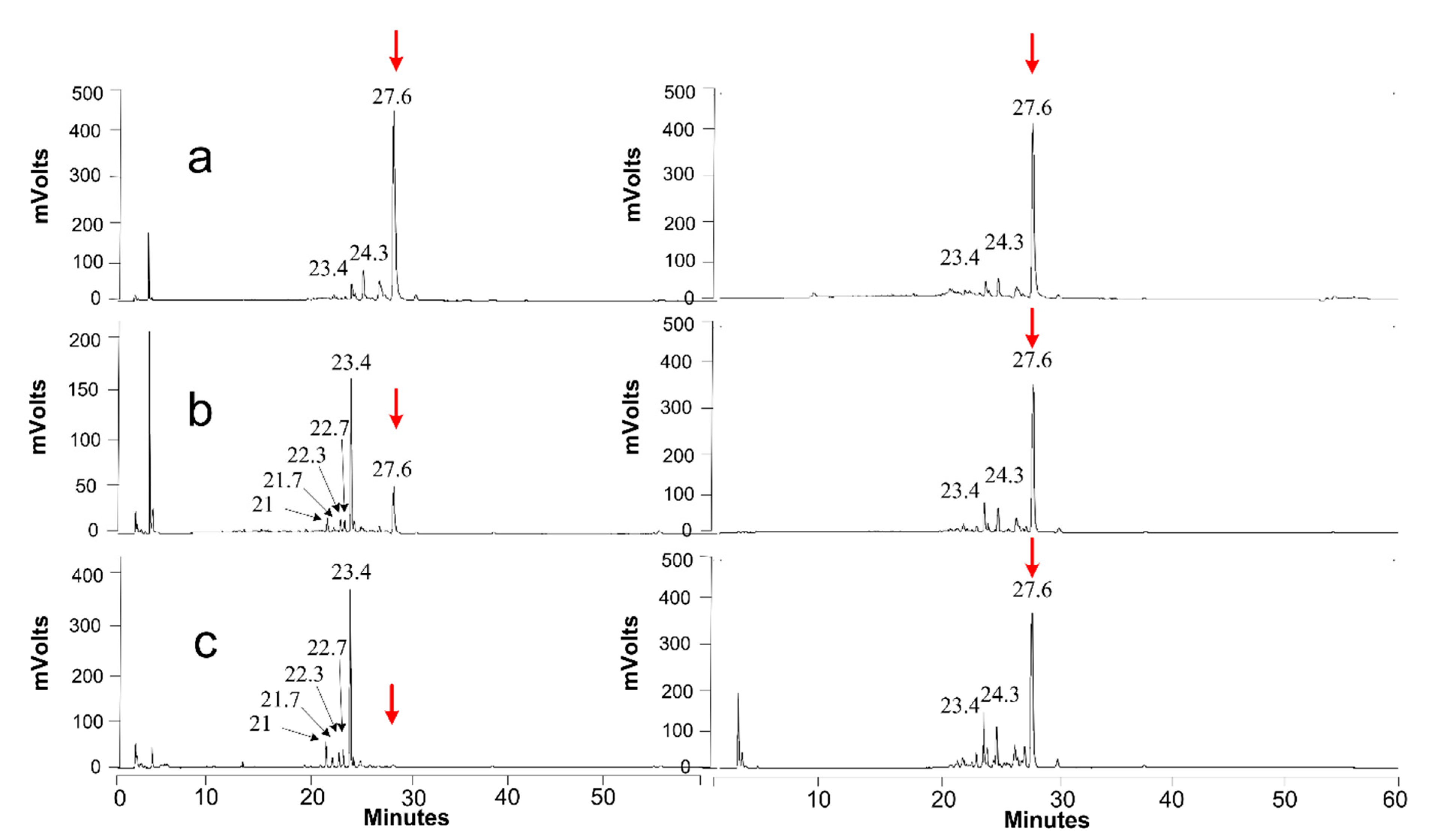
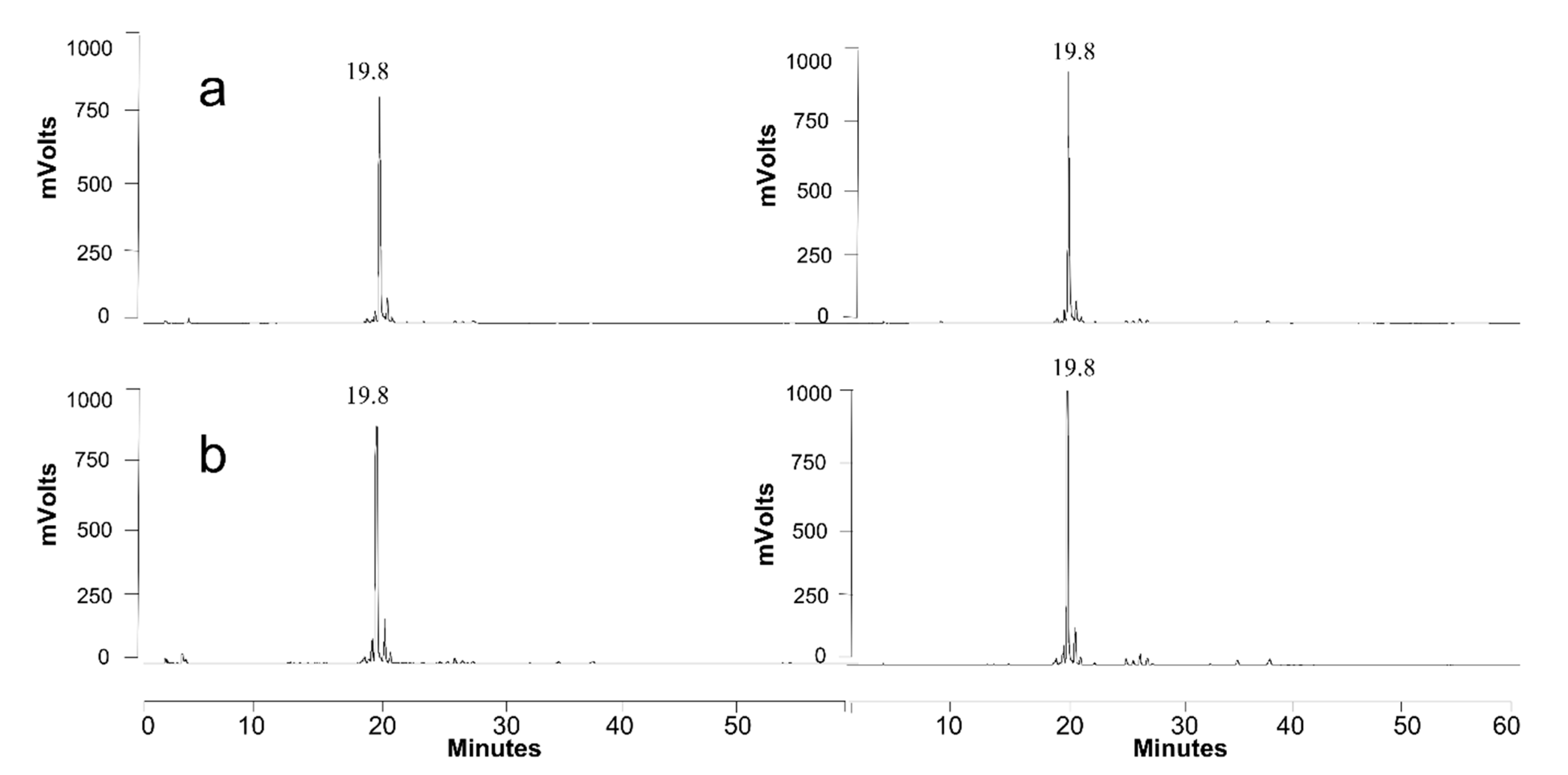
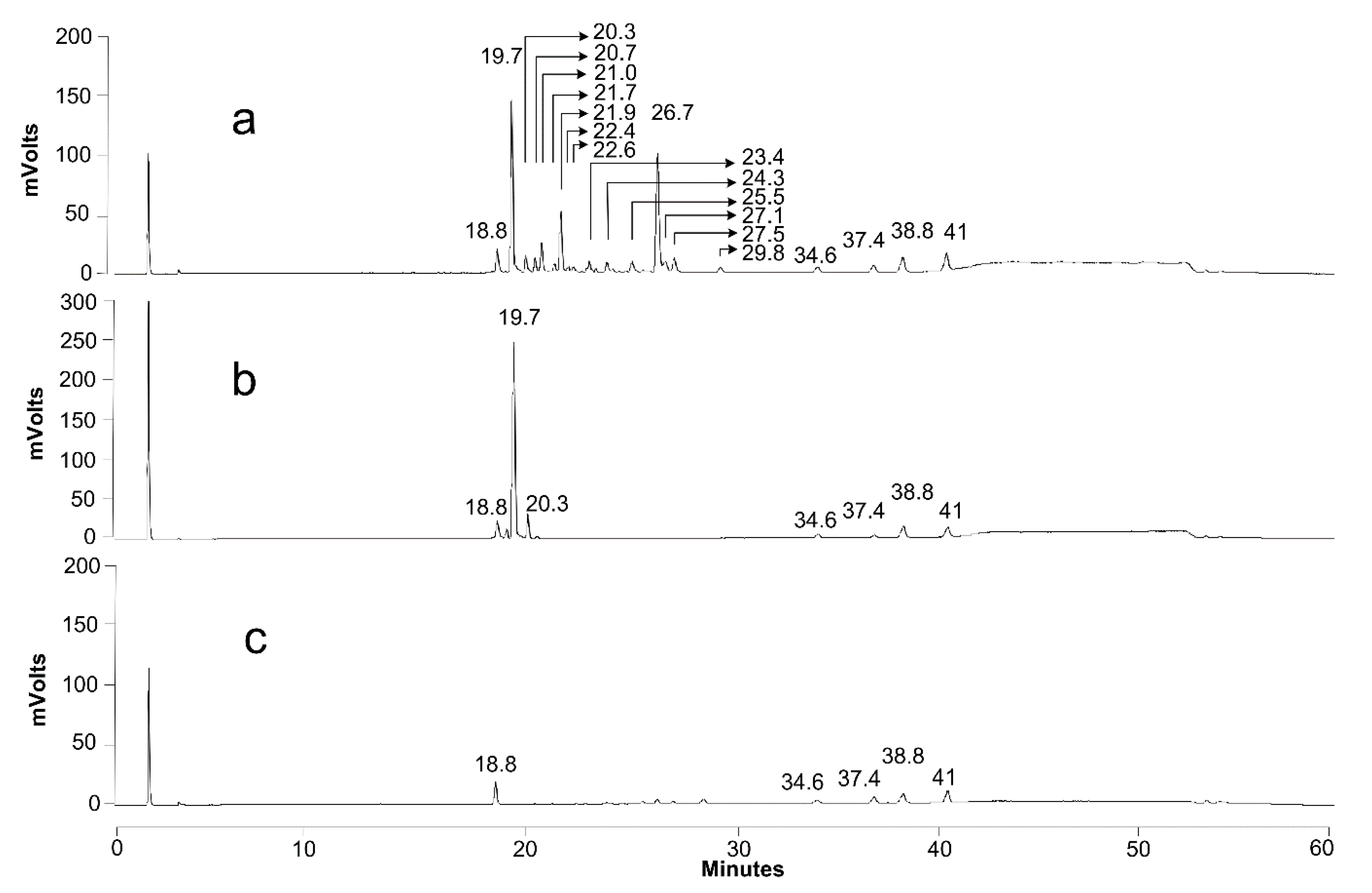
2.7. Product Identification and Quantification
3. Results
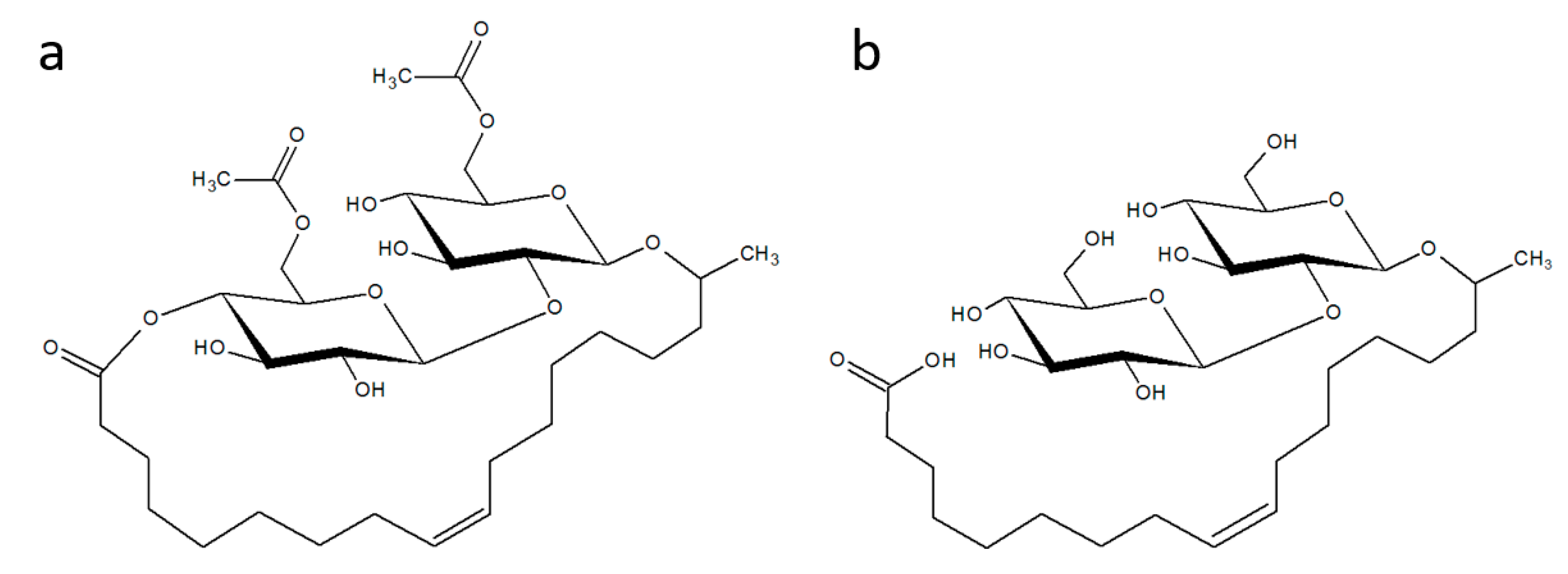
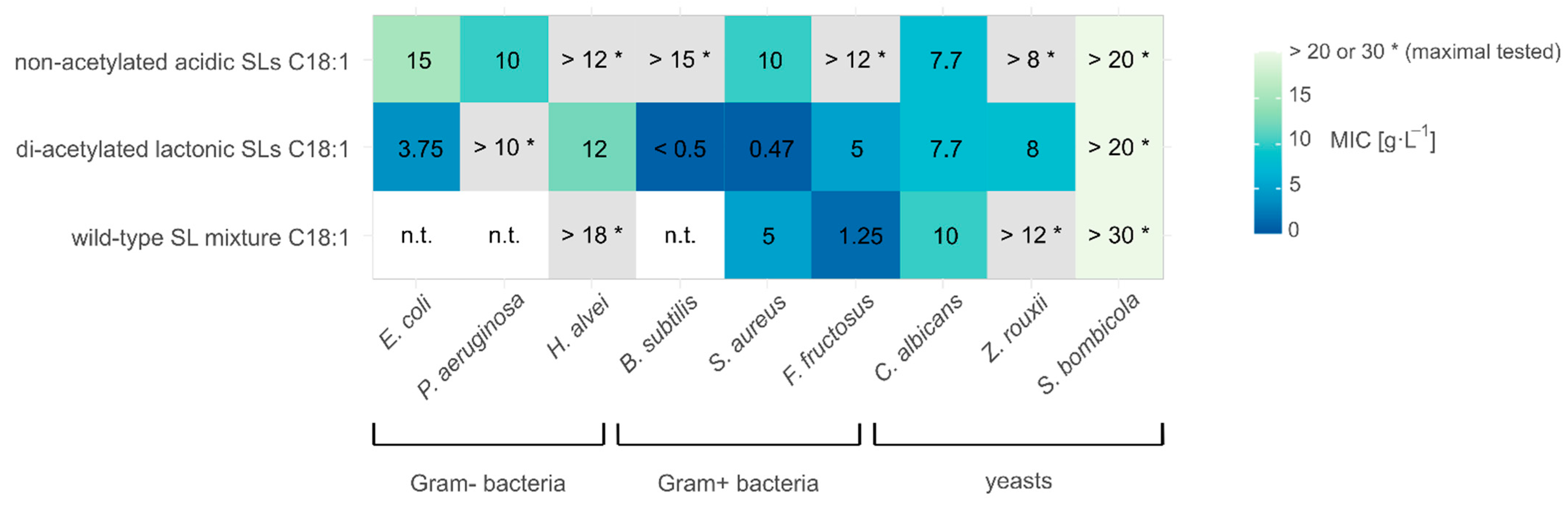
3.1. Antimicrobial Activity
3.2. Protection against Osmotic Pressure
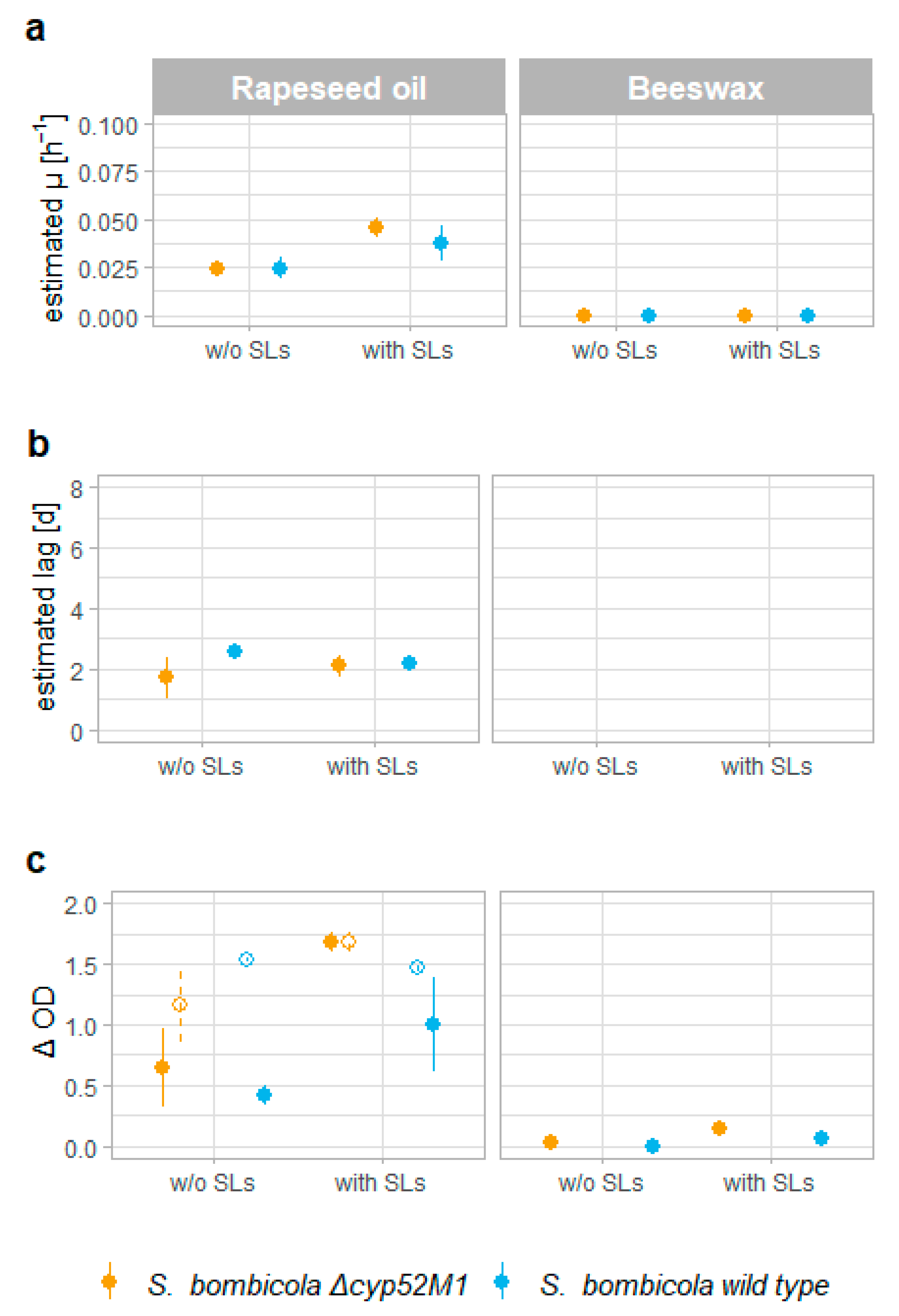
3.3. Uptake of Hydrophobic Substrates
3.4. Exclusive Storage Compound
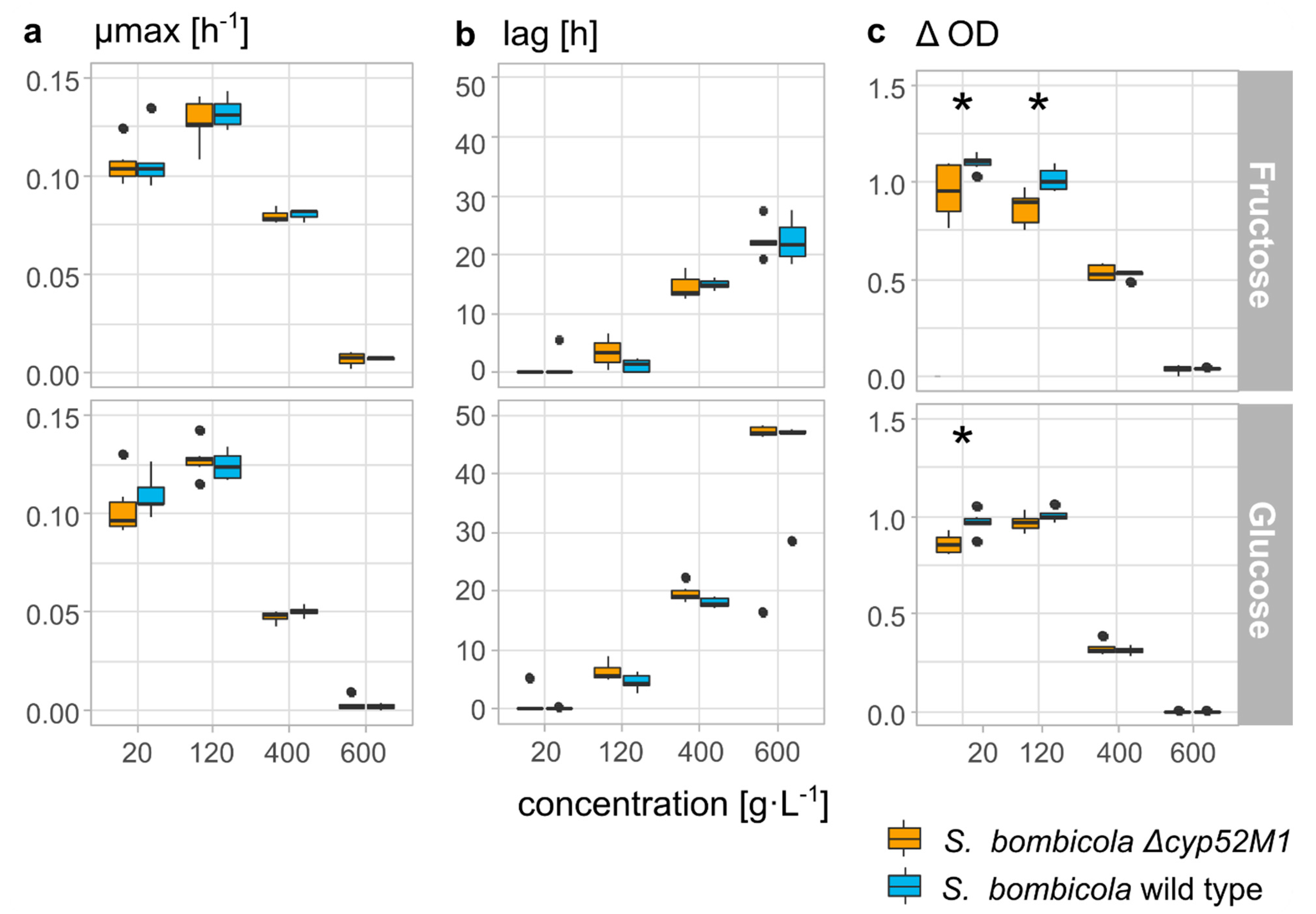
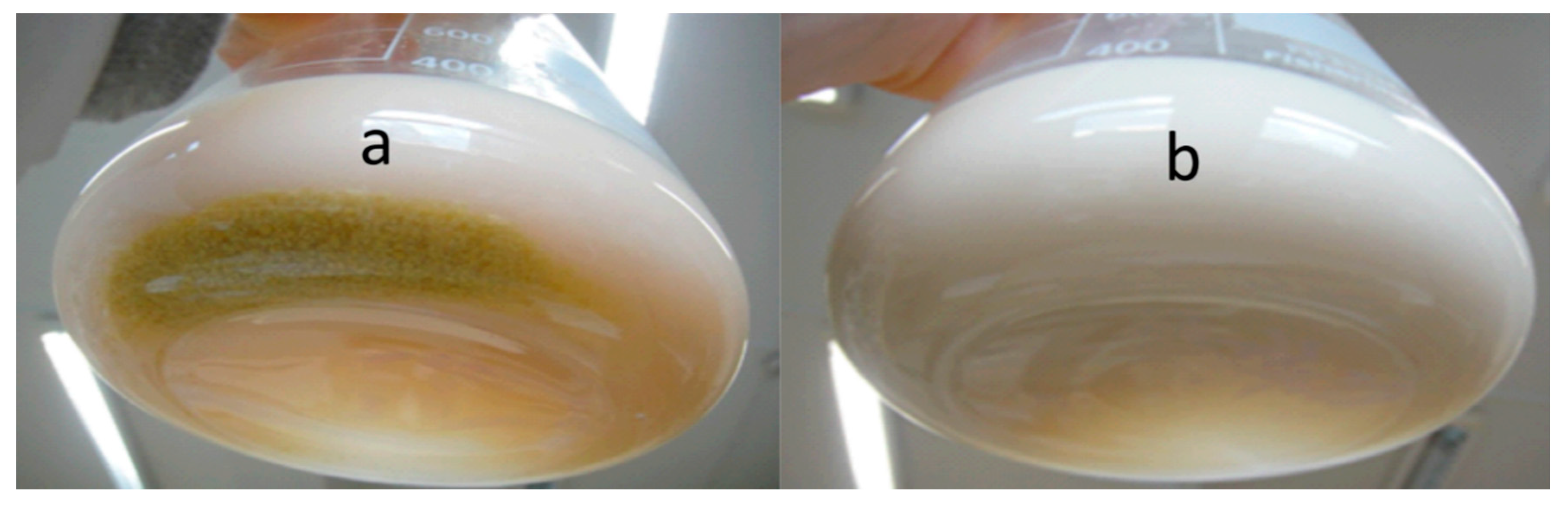
3.4.1. Growth on Production Medium
3.4.2. Growth on Sophorolipids as the Sole Carbon Source
3.4.3. Extracellular Activity (of the Secretome)
3.4.4. Intracellular Activity (of Cell Lysate)
4. Discussion
4.1. Antimicrobial Activity
4.2. Protection against Osmotic Pressure
4.3. Uptake of Hydrophobic Substrates
4.4. Exclusive Storage Compound
4.5. SLs and Their Possible Relation to Overwintering
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Oliveira, M.R.; Camilios-Neto, D.; Baldo, C.; Magri, A.; Pedrine, M.A.; Celligoi, C. Biosynthesis and Production of Sophorolipids. Int. J. Sci. Technol. Res. 2014, 3, 133–146. [Google Scholar]
- De Graeve, M.; de Maeseneire, S.L.; Roelants, S.L.K.W.; Soetaert, W. Starmerella bombicola, an industrially relevant, yet fundamentally underexplored yeast. FEMS Yeast Res. 2018, 18, foy072. [Google Scholar] [CrossRef]
- Asmer, H.-J.; Lang, S.; Wagner, F.; Wray, V. Microbial production, structure elucidation and bioconversion of sophorose lipids. J. Am. Oil Chem. Soc. 1988, 65, 1460–1466. [Google Scholar] [CrossRef]
- Cavalero, D.A.; Cooper, D.G. The effect of medium composition on the structure and physical state of sophorolipids produced by Candida bombicola ATCC 22214. J. Biotechnol. 2003, 103, 31–41. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Price, N.P.J.; Ray, K.J.; Kuo, T.-M. Production of sophorolipid biosurfactants by multiple species of the Starmerella (Candida) bombicola yeast clade. FEMS Microbiol. Lett. 2010, 311, 140–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davila, A.M.; Marchal, R.; Monin, N.; Vandecasteele, J.P. Identification and determination of individual sophorolipids in fermentation products by gradient elution high-performance liquid chromatography with evaporative light-scattering detection. J. Chromatogr. A 1993, 648, 139–149. [Google Scholar] [CrossRef]
- Chen, M.; Dong, C.; Penfold, J.; Thomas, R.K.; Smyth, T.J.P.; Perfumo, A.; Marchant, R.; Banat, I.M.; Stevenson, P.; Parry, A.; et al. Adsorption of Sophorolipid Biosurfactants on Their Own and Mixed with Sodium Dodecyl Benzene Sulfonate, at the Air/Water Interface. Langmuir 2011, 27, 8854–8866. [Google Scholar] [CrossRef] [PubMed]
- Imura, T.; Kawamura, D.; Taira, T.; Morita, T.; Fukuoka, T.; Aburai, K.; Sakai, H.; Abe, M.; Kitamoto, D. Monolayer Behavior of Binary Systems of Lactonic and Acidic Forms of Sophorolipids: Thermodynamic Analyses of Langmuir Monolayers and AFM Study of Langmuir-Blodgett Monolayers. J. Oleo Sci. 2014, 63, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Tulloch, A.P.; Hill, A.; Spencer, J.F.T. Structure and reactions of lactonic and acidic sophorosides of 17-hydroxyoctadecanoic acid. Can. J. Chem. 1968, 46, 3337–3351. [Google Scholar] [CrossRef]
- Hu, Y.; Ju, L.K. Sophorolipid production from different lipid precursors observed with LC-MS. Enzyme Microb. Technol. 2001, 29, 593–601. [Google Scholar] [CrossRef]
- Hall, P.J.; Haverkamp, J.; van Kralingen, C.G.; Schmidt, M. Synergistic Dual-Surfactant Detergent Composition Containing Sophoroselipid 1993. U.S. Patent 5,417,879, 23 May 1995. [Google Scholar]
- Furuta, T.; Igarashi, K.; Hirata, Y. Low-Foaming Detergent Compositions 2002. U.S. Patent Application Publication US20040171512A1, 2 September 2004. [Google Scholar]
- Shete, A.M.; Wadhawa, G.; Banat, I.M.; Chopade, B.A. Mapping of patents on bioemulsifier and biosurfactant: A review. J. Sci. Ind. Res. 2006, 65, 91–115. [Google Scholar]
- Roberto de Oliveira, M.; Magri, A.; Baldo, C.; Camilios-Neto, D.; Minucelli, T.; Pedrine, M.A.; Celligoi, C. Review: Sophorolipids A Promising Biosurfactant and it’s Applications. Int. J. Adv. Biotechnol. Res. 2015, 6, 161–174. [Google Scholar]
- Lydon, H.L.; Baccile, N.; Callaghan, B.; Marchant, R.; Mitchell, C.A.; Banat, I.M. Adjuvant antibiotic activity of acidic sophorolipids with potential for facilitating wound healing. Antimicrob. Agents Chemother. 2017, 61, e02547-16. [Google Scholar] [CrossRef] [Green Version]
- Van Renterghem, L.; Roelants, S.L.K.W.; Baccile, N.; Uyttersprot, K.; Taelman, M.C.; Everaert, B.; Mincke, S.; Ledegen, S.; Debrouwer, S.; Scholtens, K.; et al. From lab to market: An integrated bioprocess design approach for new-to-nature biosurfactants produced by Starmerella bombicola. Biotechnol. Bioeng. 2018, 115, 1195–1206. [Google Scholar] [CrossRef] [Green Version]
- Arab, F.; Mulligan, C.N. An eco-friendly method for heavy metal removal from mine tailings. Environ. Sci. Pollut. Res. 2018, 25, 16202–16216. [Google Scholar] [CrossRef]
- Lodens, S.; de Graeve, M.; Roelants, S.L.K.W.; de Maeseneire, S.L.; Soetaert, W. Transformation of an exotic yeast species into a platform organism: A case study for engineering glycolipid production in the yeast starmerella bombicola. In Synthetic Biology. Methods in Molecular Biology; Braman, J., Ed.; Humana Press: New York, NY, USA, 2018; Volume 1772, pp. 95–123. [Google Scholar]
- Van Bogaert, I.N.A.; Buyst, D.; Martins, J.C.; Roelants, S.L.K.W.; Soetaert, W.K. Synthesis of bolaform biosurfactants by an engineered Starmerella bombicola yeast. Biotechnol. Bioeng. 2016, 113, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- Saerens, K.M.J.; Zhang, J.; Saey, L.; van Bogaert, I.N.A.; Soetaert, W. Cloning and functional characterization of the UDP-glucosyltransferase UgtB1 involved in sophorolipid production by Candida bombicola and creation of a glucolipid-producing yeast strain. Yeast 2011, 28, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Lodens, S.; Roelants, S.L.K.W.; Ciesielska, K.; Geys, R.; Derynck, E.; Maes, K.; Pattyn, F.; van Renterghem, L.; Mottet, L.; Dierickx, S.; et al. Unraveling and resolving inefficient glucolipid biosurfactants production through quantitative multiomics analyses of Starmerella bombicola strains. Biotechnol. Bioeng. 2020, 117, 453–465. [Google Scholar] [CrossRef]
- Spencer, J.F.; Gorin, P.A.; Tulloch, A.P. Torulopsis bombicola sp.n. Antonie Van Leeuwenhoek 1970, 36, 129–133. [Google Scholar] [CrossRef]
- Yarrow, D.; Meyer, S.A. Proposal for amendment of the diagnosis of the genus Candida Berkhout nom. cons. Int. J. Syst. Bacteriol. 1978, 28, 611–615. [Google Scholar] [CrossRef] [Green Version]
- McNeill, J.; Barrie, F.R.; Buck, W.R.; Demoulin, V.; Greuter, W.; Hawksworths, D.L.; Herendeen, P.S.; Knapp, S.; Marhold, K.; Prado, J.; et al. International Code of Nomenclature for Algae, Fungi and Plants (Melbourne Code) Adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011; Koeltz Scientific Books: Königstein, Germany, 2012; Volume 154, ISBN 9783874294256. [Google Scholar]
- Crane, E. Honey from honeybees and other insects. Ethol. Ecol. Evol. 1991, 3, 100–105. [Google Scholar] [CrossRef]
- Chalcoff, V.R.; Aizen, M.A.; Galetto, L. Nectar concentration and composition of 26 species from the temperate forest of South America. Ann. Bot. 2006, 97, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Aleklett, K.; Hart, M.; Shade, A. The microbial ecology of flowers: An emerging frontier in phyllosphere research1. Botany 2014, 92, 253–266. [Google Scholar] [CrossRef]
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Beeswax: A minireview of its antimicrobial activity and its application in medicine. Asian Pac. J. Trop. Med. 2016, 9, 839–843. [Google Scholar] [CrossRef]
- Hammond, E.W. Vegetable Oils|Types and Properties. In Encyclopedia of Food Sciences and Nutrition; Academic Press: Cambridge, MA, USA, 2003; pp. 5899–5904. [Google Scholar]
- Davila, A.M.; Marchal, R.; Vandecasteele, J.P. Sophorose lipid fermentation with differentiated substrate supply for growth and production phases. Appl. Microbiol. Biotechnol. 1997, 47, 496–501. [Google Scholar] [CrossRef]
- Ito, S.; Kinta, M.; Inoue, S. Growth of yeasts on n-alkanes: Inhibition by a lactonic sophorolipid produced by Torulopsis bombicola. Agric. Biol. Chem. 1980, 44, 2221–2223. [Google Scholar] [CrossRef]
- Tran, H.G. Improving the Glycosylation Activity of Cellobiose/Cellodextrin Phosphorylase through Enzyme Engineering; Ghent University: Ghent, Belgium, 2012. [Google Scholar]
- Gross, R.A.; Shah, V. Antifungal Properties of Various Forms of Sophorolipids 2003. World Intellectual Property Organization. WO2004044216A1, 27 May 2004. [Google Scholar]
- Díaz de Rienzo, M.A.; Stevenson, P.; Marchant, R.; Banat, I.M. Antibacterial properties of biosurfactants against selected Gram-positive and -negative bacteria. FEMS Microbiol. Lett. 2016, 363, fnv224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Yoo, D.; Kim, Y.; Lee, B.-S.; Shin, D.; Kim, E.K. Characteristics of sophorolipid as an antimicrobial agent. J. Microbiol. Biotechnol. 2002, 12, 235–241. [Google Scholar]
- Haque, F.; Alfatah, M.; Ganesan, K.; Bhattacharyya, M.S. Inhibitory Effect of Sophorolipid on Candida albicans Biofilm Formation and Hyphal Growth. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hipólito, A.; Alves da Silva, R.A.; de Oliveira Caretta, T.; Itakura Silveira, V.A.; Amador, I.R.; Panagio, L.A.; Borsato, D.; Pedrine Colabone Celligoi, M.A. Evaluation of the antifungal activity of sophorolipids from Starmerella bombicola against food spoilage fungi. Biocatal. Agric. Biotechnol. 2020, 29, 101797. [Google Scholar] [CrossRef]
- Hommel, R.K.; Weber, L.; Weiss, A.; Himmelreich, U.; Rilke, O.; Kleber, H.P. Production of sophorose lipid by Candida (Torulopsis) apicola grown on glucose. J. Biotechnol. 1994, 33, 147–155. [Google Scholar] [CrossRef]
- Ito, S.; Inoue, S. Sophorolipids from Torulopsis bombicola: Possible relation to alkane uptake. Appl. Environ. Microbiol. 1982, 43, 1278–1283. [Google Scholar] [CrossRef] [Green Version]
- Hommel, R.K. Formation and physiological role of biosurfactants produced by hydrocarbon-utilizing microorganisms—Biosurfactants in hydrocarbon utilization. Biodegradation 1990, 1, 107–119. [Google Scholar] [CrossRef]
- Linton, J.D. Metabolite production and growth efficiency. Antonie Van Leeuwenhoek 1991, 60, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Dierickx, S.; Castelein, M.; Remmery, J.; De Clercq, V.; Lodens, S.; Baccile, N.; De Maeseneire, S.L.; Roelants, S.L.K.W.; Soetaert, W.K. From bumblebee to bioeconomy: Recent developments and perspectives for sophorolipid biosynthesis. Biotechnol. Adv. 2021, 107788. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically (CLSI Standard M07), 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elshikh, M.; Moya-Ramírez, I.; Moens, H.; Roelants, S.; Soetaert, W.; Marchant, R.; Banat, I.M. Rhamnolipids and lactonic sophorolipids: Natural antimicrobial surfactants for oral hygiene. J. Appl. Microbiol. 2017, 123, 1111–1123. [Google Scholar] [CrossRef]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Bogaert, I.N.A.; Holvoet, K.; Roelants, S.L.K.W.; Li, B.; Lin, Y.C.; van de Peer, Y.; Soetaert, W. The biosynthetic gene cluster for sophorolipids: A biotechnological interesting biosurfactant produced by Starmerella bombicola. Mol. Microbiol. 2013, 88, 501–509. [Google Scholar] [CrossRef]
- Ciesielska, K.; van Bogaert, I.N.; Chevineau, S.; Li, B.; Groeneboer, S.; Soetaert, W.; van de Peer, Y.; Devreese, B. Exoproteome analysis of Starmerella bombicola results in the discovery of an esterase required for lactonization of sophorolipids. J. Proteomics 2014, 98, 159–174. [Google Scholar] [CrossRef]
- Roelants, S.L.K.W.; Ciesielska, K.; de Maeseneire, S.L.; Moens, H.; Everaert, B.; Verweire, S.; Denon, Q.; Vanlerberghe, B.; van Bogaert, I.N.A.; van der Meeren, P.; et al. Towards the Industrialization of New Biosurfactants: Biotechnological Opportunities for the Lactone Esterase Gene From Starmerella bombicola. Biotechnol. Bioeng. 2016, 113, 550–559. [Google Scholar] [CrossRef]
- Lang, S.; Brakemeier, A.; Heckmann, R.; Spockner, S.; Rau, U. Production of native and modified sophorose lipids. Chim. Oggi-Chem. Today 2000, 18, 76–79. [Google Scholar]
- Saerens, K.M.J.; Roelants, S.L.K.W.; van Bogaert, I.N.A.; Soetaert, W. Identification of the UDP-glucosyltransferase gene UGTA1, responsible for the first glucosylation step in the sophorolipid biosynthetic pathway of Candida bombicola ATCC 22214. FEMS Yeast Res. 2011, 11, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Pozo, M.I.; Lievens, B.; Jacquemyn, H. Impact of Microorganisms on Nectar Chemistry, Pollinator Attraction and Plant Fitness. In Nectar: Production, Chemical Composition and Benefits to Animals and Plants; Nova Science Publishers Inc.: New York, NY, USA, 2015; pp. 1–45. [Google Scholar]
- Vit, P.; Roubik, D.W.; Pedro, S.R.M. Pot-Honey: A Legacy of Stingless Bees; Springer: New York, NY, USA, 2012; ISBN 9781461449607. [Google Scholar]
- De Vega, C.; Albaladejo, R.G.; Guzmán, B.; Steenhuisen, S.; Johnson, S.D.; Herrera, C.M.; Lachance, M. Flowers as a reservoir of yeast diversity: Description of Wickerhamiella nectarea f.a. sp. nov., and Wickerhamiella natalensis f.a. sp. nov. from South African flowers and pollinators, and transfer of related Candida species to the genus Wickerhamiella as new combinations. FEMS Yeast Res. 2017, 17, fox054. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, D.K.; Waraich, M.K. Yeasts Associated with Pollinating Bees and Flower Nectar. Microb. Ecol. 1985, 11, 51–58. [Google Scholar] [CrossRef]
- Praet, J.; Parmentier, A.; Schmid-Hempel, R.; Meeus, I.; Smagghe, G.; Vandamme, P. Large-scale cultivation of the bumblebee gut microbiota reveals an underestimated bacterial species diversity capable of pathogen inhibition. Environ. Microbiol. 2018, 20, 214–227. [Google Scholar] [CrossRef]
- Brysch-Herzberg, M. Ecology of yeasts in plant-bumblebee mutualism in Central Europe. FEMS Microbiol. Ecol. 2004, 50, 87–100. [Google Scholar] [CrossRef]
- Endo, A. Fructophilic lactic acid bacteria inhabit fructose-rich niches in nature. Microb. Ecol. Health Dis. 2012, 23, 18563. [Google Scholar] [CrossRef]
- Babazadeh, R.; Lahtvee, P.J.; Adiels, C.B.; Goksör, M.; Nielsen, J.B.; Hohmann, S. The yeast osmostress response is carbon source dependent. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sleator, R.D.; Hill, C. Bacterial osmoadaptation: The role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 2002, 26, 49–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, C.; Wisecaver, J.H.; Kominek, J.; Salema Oom, M.; Leandro, M.J.; Shen, X.X.; Opulente, D.A.; Zhou, X.; Peris, D.; Kurtzman, C.P.; et al. Evidence for loss and reacquisition of alcoholic fermentation in a fructophilic yeast lineage. elife 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Hommel, R.K.; Huse, K. Regulation of sophorose lipid production by Candida (Torulopsis) apicola. Biotechnol. Lett. 1993, 15, 853–858. [Google Scholar] [CrossRef]
- Jules, M.; Beltran, G.; François, J.; Parrou, J.L. New insights into trehalose metabolism by Saccharomyces cerevisiae: NTH2 encodes a functional cytosolic trehalase, and deletion of TPS1 reveals Ath1p-dependent trehalose mobilization. Appl. Environ. Microbiol. 2008, 74, 605–614. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Ochoa, F.; Casas, J.A. Process for the Production of Sophorose by Candida Bombicola 1996. Spanish Patent ES2103688A1, 16 September 1997. [Google Scholar]
- Hu, Y. Production, Purification and Enzymatic Polymerization of Sophorolipids; University of Akron: Akron, OH, USA, 2000. [Google Scholar]
- Lo, C.M.; Ju, L.K. Sophorolipids-induced cellulase production in cocultures of Hypocrea jecorina Rut C30 and Candida bombicola. Enzyme Microb. Technol. 2009, 44, 107–111. [Google Scholar] [CrossRef]
- Mimee, B.; Labbé, C.; Bélanger, R.R. Catabolism of flocculosin, an antimicrobial metabolite produced by Pseudozyma flocculosa. Glycobiology 2009, 19, 995–1001. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Mawgoud, A.M.; Aboulwafa, M.M.; Hassouna, N.A.H. Optimization of surfactin production by bacillus subtilis isolate BS5. Appl. Biochem. Biotechnol. 2008, 150, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, D.; Nakane, T.; Nakao, N.; Nakahara, T.; Tabuchi, T. Intracellular accumulation of mannosylerythritol lipids as storage materials by Candida antarctica. Appl. Microbiol. Biotechnol. 1992, 36, 768–772. [Google Scholar] [CrossRef]
- Deinema, M. Intra- and Extra-Cellular Lipid Production by Yeasts; University of Wageningen: Wageningen, The Netherlands, 1961. [Google Scholar]
- Stüwer, O.; Hommel, R.; Haferburg, D.; Kleber, H.P. Production of crystalline surface-active glycolipids by a strain of torulopsis apicola. J. Biotechnol. 1987, 6, 259–269. [Google Scholar] [CrossRef]
- Albrecht, A.; Rau, U.; Wagner, F. Initial steps of sophoroselipid biosynthesis by Candida bombicola ATCC 22214 grown on glucose. Appl. Microbiol. Biotechnol. 1996, 46, 67–73. [Google Scholar] [CrossRef]
- Saerens, K.M.J.; Saey, L.; Soetaert, W. One-step production of unacetylated sophorolipids by an acetyltransferase negative Candida bombicola. Biotechnol. Bioeng. 2011, 108, 2923–2931. [Google Scholar] [CrossRef]
- Joshi-Navare, K.; Khanvilkar, P.; Prabhune, A. Jatropha Oil Derived Sophorolipids: Production and Characterization as Laundry Detergent Additive. Biochem. Res. Int. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Luong, N.; Hoa, H.; Loan, L.Q.; Eun-ki, K.; Ha, T.T.; Duy, N.D.; Khanh, H.Q.; Dung, N.H. Production and characterization of sophorolipids produced by Candida bombicola using sugarcane molasses and coconut oil. Asia-Pacific J. Sci. Technol. 2017, 22, 1–8. [Google Scholar] [CrossRef]
- Akemi, V.; Silveira, I.; Urzedo, C.A.; Freitas, Q.; Pedrine, M.A.; Celligoi, C. Antimicrobial applications of sophorolipid from Candida bombicola: A promising alternative to conventional drugs. J. Appl. Biol. Biotechnol. 2018, 6, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Dengle-Pulate, V.; Chandorkar, P.; Bhagwat, S.; Prabhune, A.A. Antimicrobial and SEM studies of sophorolipids synthesized using lauryl alcohol. J. Surfactants Deterg. 2014, 17, 543–552. [Google Scholar] [CrossRef]
- Pohl, A.; Lübke-Becker, A.; Heuwieser, W. Minimum inhibitory concentrations of frequently used antibiotics against Escherichia coli and Trueperella pyogenes isolated from uteri of postpartum dairy cows. J. Dairy Sci. 2018, 101, 1355–1364. [Google Scholar] [CrossRef]
- Detry, R.; Simon-Delso, N.; Bruneau, E.; Daniel, H.-M. Specialisation of Yeast Genera in Different Phases of Bee Bread Maturation. Microorganisms 2020, 8, 1789. [Google Scholar] [CrossRef] [PubMed]
- Esders, T.W.; Light, R.J. Characterization and in vivo production of three glycolipids from Candida Bogoriensis: 13-glucopyranosylglucopyranosyloxydocosanoic acid and its mono- and diacetylated derivatives. J. Lipid Res. 1972, 13, 663–671. [Google Scholar] [CrossRef]
- Bucholtz, M.L.; Light, R.J. Hydrolysis of 13-sophorosyloxydocosanoic acid esters by acetyl- and carboxylesterases isolated from Candida bogoriensis. J. Biol. Chem. 1976, 251, 431–437. [Google Scholar] [CrossRef]
- Danchin, A. Cells need safety valves. BioEssays 2009, 31, 769–773. [Google Scholar] [CrossRef]
- Rosa, C.A.; Lachance, M.A.; Silva, J.O.C.; Teixeira, A.C.P.; Marini, M.M.; Antonini, Y.; Martins, R.P. Yeast communities associated with stingless bees. FEMS Yeast Res. 2003, 4, 271–275. [Google Scholar] [CrossRef]
- Santos, A.R.O.; Leon, M.P.; Barros, K.O.; Freitas, L.F.D.; Hughes, A.F.S.; Morais, P.B.; Lachance, M.A.; Rosa, C.A. Starmerella camargoi f.A., sp. nov., starmerella ilheusensis f.a., sp. nov., Starmerella litoralis f.a., sp. nov., starmerella opuntiae f.a., sp. nov., Starmerella roubikii f.a., sp. nov. and Starmerella vitae f.a., sp. nov., isolated from flowers and bee. Int. J. Syst. Evol. Microbiol. 2018, 68, 1333–1343. [Google Scholar] [CrossRef]
- Pozo, M.I.; Bartlewicz, J.; van Oystaeyen, A.; Benavente, A.; van Kemenade, G.; Wäckers, F.; Jacquemyn, H. Surviving in the absence of flowers: Do nectar yeasts rely on overwintering bumblebee queens to complete their annual life cycle? FEMS Microbiol. Ecol. 2018, 94, fiy196. [Google Scholar] [CrossRef]
- Spencer, J.F.T.; Spencer, D.M. Ecology: Where Yeasts Live. In Yeasts in Natural and Artificial Habitats; Springer: Berlin/Heidelberg, Germany, 1997; pp. 33–58. [Google Scholar]
- Spencer, J.F.T.; Gorin, P.A.J.; Hobbs, G.A.; Cooke, D.A. Yeasts isolated from bumblebee honey from Western Canada: Identification with the aid of proton magnetic resonance spectra of their mannose-containing polysaccharides. Can. J. Microbiol. 1970, 16, 117–119. [Google Scholar] [CrossRef]
- Crane, E. Bee Products. Bee World 1972, 53, 38–39. [Google Scholar] [CrossRef]
- Johansson, T.S.K.; Johansson, M.P. The Honeybee Colony in Winter. Bee World 1979, 60, 155–170. [Google Scholar] [CrossRef]
- Alford, D.V. A Study of the Hibernation of Bumblebees (Hymenoptera:Bombidae) in Southern England. J. Anim. Ecol. 1969, 38, 149. [Google Scholar] [CrossRef]
- Klaps, J.; Lievens, B.; Álvarez-Pérez, S. Towards a better understanding of the role of nectar-inhabiting yeasts in plant-animal interactions. Fungal Biol. Biotechnol. 2020, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| % w/w | Honey of A. mellifera | Honey of Bombus sp. | Nectar of B. darwinii |
|---|---|---|---|
| Sucrose | 1–3 | 0.6–3 | 33.1 |
| Glucose | 30–48 | 5–54 | 1.2 |
| Fructose | 41–54 | 37–79 | 0.6 |
| Water (and other) | 13.4–26.6 | 13–30 | 63.7 |
| Component (g·L−1) | Production Medium 1,a | SL Medium 1,b | SD CSM Medium 2 | SD CSM Medium w/o C-source 3 |
|---|---|---|---|---|
| Glucose (Cargill) | 120 | / | 20 | / |
| YNB w/o AA (BD Difco) | / | 4 | 6.7 | 6.7 |
| CSM (MP biomedicals) | / | / | 0.79 | 0.79 |
| Yeast extract (Brenntag) | 4 | / | / | / |
| 3Na-citraat.2H2O (Sigma-Aldrich) | 5 | / | / | / |
| NH4Cl (Sigma-Aldrich) | 1.5 | 1.5 | / | / |
| KH2PO4 (Sigma-Aldrich) | 1 | 1 | / | / |
| K2HPO4 (Sigma-Aldrich) | 0.16 | 0.16 | / | / |
| MgSO4·7H2O (Sigma-Aldrich) | 0.7 | 0.7 | / | / |
| NaCl (Esco) | 0.5 | 0.5 | / | / |
| CaCl2·2H2O (Merck) | 0.27 | 0.27 | / | / |
| Sophorolipids | / | 20 | / | / |
| Retention Time | m/z | Identity | Acetylation | |
|---|---|---|---|---|
| 18.8 | 215 | hydroxylated fatty acid | C12:0 | |
| 19.7 | 621 | acidic SL | C18:1 | non |
| 20.3 | 623 | acidic SL | C18:0 | non |
| 20.7 | 663 | acidic SL | C18:1 | mono |
| 21 | 459 | glucolipid | C18:1 | non |
| 21.7 | 665 | acidic SL | C18:0 | mono |
| 21.9 | 459 | glucolipid | C18:1 | non |
| 22.4 | 459 | glucolipid | C18:1 | non |
| 23.4 | 705 | acidic SL | C18:1 | di |
| 24.3 | 501 | glucolipid | C18:1 | mono |
| 25.5 | 295 | hydroxylated fatty acid | C18:2 | |
| 26 | 271 | hydroxylated fatty acid | C16:0 | |
| 26.7 | 297 | hydroxylated fatty acid | C18:1 | |
| 27.1 | 299 | hydroxylated fatty acid | C18:0 | |
| 27.5 | 297 | hydroxylated fatty acid | C18:1 | |
| 29.8 | 299 | hydroxylated fatty acid | C18:0 | |
| 38.8 | 255 | fatty acid | C16:0 | |
| 41 | 283 | fatty acid | C18:0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Clercq, V.; Roelants, S.L.K.W.; Castelein, M.G.; De Maeseneire, S.L.; Soetaert, W.K. Elucidation of the Natural Function of Sophorolipids Produced by Starmerella bombicola. J. Fungi 2021, 7, 917. https://doi.org/10.3390/jof7110917
De Clercq V, Roelants SLKW, Castelein MG, De Maeseneire SL, Soetaert WK. Elucidation of the Natural Function of Sophorolipids Produced by Starmerella bombicola. Journal of Fungi. 2021; 7(11):917. https://doi.org/10.3390/jof7110917
Chicago/Turabian StyleDe Clercq, Veerle, Sophie L. K. W. Roelants, Martijn G. Castelein, Sofie L. De Maeseneire, and Wim K. Soetaert. 2021. "Elucidation of the Natural Function of Sophorolipids Produced by Starmerella bombicola" Journal of Fungi 7, no. 11: 917. https://doi.org/10.3390/jof7110917
APA StyleDe Clercq, V., Roelants, S. L. K. W., Castelein, M. G., De Maeseneire, S. L., & Soetaert, W. K. (2021). Elucidation of the Natural Function of Sophorolipids Produced by Starmerella bombicola. Journal of Fungi, 7(11), 917. https://doi.org/10.3390/jof7110917








