Effectiveness of Augmentative Biological Control of Streptomyces griseorubens UAE2 Depends on 1-Aminocyclopropane-1-Carboxylic Acid Deaminase Activity against Neoscytalidium dimidiatum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Culture and Isolation of Actinobacteria from Soil Samples
2.2. In Vitro Screening for Diffusible Antifungal and CWDE Activities
2.3. In Vivo Apple Fruit Bioassay
2.4. Qualitative and Quantitative Determination of ACCD Activity by Actinobacterial Isolates
2.5. Production of Volatile Antifungal Compounds, Siderophores and Hydrogen Cyanide
2.6. Greenhouse Experiments
2.7. Production of Antifungal Compounds and CWDEs
2.8. Quantitative Production of CWDEs by BCAs
2.9. Effect of Crude Culture Filtrates of BCAs on N. dimidiatum
2.10. Cultural, Morphological and Molecular Identification of the BCA
2.11. Container Nursery Experiments
- (i)
- Healthy controls (C): Non-inoculated control seedlings;
- (ii)
- Diseased controls (Nd): Seedlings inoculated with N. dimidiatum only;
- (iii)
- Isolate #9 treatment: Seedlings inoculated with the non-BCA, ACCD-producing isolate #9 followed by N. dimidiatum inoculation (positive control);
- (iv)
- BCA3 treatment: Seedlings inoculated with the BCA and ACCD-producing isolate #21 followed by N. dimidiatum inoculation; and
- (v)
- BCA5: Seedlings inoculated with the BCA and non-ACCD-producing isolate #45 followed by N. dimidiatum inoculation.
2.12. Extraction and Measurement of Endogenous ACC from Stem Tissues
2.13. Statistical Analyses
3. Results
3.1. In Vitro Production of Diffusible Antifungal Metabolites and CWDEs by SA and NSA
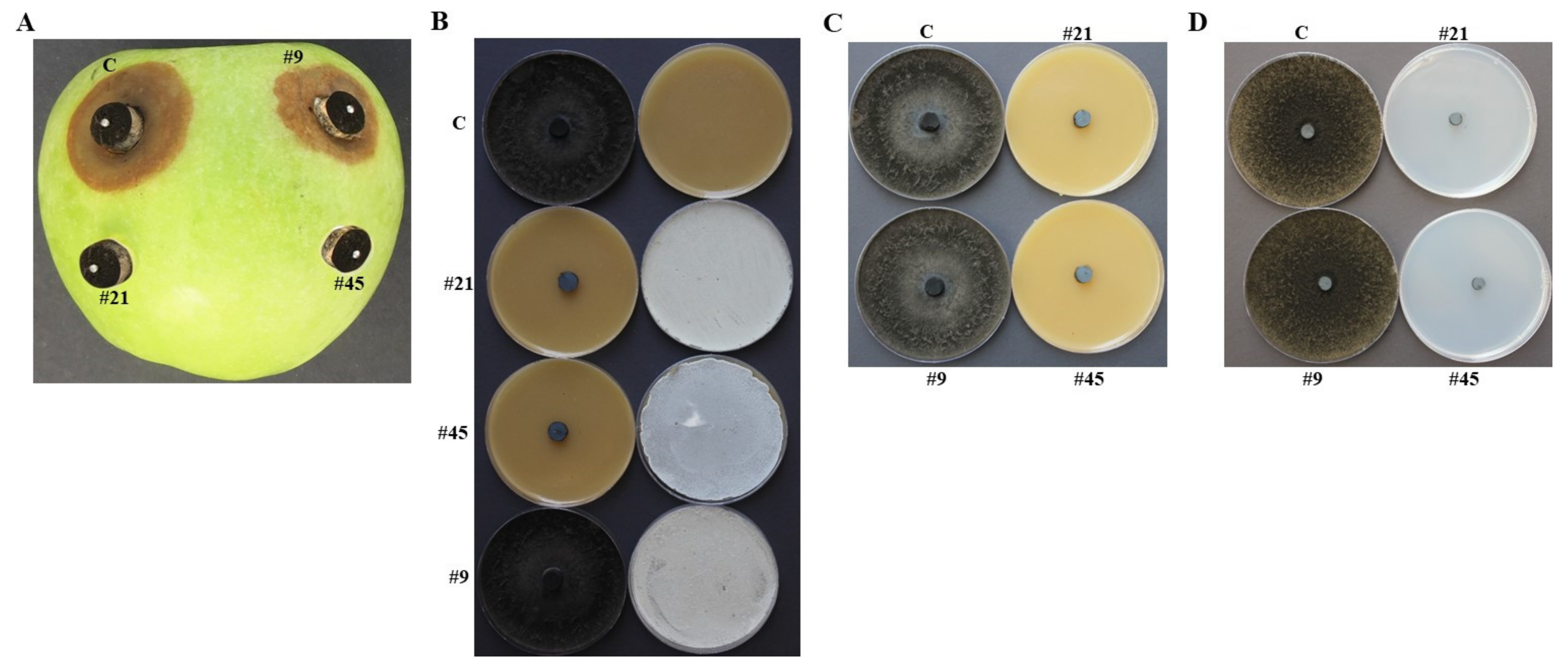
3.2. In Vivo Selection of the Most Likely BCA Candidates to N. dimidiatum
3.3. Evaluation of Potential BCA Candidates on N. dimidiatum in the Greenhouse
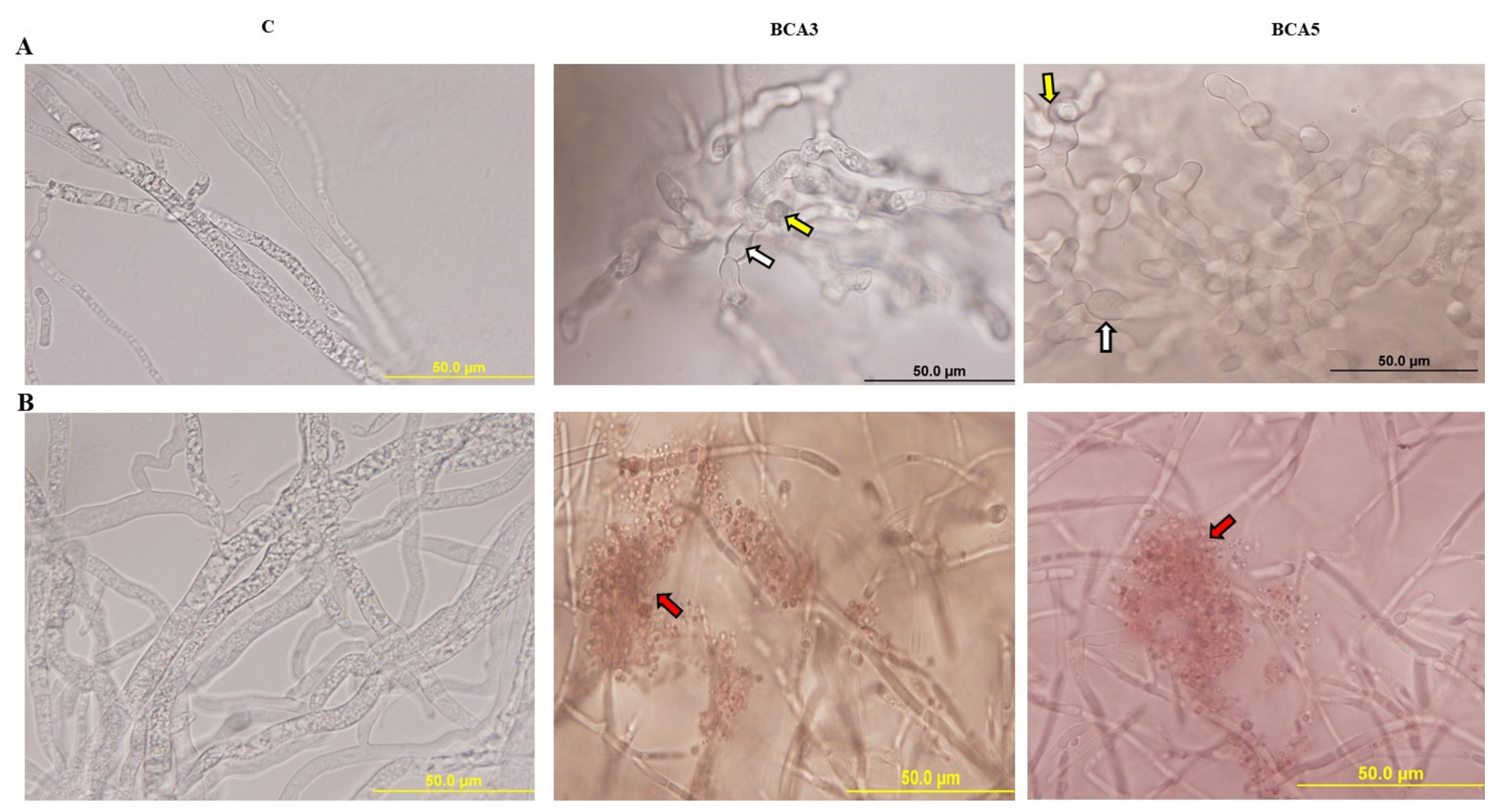
3.4. In Vitro Comparisons of Antagonistic Activities between BCA3 and BCA5
3.5. Effect of Crude Antifungal Culture Substances of BCA Candidates on N. dimidiatum
3.6. Identification and Phylogeny of the Candidate BCA Species
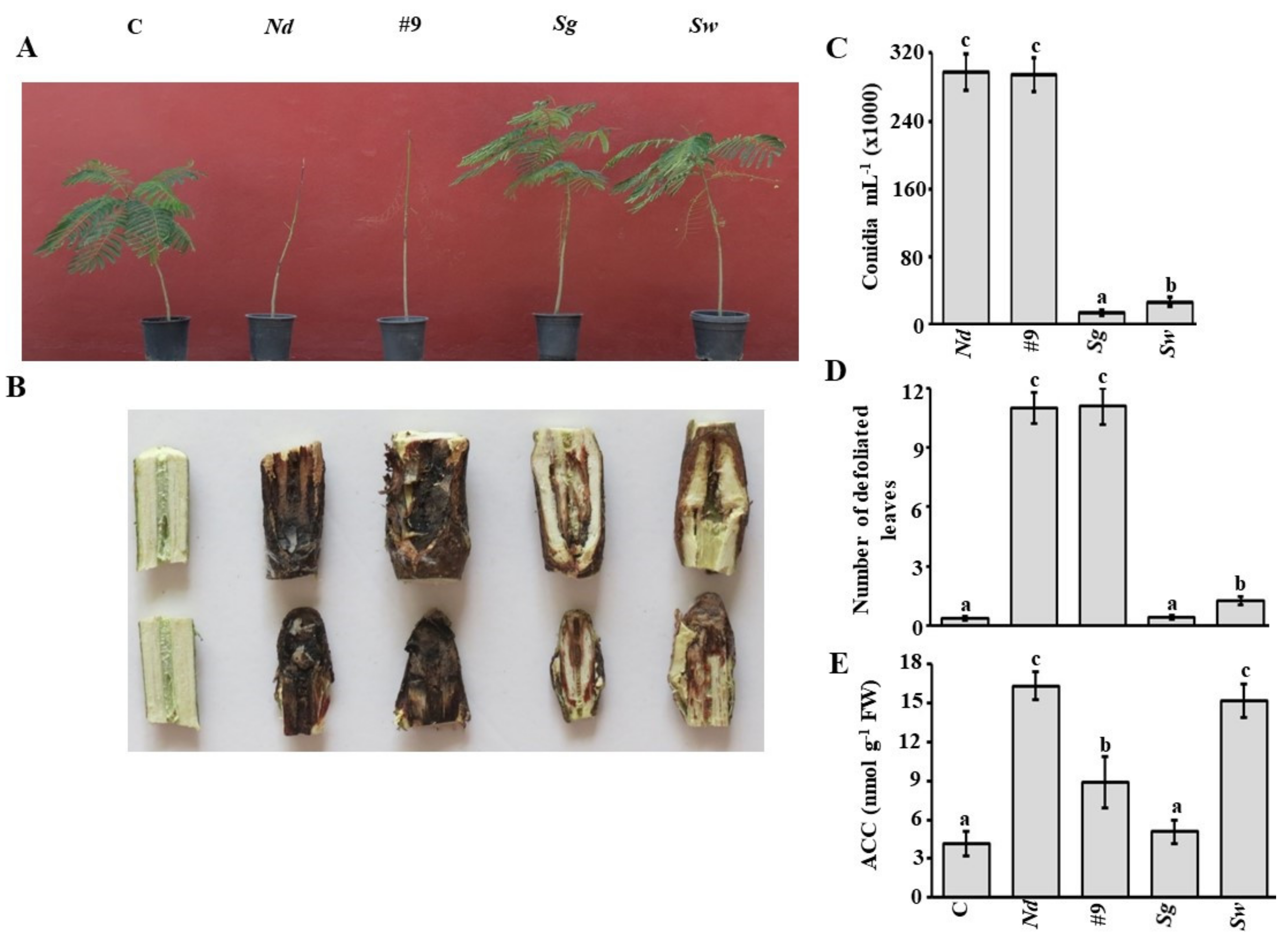
3.7. Additive Effect of S. griseorubens UAE2 on N. dimidiatum under Container Nursery Conditions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilman, E.F.; Watson, D.G.; Klein, R.W.; Koeser, A.K.; Hilbert, D.R.; McLean, D.C. Delonix Regia: Royal Poinciana; The Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2019; Available online: https://edis.ifas.ufl.edu/pdffiles/ST/ST22800.pdf (accessed on 14 October 2021).
- Ali, S.; Renderos, W.; Bevis, E.; Hebb, J.; Abbasi, P.A. Diaporthe eres causes stem cankers and death of young apple rootstocks in Canada. Can. J. Plant Pathol. 2020, 42, 218–227. [Google Scholar] [CrossRef]
- Reckhaus, P. Hendersonula dieback of mango in Niger. Plant Dis. 1987, 71, 1045. [Google Scholar] [CrossRef]
- Ray, J.D.; Burgess, T.; Lanoiselet, V.M. First record of Neoscytalidium dimidiatum and N. novaehollandiae on Mangifera indica and N. dimidiatum on Ficus carica in Australia. Australas. Plant Dis. Notes 2010, 5, 48–50. [Google Scholar] [CrossRef] [Green Version]
- Hajlaoui, M.R.; Nouri, M.T.; Hamrouni, N.; Trouillas, F.P.; Ben Yahmed, N.; Eddouzi, J.; Mnari-Hattab, M. First record of dieback and decline of plum caused by Neoscytalidium dimidiatum in Tunisia. New Dis. Rep. 2018, 38, 20. [Google Scholar] [CrossRef] [Green Version]
- Nouri, M.T.; Lawrence, D.P.; Yaghmour, M.A.; Michailides, T.J.; Trouillas, F.P. Neoscytalidium dimidiatum causing canker, shoot blight and fruit rot of almond in California. Plant Dis. 2018, 102, 1638–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Raish, S.M.; Saeed, E.E.; Sham, A.; Alblooshi, K.; El-Tarabily, K.A.; AbuQamar, S.F. Molecular characterization and disease control of stem canker on royal poinciana (Delonix regia) caused by Neoscytalidium dimidiatum in the United Arab Emirates. Int. J. Mol. Sci. 2020, 21, 1033. [Google Scholar] [CrossRef] [Green Version]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants; International Book Distributors: New Delhi, India, 1999. [Google Scholar]
- Saeed, E.E.; Sham, A.; El-Tarabily, K.A.; Abu Elsamen, F.; Iratni, R.; AbuQamar, S.F. Chemical control of black scorch disease on date palm caused by the fungal pathogen, Thielaviopsis punctulata in United Arab Emirates. Plant Dis. 2016, 100, 2370–2376. [Google Scholar] [CrossRef] [Green Version]
- Saeed, E.E.; Sham, A.; Salmin, Z.; Abdelmowla, Y.; Iratni, R.; El-Tarabily, K.A.; AbuQamar, S.F. Streptomyces globosus UAE1, a potential effective biocontrol agent for black scorch disease in date palm plantations. Front. Microbiol. 2017, 8, 1455. [Google Scholar] [CrossRef] [PubMed]
- Behlau, F.; Lanza, F.E.; da Silva Scapin, M.; Scandelai, L.H.M.; Silva, G.J., Jr. Spray volume and rate based on the tree row volume for a sustainable use of copper in the control of citrus canker. Plant Dis. 2021, 105, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Escudero, F.J.; Mercado-Blanco, J. Verticillium wilt of olive: A case study to implement an integrated management strategy to control a soil-borne pathogen. Plant Soil 2011, 344, 1–50. [Google Scholar] [CrossRef] [Green Version]
- Saeed, E.E.; Sham, A.; AbuZarqa, A.; Al Shurafa, K.A.; Al Naqbi, T.S.; Iratni, R.; El-Tarabily, K.; AbuQamar, S.F. Detection and management of mango dieback disease in the United Arab Emirates. Int. J. Mol. Sci. 2017, 18, 2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamil, F.H.; Saeed, E.E.; El-Tarabily, K.A.; AbuQamar, S.F. Biological control of mango dieback disease caused by Lasiodiplodia theobromae using streptomycete and non-streptomycete actinobacteria in the United Arab Emirates. Front. Microbiol. 2018, 9, 829. [Google Scholar] [CrossRef] [PubMed]
- Vinchira-Villarraga, D.M.; Castellanos, L.; Moreno-Sarmiento, N.; Suarez-Moreno, Z.R.; Ramos, F.A. Antifungal activity of marine-derived Paenibacillus sp. PNM200 against Fusarium oxysporum f. sp. lycopersici, the causal agent of tomato vascular wilt. Biol. Control. 2021, 154, 104501. [Google Scholar]
- AbuQamar, S.F.; Moustafa, K.; Tran, L.S. Mechanisms and strategies of plant defense against Botrytis cinerea. Crit. Rev. Biotechnol. 2017, 37, 262–274. [Google Scholar] [CrossRef]
- Ab Rahman, S.F.S.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alwan, S.F.; Hussein, H.N. Efficacy of ecofriendly biocontrol Azotobacter chroococcum and Lactobacillus rhamnosus for enhancing plant growth and reducing infection by Neoscytalidium spp. in fig (Ficus carica L.) saplings. J. Kerbala Agric. Sci. 2019, 6, 16–25. [Google Scholar]
- Rusmarini, W.; Shah, U.K.M.; Abdullah, M.P.; Mamat, S.; Hun, T.G. Identification of Trichoderma harzianum T3.13 and its interaction with Neoscytalidium dimidiatum U1, a pathogenic fungus isolated from dragon fruit (Hylocereus polyrhizus) in Malaysia. Int. J. Agric. Environ. Res. 2017, 3, 3205–3228. [Google Scholar]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Meier-Kolthoff, J.P.; Klenk, H.P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Ayswaria, R.; Vasu, V.; Krishna, R. Diverse endophytic Streptomyces species with dynamic metabolites and their meritorious applications: A critical review. Crit. Rev. Microbiol. 2020, 46, 750–758. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; Ramadan, G.A.; Elbadawi, A.A.; Hassan, A.H.; Tariq, S.; Ghazal, E.W.; Abo Gamar, M.I.; AbuQamar, S.F. The marine endophytic polyamine-producing Streptomyces mutabilis UAE1 isolated from extreme niches in the Arabian Gulf promotes the performance of mangrove (Avicennia marina) seedlings under greenhouse conditions. Front. Mar. Sci. 2021, 8, 710200. [Google Scholar] [CrossRef]
- El-Tarabily, K.A. Rhizosphere-competent isolates of streptomycete and non-streptomycete actinomycetes capable of producing cell-wall-degrading enzymes to control Pythium aphanidermatum damping-off disease of cucumber. Can. J. Bot. 2006, 84, 211–222. [Google Scholar] [CrossRef]
- Palaniyandi, S.A.; Yang, S.H.; Zhang, L.; Suh, J.W. Effects of actinobacteria on plant disease suppression and growth promotion. Appl. Microbiol. Biotechnol. 2013, 97, 9621–9636. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; Hardy, G.E.S.J.; Sivasithamparam, K.; Hussein, A.M.; Kurtböke, D.I. The potential for the biological control of cavity spot disease of carrots caused by Pythium coloratum by streptomycete and non-streptomycete actinomycetes in Western Australia. New Phytol. 1997, 137, 495–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, R.J.; Baker, K.F. The Nature and Practice of Biological Control of Plant Pathogens; The American Phytopathological Society: St. Paul, MN, USA, 1983. [Google Scholar]
- Martínez-Hidalgo, P.; García, J.M.; Pozo, M.J. Induced systemic resistance against Botrytis cinerea by Micromonospora strains isolated from root nodules. Front. Microbiol. 2015, 6, 922. [Google Scholar] [CrossRef] [Green Version]
- Al Raish, S.M.; Saeed, E.E.; Alyafei, D.M.; El-Tarabily, K.A.; AbuQamar, S.F. Evaluation of streptomycete actinobacterial isolates as biocontrol agents against royal poinciana stem canker disease caused by Neoscytalidium dimidiatum. Biol. Control. 2021. accepted. [Google Scholar]
- Glick, B.R. Promotion of plant growth by bacterial ACC deaminase. Crit. Rev. Plant Sci. 2007, 26, 227–242. [Google Scholar] [CrossRef]
- Acuna, J.J.; Campos, M.; Mora, M.L.; Jaisi, D.P.; Jorquera, M.A. ACCD-producing rhizobacteria from an Andean Altiplano native plant (Parastrephia quadrangularis) and their potential to alleviate salt stress in wheat seedlings. Appl. Soil Ecol. 2019, 136, 184–190. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [Green Version]
- El-Tarabily, K.A.; ElBaghdady, K.Z.; AlKhajeh, A.S.; Ayyash, M.M.; Aljneibi, R.S.; El-Keblawy, A.; AbuQamar, S.F. Polyamine-producing actinobacteria enhance biomass production and seed yield in Salicornia bigelovii. Biol. Fertil. Soils 2020, 56, 499–519. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; Sham, A.; Elbadawi, A.A.; Hassan, A.H.; Alhosani, B.K.K.; El-Esawi, M.A.; AbuQamar, S.F. A consortium of rhizosphere-competent actinobacteria with multiple plant growth promoting traits contributes to improve growth and performance of Avicennia marina. Front. Mar. Sci. 2021, 8, 715123. [Google Scholar] [CrossRef]
- Wang, C.X.; Knill, E.; Glick, B.R.; Defago, G. Effect of transferring 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase genes into Pseudomonas fluorescens strain CHA0 and its gacA derivative CHA96 on their growth-promoting and disease-suppressive capacities. Can. J. Microbiol. 2000, 46, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Changes in plant growth and development by rhizosphere bacteria that modify plant ethylene levels. Acta Hortic. 2004, 631, 265–273. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Vicente, C.S.L.; Barbosa, P.; Espada, M.; Glick, B.R.; Mota, M.; Oliveira, S. Evidence for the involvement of ACC deaminase from Pseudomonas putida UW4 in the biocontrol of pine wilt disease caused by Bursaphelenchus xylophilus. BioControl 2013, 58, 427–433. [Google Scholar] [CrossRef]
- Yim, W.; Seshadri, S.; Kim, K.; Lee, G.; Sa, T. Ethylene emission and PR protein synthesis in ACC deaminase producing Methylobacterium spp. inoculated tomato plants (Lycopersicon esculentum Mill.) challenged with Ralstonia solanacearum under greenhouse conditions. Plant Physiol. Biochem. 2013, 67, 95–104. [Google Scholar] [CrossRef]
- Williams, S.T.; Shameemullah, M.; Watson, E.T.; Mayfield, C.I. Studies on the ecology of actinomycetes in soil—VI. The influence of moisture tension on growth and survival. Soil Biol. Biochem. 1972, 4, 215–225. [Google Scholar] [CrossRef]
- Johnson, L.F.; Curl, E.A. Methods for Research on the Ecology of Soil-Borne Plant Pathogens; Burgess Publishing Company: Minneapolis, MN, USA, 1972. [Google Scholar]
- Küster, E. Outline of a comparative study of criteria used in characterization of the actinomycetes. Int. Bull. Bact. Nomen. Taxon. 1959, 9, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, M.; Nonomura, H. Humic acid-vitamin agar, a new medium for the selective isolation of soil actinomycetes. J. Ferment. Technol. 1987, 65, 501–509. [Google Scholar] [CrossRef]
- Nonomura, H.; Ohara, Y. Distribution of actinomycetes in soil (VI). A culture method effective for both preferential isolation and enumeration of Microbispora and Streptosporangium strains in soil (part 1). J. Ferment. Technol. 1969, 47, 463–469. [Google Scholar]
- Nonomura, H.; Hayakawa, M. New methods for the selective isolation of soil actinomycetes. In Biology of Actinomycetes; Okami, Y., Beppu, T., Oegawara, H., Eds.; Japan Scientific Societies Press: Tokyo, Japan, 1988; pp. 288–293. [Google Scholar]
- Kurtböke, D.I.; Chen, C.F.; Williams, S.T. Use of polyvalent phage for reduction of streptomycetes on soil dilution plates. J. Appl. Bacteriol. 1992, 72, 103–111. [Google Scholar] [CrossRef]
- Cross, T. Growth and examination of actinomycete-some guidelines. In Bergey’s Manual of Systematic Bacteriology; Williams, S.T., Sharpe, M.E., Holt, J.G., Eds.; Williams & Wilkins: Baltimore, MD, USA, 1989; pp. 2340–2343. [Google Scholar]
- Pridham, T.G.; Lindenfelser, L.A.; Shotwell, O.L.; Stodola, F.H.; Benedict, R.G.; Foley, C.; Jacksom, P.W.; Zaumeyer, W.J.; Perston, W.H.; Mitchell, J.W. Antibiotics against plant disease. I. Laboratory and greenhouse survey. Phytopathology 1956, 46, 568–575. [Google Scholar]
- Valois, D.; Fayad, K.; Barbasubiye, T.; Garon, M.; Dery, C.; Brzezinski, R.; Beaulieu, C. Glucanolytic actinomycetes antagonistic to Phytophthora fragariae var. rubi, the causal agent of raspberry root rot. Appl. Environ. Microbiol. 1996, 62, 1630–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.; Saxena, R.K.; Chaturvedi, P.; Virdi, J.S. Chitinase production by Streptomyces viridificans: Its potential in fungal cell wall lysis. J. Appl. Bacteriol. 1995, 78, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, M.; Foster, J. Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 1958, 75, 592–601. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.; Li, J.; Moffatt, B.A.; Glick, B.R. Isolation and characterization of ACC deaminase gene from two different plant growth-promoting rhizobacteria. Can. J. Microbiol. 1998, 44, 833–843. [Google Scholar] [CrossRef]
- Honma, M.; Shimomura, T. Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric. Biol. Chem. 1978, 42, 1825–1831. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–258. [Google Scholar] [CrossRef]
- Payne, C.; Bruce, A.; Staines, H. Yeast and bacteria as biological control agents against fungal discolouration of Pinus sylvestris blocks in laboratory-based tests and the role of antifungal volatiles. Holzforschung 2000, 54, 563–569. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Bakker, A.W.; Schippers, B. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp-mediated plant-growth stimulation. Soil Biol. Biochem. 1987, 19, 451–457. [Google Scholar] [CrossRef]
- Castric, P.A. Hydrogen cyanide, a secondary metabolite of Pseudomonas aeruginosa. Can. J. Microbiol. 1975, 21, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Bacharach, A.L.; Cuthbertson, W.F.J. The cup-plate method in microbiological assay, with special reference to riboflavine and aneurine. Analyst 1948, 73, 334–340. [Google Scholar] [CrossRef]
- Gibbs, J.N. A study of the epiphytic growth habit of Fomes annosus. Ann. Bot. 1967, 31, 755–774. [Google Scholar] [CrossRef]
- Tweddell, R.J.; Jabaji-Hare, S.H.; Charest, P.M. Production of chitinases and ß-1,3-glucanases by Stachybotrys elegans, a mycoparasite of Rhizoctonia solani. Appl. Environ. Microbiol. 1994, 60, 489–495. [Google Scholar] [CrossRef] [Green Version]
- El-Tarabily, K.A. An endophytic chitinase-producing isolate of Actinoplanes missouriensis, with potential for biological control of root rot of lupin caused by Plectosporium tabacinum. Aust. J. Bot. 2003, 51, 257–266. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Lorito, M.; Di Pietro, A.; Hayes, C.K.; Woo, S.L.; Harman, G.E. Antifungal, synergistic interaction between chitinolytic enzymes from Trichoderma harzianum and Enterobacter cloacae. Phytopathology 1993, 83, 721–728. [Google Scholar] [CrossRef]
- Sneh, B. Use of rhizosphere chitinolytic bacteria for biological control of Fusarium oxysporum f. sp. dianthi in carnation. J. Phytopathol. 1981, 100, 251–256. [Google Scholar] [CrossRef]
- Rainey, F.A.; Ward-Rainey, N.; Kroppenstedt, R.M.; Stackebrandt, E. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: Proposal of Nocardiopsaceae fam. nov. Int. J. Syst. Bacteriol. 1996, 46, 1088–1092. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl. Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locci, R. Streptomycetes and related genera. In Bergey’s Manual of Systematic Bacteriology; Williams, S.T., Sharpe, M.E., Holt, J.G., Eds.; Williams & Wilkins: Baltimore, MD, USA, 1989; pp. 2451–2508. [Google Scholar]
- Lizada, M.C.; Yang, S.F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal. Biochem. 1979, 100, 140–145. [Google Scholar]
- Lanneluc-Sanson, D.; Phan, C.T.; Granger, R.L. Analysis by reverse-phase high-pressure liquid chromatography of phenylisothiocyanate-deriderivatized 1-aminocyclopropane-1-carboxylic acid in apple extracts. Anal. Biochem. 1986, 155, 322–327. [Google Scholar] [CrossRef]
- Preobrazhenskaya, T.P.; Blinov, N.O.; Ryabova, I.D. Problems in the Classification of Antagonistic Actinomycetes; Gauze, G.F., Preobrazhenskaya, T.P., Kudrina, E.S., Blinov, N.O., Ryabova, I.D., Sveshnikova, M.A., Eds.; Government Publishing House of Medical Literature, Medgiz: Moscow, Russia, 1957; p. 68.
- Pridham, T.G.; Hesseltine, C.W.; Benedict, R.G. A guide for the classification of streptomycetes according to selected groups; placement of strains in morphological sections. Appl. Microbiol. 1958, 6, 52–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.; Zheng, J.; Xin, D.; Xin, Y.; Pang, H. Streptomyces wuyuanensis sp. nov., an actinomycete from soil. Int. J. Syst. Evol. Microbiol. 2013, 63, 2945–2950. [Google Scholar] [CrossRef] [PubMed]
- Jose, P.A.; Jebakumar, S.R.D. Non-streptomycete actinomycetes nourish the current microbial antibiotic drug discovery. Front. Microbiol. 2013, 4, 240. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, M.; Sadakata, T.; Kajiura, T.; Nonomura, H. New methods for the highly selective isolation of Micromonospora and Microbispora from soil. J. Ferment. Bioeng. 1991, 72, 320–326. [Google Scholar] [CrossRef]
- Tamura, T.; Hayakawa, M.; Hatano, K. A new genus of the order actinomycetales, Spirilliplanes gen. nov., with description of Spirilliplane syamanashiensis sp. nov. Int. J. Syst. Bacteriol. 1997, 47, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Köhl, J.; Postma, J.; Nicot, P.; Ruocco, M.; Blum, B. Stepwise screening of microorganisms for commercial use in biological control of plant-pathogenic fungi and bacteria. Biol. Control. 2011, 57, 1–12. [Google Scholar] [CrossRef]
- Al-Askar, A.A.; Rashad, Y.M.; Hafez, E.E.; Abdulkhair, W.M.; Baka, Z.A.; Ghoneem, K.M. Characterization of alkaline protease produced by Streptomyces griseorubens E44G and its possibility for controlling Rhizoctonia root rot disease of corn. Biotechnol. Equip. 2015, 29, 457–462. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, Q.H.; Nguyen, H.V.; Vu, T.H.-N.; Chu-Ky, S.; Vu, T.T.; Hoang, H.; Quach, N.T.; Bui, T.L.; Chu, H.H.; Khieu, T.N.; et al. Characterization of endophytic Streptomyces griseorubens MPT42 and assessment of antimicrobial synergistic interactions of its extract and essential oil from host plant Litsea cubeba. Antibiotics 2019, 8, 197. [Google Scholar] [CrossRef] [Green Version]
- El-Tarabily, K.A.; Nassar, A.H.; Hardy, G.E.S.J.; Sivasithamparam, K. Plant growth promotion and biological control of Pythium aphanidermatum, a pathogen of cucumber, by endophytic actinomycetes. J. Appl. Microbiol. 2009, 106, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Charles, T.C.; Glick, B.R. Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol. Biochem. 2014, 80, 160–167. [Google Scholar] [CrossRef]
- Kang, S.A.; Shahzad, R.; Bilal, S.; Khan, A.L.; Park, Y.G.; Lee, K.E.; Asaf, S.; Khan, M.A.; Lee, I.-J. Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol. 2019, 19, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abiri, R.; Shaharuddin, N.A.; Maziah, M.; Yusof, Z.N.B.; Atabaki, N.; Sahebi, M.; Valdiani, A.; Kalhori, N.; Azizi, P.; Hanafi, M.M. Role of ethylene and the APETALA 2/ethylene response factor superfamily in rice under various abiotic and biotic stress conditions. Environ. Exp. Bot. 2017, 134, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, P.K.; De, T.K.; Maiti, T.K. Role of ACC deaminase as a stress ameliorating enzyme of plant growth-promoting rhizobacteria useful in stress agriculture: A review. In Role of Rhizospheric Microbes in Soil: Volume 1: Stress Management and Agricultural Sustainability; Meena, V.S., Ed.; Springer: Singapore, 2018; pp. 57–106. [Google Scholar]
- Toklikishvili, N.; Dandurishvili, N.; Vainstein, A.; Tediashvili, M.; Giorgobiani, N.; Lurie, S.; Szegedi, E.; Glick, B.R.; Chernin, L. Inhibitory effect of ACC deaminase-producing bacteria on crown gall formation in tomato plants infected by Agrobacterium tumefaciens or A. vitis. Plant Pathol. 2010, 59, 1023–1030. [Google Scholar] [CrossRef]
- Goodfellow, M.; Williams, S. Ecology of actinomycetes. Annu. Rev. Microbiol. 1983, 37, 189–216. [Google Scholar] [CrossRef] [PubMed]


| Species | Isolate | In Vitro | In Vivo | In Vitro | |
|---|---|---|---|---|---|
| Inhibition Zone Diameter (mm) a | Clearing Zone Diameter (mm) b | Lesion Diameter (mm) c | ACCD Activity (Nmol α-Ketobutyrate/mg Protein/h) d | ||
| N. dimidiatum (negative control) | 0.00 | 0.00 | 32.34 ± 0.29 e | 0.00 a | |
| #9 (positive control) | 0.00 | 0.00 | 31.98 ± 0.32 e | 406.06 ± 13.12 e | |
| Streptomyces | #4 (BCA1) | 47.80 ± 2.22 e | 54.23 ± 1.83 d | 0.34 ± 0.04 a | 41.88 ± 2.62 b |
| #8 | 35.10 ± 0.24 c | 26.32 ± 0.18 a | 14.34 ± 0.03 c | 90.08 ± 4.04 c | |
| #10 (BCA2) | 45.26 ± 2.90 d | 53.73 ± 1.88 d | 0.16 ± 0.02 a | 0.00 a | |
| #19 | 25.45 ± 0.15 a | 26.34 ± 0.52 a | 25.16 ± 1.10 a | 81.18 ± 3.24 c | |
| #21 (BCA3) | 48.01 ± 2.07 e | 55.64 ± 1.78 d,e | 0.18 ± 0.04 a | 527.42 ± 15.84 f | |
| #23 (BCA4) | 45.94 ± 3.06 d | 54.87 ± 1.62 de | 0.86 ± 0.05 a | 0.00 a | |
| #29 | 45.76 ± 3.46 d | 56.67 ± 2.35 e | 26.36 ± 1.67 d | 0.00 a | |
| #32 | 46.98 ± 0.88 de | 27.06 ± 0.08 a | 7.40 ± 0.05 b | 0.00 a | |
| #37 | 46.78 ± 0.56 d | 37.22 ± 0.82 b | 8.16 ± 0.22 b | 80.88 ± 4.26 c | |
| #44 | 47.30 ± 2.32 e | 60.16 ± 3.04 f | 32.65 ± 1.34 | 25.40 ± 2.00 b | |
| #45 (BCA5) | 48.66 ± 0.26 e | 55.24 ± 1.08 d,e | 0.14 ± 0.02 a | 0.00 a | |
| #53 | 46.68 ± 2.54 d | 56.52 ± 0.56 e | 31.17 ± 0.61 e | 0.00 a | |
| Micromonospora | #7 | 46.17 ± 3.52 d | 52.84 ± 2.3 d | 31.18 ± 0.18 e | 30.50 ± 2.08 b |
| #42 (BCA6) | 46.62 ± 1.46 d | 55.35 ± 2.18 d,e | 0.10 ± 0.01 a | 0.00 a | |
| Actinomadura | #14 | 26.16 ± 0.17 a | 44.82 ± 1.12 c | 15.15 ± 0.34 c | 26.54 ± 1.98 b |
| Dactylosporangium | #38 (BCA7) | 46.46 ± 2.26 d | 54.84 ± 2.14 d,e | 0.25 ± 0.05 a | 280.18 ± 10.60 d |
| Streptosporangium | #41 | 35.36 ± 0.92 c | 56.86 ± 1.88 e | 6.68 ± 0.04 b | 0.00 a |
| Microbispora | #48 | 33.81 ± 1.25 c | 35.83 ± 3.46 b | 13.18 ± 0.06 c | 28.70 ± 1.66 b |
| Microtetraspora | #25 | 28.82 ± 0.76 b | 27.66 ± 0.18 a | 32.02 ± 0.10 e | 439.68 ± 13.16 e |
| Actinoplanes | #56 | 47.78 ± 0.22 e | 26.06 ± 0.12 a | 8.08 ± 1.02 b | 0.00 a |
| Media | BCA | Culture Filtrate (%) | Colony Diameter (mm) | Mycelial Dry Weight (g) | Conidia Germination (%) | Germ Tube Length (µm) |
|---|---|---|---|---|---|---|
| FMEB | BCA3 | 0 | 97.94 ± 0.64 c | 76.95 ± 3.04 c | 87.62 ± 1.16 c | 48.45 ± 2.40 c |
| 50 | 12.43 ± 1.16 b | 6.28 ± 1.56 b | 15.96 ± 2.20 b | 14.24 ± 2.03 b | ||
| 100 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.94 ± 0.26 a | 2.04 ± 0.32 a | ||
| BCA5 | 0 | 96.78 ± 0.32 c | 79.54 ± 2.74 c | 85.82 ± 1.72 c | 55.78 ± 3.00 c | |
| 50 | 10.86 ± 1.04 b | 4.96 ± 1.65 b | 17.78 ± 1.56 b | 13.34 ± 1.62 b | ||
| 100 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.74 ± 0.32 a | 1.43 ± 0.29 a | ||
| CCB | BCA3 | 0 | 96.78 ± 1.12 c | 78.22 ± 4.00 c | 91.92 ± 1.88 c | 66.60 ± 2.60 c |
| 50 | 14.86 ± 1.38 b | 12.56 ± 0.99 b | 19.28 ± 1.04 b | 9.67 ± 1.08 b | ||
| 100 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.97 ± 0.12 a | 0.00 ± 0.00 a | ||
| BCA5 | 0 | 97.12 ± 1.46 c | 80.92 ± 2.86 c | 88.62 ± 2.08 c | 62.99 ± 3.12 c | |
| 50 | 16.92 ± 0.62 b | 13.10 ± 1.66 b | 20.67 ± 0.88 b | 10.32 ± 0.75 b | ||
| 100 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 1.82 ± 0.34 a | 0.00 ± 0.00 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Hamad, B.M.; Al Raish, S.M.; Ramadan, G.A.; Saeed, E.E.; Alameri, S.S.A.; Al Senaani, S.S.; AbuQamar, S.F.; El-Tarabily, K.A. Effectiveness of Augmentative Biological Control of Streptomyces griseorubens UAE2 Depends on 1-Aminocyclopropane-1-Carboxylic Acid Deaminase Activity against Neoscytalidium dimidiatum. J. Fungi 2021, 7, 885. https://doi.org/10.3390/jof7110885
Al Hamad BM, Al Raish SM, Ramadan GA, Saeed EE, Alameri SSA, Al Senaani SS, AbuQamar SF, El-Tarabily KA. Effectiveness of Augmentative Biological Control of Streptomyces griseorubens UAE2 Depends on 1-Aminocyclopropane-1-Carboxylic Acid Deaminase Activity against Neoscytalidium dimidiatum. Journal of Fungi. 2021; 7(11):885. https://doi.org/10.3390/jof7110885
Chicago/Turabian StyleAl Hamad, Bader M., Seham M. Al Raish, Gaber A. Ramadan, Esam Eldin Saeed, Shaikha S. A. Alameri, Salima S. Al Senaani, Synan F. AbuQamar, and Khaled A. El-Tarabily. 2021. "Effectiveness of Augmentative Biological Control of Streptomyces griseorubens UAE2 Depends on 1-Aminocyclopropane-1-Carboxylic Acid Deaminase Activity against Neoscytalidium dimidiatum" Journal of Fungi 7, no. 11: 885. https://doi.org/10.3390/jof7110885
APA StyleAl Hamad, B. M., Al Raish, S. M., Ramadan, G. A., Saeed, E. E., Alameri, S. S. A., Al Senaani, S. S., AbuQamar, S. F., & El-Tarabily, K. A. (2021). Effectiveness of Augmentative Biological Control of Streptomyces griseorubens UAE2 Depends on 1-Aminocyclopropane-1-Carboxylic Acid Deaminase Activity against Neoscytalidium dimidiatum. Journal of Fungi, 7(11), 885. https://doi.org/10.3390/jof7110885







