1. Introduction
Aspergillus section
Fumigati is associated with a high mortality rate in at risk patients, such as those with asthma, cystic fibrosis, and chronic obstructive lung disease or with immune suppression, mostly due to invasive pulmonary aspergillosis—a fatal disease [
1]. It is predictable that over 30 million patients are at risk of developing invasive aspergillosis worldwide, mainly due to the use of immunosuppressive therapies [
2]. Healthcare-associated aspergillosis is most often acquired by inhalation of airborne spores causing pulmonary aspergillosis, which the fungus can disseminate in the bloodstream and reach other organs [
3]. Airway colonization by
Aspergillus spp. Has also been observed in approximately half of patients in an adult pneumology ward with no symptoms of aspergillosis [
4]. Exposure to
Aspergillus section
Fumigati can be, therefore, considered a risk for both patients and health staff in health care environments (HCE) [
5].
The genus
Aspergillus is classified into four subgenera (
Aspergillus,
Circumdati,
Fumigati, and
Nidulantes) and 20 sections, each including a number of related species [
6,
7]. The most prevalent in HCE, as in other environments, are
Aspergillus sections
Fumigati,
Flavi,
Nigri, and
Nidulantes [
8,
9]. Section
Fumigati is the most frequently isolated section both from respiratory samples from patients and from HCE sampling [
10]. Section
Fumigati is one of the most species-rich sections in the
Aspergillus genus, comprising about 50 to 60 potentially pathogenic species for humans [
11]. It is the
Aspergillus section that has the most reported clinical relevance and is more often associated with respiratory symptoms (mainly
A. fumigatus sensu stricto).
The adverse effects resulting from exposure to airborne toxigenic fungi are well known. Long-term exposure to toxigenic fungi interferes with natural killer cell activity, and may cause symptoms such as cough, fever, headache, anxiety, or depression [
12]. The induction of immunosuppression and inflammation by exposure to fungi and bioaerosols is also described [
13,
14]. An additional concern regarding
A. fumigatus sensu stricto is the emergence of acquired azole-resistance in clinical practice and in the environment [
10,
15,
16,
17,
18,
19].
Aspergillus section
Fumigati’s clinical relevance has been related to the small size of conidia and other virulence factors [
9,
20,
21]. The cytotoxicity effect of
Aspergillus section
Fumigati toxins, such as gliotoxin, has been described [
22,
23,
24]. While a more recent study described cytotoxic and apoptotic effects of
Aspergillus section
Fumigati conidia in lung epithelial cells and fibroblasts [
25].
In the present study, to evaluate the cytotoxicity of Aspergillus section Fumigati from ten primary health care centers and one central hospital, isolates were obtained by air, passive sampling and workers’ nasal swabbing and were co-cultured with lung epithelial cells and kidney cells. An MTT assay was used to determine IC50 levels, and correlational statistical analysis was performed to explore relations between isolates growth in different media, including azoles, sampling, and the cytotoxicity effect.
2. Materials and Methods
2.1. Health Care Facilities and Sampling Campaign
Ten Primary Health Care Centres (PHCC) and one Central Hospital (CH) were assessed in Lisbon and Oporto, respectively, from June to July 2018, as part of a wider study aiming to propose new procedures to determine exposure to bioburden at HCE [
26]. The project protocol was first approved by scientific councils from HCE (ref: 064/CES/INV/2017) and by the Ethical Committee of Escola Superior de Tecnologia da Saúde de Lisboa (ref: CE-ESTeSL-No 45-2018). The protocol was in accordance with the World Medical Association Declaration of Helsinki and the Oviedo Convention, and in agreement with the Portuguese law no 58/2019 of 8 August, regarding data protection [
26]. A prior evaluation by a certified exposure assessor was developed on site at each HCE to identify critical control points in workplaces which could involve higher exposure to microbial contamination. A comprehensive sampling campaign was then held, using active and passive sampling methods in both indoor environments (PHCC and CH) [
27,
28]. Active sampling comprised air sampling by impaction (N = 201). Air sampling by impinger was also performed (N = 56) for molecular detection purposes (not presented). Passive sampling included surface swabs (N = 126), electrostatic dust cloths (EDC, N = 96), settled dust (N = 15), and filters from HVAC system (N = 12) (
Table 1).
2.2. Volunteers Enrolment and Biological Sampling
Nasal swabs were collected from volunteer health care workers in the ten PHCC (N = 25) and in the CH (N = 22). A control group of 25 healthy volunteers with no occupational contact with health care facilities was also evaluated in Lisbon. All volunteers signed informed consent prior to enrollment in the study. All inherent ethical principles were duly observed. Biological samples were obtained through a nasopharyngeal swab procedure using transport swabs with Stuart media when necessary. For nasal sampling, a swab was inserted about one centimeter into the nostril and rubbed in a circular way. The same swab was then used to sample the other nostril following the same procedure. For the samples collection, transport swabs with Stuart media were used that were immediately transported to the laboratory after being used.
2.3. Fungal Culture and Screening of Azole Resistance
Malt extract agar (MEA) supplemented with chloramphenicol (0.05%), and dichloran-glycerol agar (DG18) were used to increase selectivity for fungal growth. Sabouraud dextrose agar (SDA) and SDA supplemented with 4 mg/L itraconazole (ITR), 1 mg/L voriconazole (VOR) or 0.5 mg/L posaconazole (POS) were used for screening of azole resistance (adapted from EUCAST 2018) and following the procedures already reported [
5,
18,
19]. The reference strain
A. fumigatus ATCC 204,305 was used as a negative control and a pan-azole-resistant strain was used as a positive control (both kindly provided by Reference Unit for Parasitic and Fungal Infections, Department of Infectious Diseases of the National Institute of Health, from Dr. Ricardo Jorge).
After incubation at 27 °C for 5 to 7 days (MEA and DG18) and 27 °C for 4 days (SDA, ITR, VOR, and POS), fungal burden densities found in environmental samples (colony-forming units, CFU/m
2) were calculated as previously described [
18,
27,
28]. Fungal species were identified microscopically using tease mount or Scotch tape mount and lactophenol cotton blue mount procedures. Morphological identification was achieved through macro and microscopic characteristics as noted by De Hoog [
29].
2.4. Aspergillus Section Fumigati Isolation
After identifying the Fumigati section in any of the media used, an isolate was obtained from each sample in MEA, and the one with the highest possibility of obtaining a pure culture of the Fumigati isolate was selected. Aspergillus section Fumigati isolates in MEA were retested in azole supplemented media. Before the cytotoxicity assay, Aspergillus section Fumigati was inoculated on the yeast extract glucose chloramphenicol (YGC) medium in order to revive the colony. Of note, some Fumigati isolates were unable to grow at this stage and could not be further analyzed. Then the isolates were inoculated on the Czapek’s agar (CZA) medium (final pH = 6.0 ± 0.2, at 25 °C) and grown for 10 days at 25 °C and 10 days at 6 °C. The composition of the CZA medium was as follows: sucrose-30.00 g/L, sodium nitrate-3.00 g/L, dipotassium phosphate-1.00 g/L, potassium chloride-0.50 g/L, magnesium sulphate-0.50 g/L, ferrous sulphate-0.01 g/l, Agar-15.00 g/L.
2.5. Cell Culture
Human A549 lung epithelial cells and swine kidney (SK) cells were maintained in Eagle’s minimum essential medium (MEM) supplemented with 10,000 units of penicillin and 10 mg of streptomycin per mL in 0.9% NaCl (Sigma-Aldrich, Portugal)) and fetal bovine serum (Sigma-Aldrich, USA). Cells were detached from the bottom of the culture vessel with 0.25% (w/v) Trypsin 0.53 mM EDTA, suspended in the culture medium, and the number of cells was counted using Scepter™ 2.0 cell counter (Merck).
2.6. Cytotoxicity Evaluation by the MTT Assay
The cytotoxicity effect was measured by reduction of MTT tetrazolium salt to formazan at 510 nm (Hanelt et al. 1994) in A549 and SK cells, using several dilutions of Aspergillus section Fumigati isolates. Fumigati isolates were exposed to thermal shock by being placed in the temperature of 4 °C for 96 h. From the strains of molds grown in the Petri dishes (Czapek-Dox medium) extracts were prepared to be evaporated later to dryness under a stream of nitrogen. The extracts contained the equivalent of Aspergillus section Fumigati from one Petri dish (62.5 cm2). Next, a series of test dilutions was prepared. The first dilution on assay plate was 31.25 cm2/mL. After the cell count, A549 and SK cells were transferred (100 µL) to a 96-well plate (densities of 2.5 × 105 cells/mL) and exposed to the several dilutions of Aspergillus section Fumigati isolates for 48 h at 5% CO2, 37 °C, and humid atmosphere. The lowest concentration of the isolates causing a drop in absorption to <50% of cell division activity (IC50) was considered the threshold toxicity level.
2.7. Statistical Analysis
Data were analyzed using SPSS V26.0 statistical software for windows. The results were considered significant at the 5% significance level. To characterize the sample, frequency analysis (n, %) was used for qualitative data and graphical representations appropriate to the nature of the data. To test the normality of the data, the Shapiro-Wilk test was used. To study the association between the growth of azoles (no/yes in ITR, VOR, and POS) and the medium (MEA, DG18, and SAB) the Chi-Square test by Monte Carlo simulation was used, since the assumptions of applicability of the Chi-Square test were not verified. To compare the cytotoxicity of IC50 cells (SK and A549) between the MEA and DG18 media (once in the SDA media there were only three observations) and between the growth of azoles (no/yes in ITR, VOR, and POS) the Mann–Whitney test was used. To compare the cytotoxicity of IC50 cells (SK and A459) between the type of environmental samples (air impaction, nasal swabs (PHCC), and nasal swabs (Control)—the others were not considered in the analysis, due to the small number of observations), a Kruskal-Wallis test was used.
4. Discussion
In the present study, we exposed human lung epithelial A549 cells and swine kidney (SK) cells to
Aspergillus section
Fumigati isolates from the HCE and health care workers and found that all
Aspergillus section
Fumigati isolates tested reduced cell viability, presenting a medium to high cytotoxicity effect in culture. Human lung epithelial cells were used as a model for exposure by inhalation [
31], and swine kidney cells as a model for mammal nephrotoxicity [
32], considering the reported nephrotoxicity of some
Aspergillus section
Fumigati toxins [
24].
Furthermore, we performed correlational statistical analysis and detected a higher cytotoxic effect in A549 cells, regardless the culture media used. This is particularly concerning regarding the cytotoxicity effect of
Aspergillus section
Fumigati isolates from air impaction samples and from nasal swabs of PHCC workers and controls, suggesting the inhalation route as a risk factor, especially for individuals suffering from asthma [
33] and immunocompromised individuals [
9,
34,
35].
Indeed, cytotoxic toxins of
Aspergillus section
Fumigati act on different cells to induce cell death. The cytotoxicity and apoptotic effects of gliotoxin, the main secondary metabolite of
Aspergillus section
Fumigati have been reported in macrophages [
22,
24]. Trypacidin, another toxin from
Aspergillus section
Fumigati, was also reported to have a cytotoxicity effect on lung cells [
23]. Other studies using lung epithelial cells to address the association between
Fumigati conidia and airways colonization revealed contradictory results on the pro-inflammatory effect [
36,
37].
Very few studies focus on the cytotoxicity of fungi from environments occupied by humans, namely, in HCE. Most available studies focus on the cytotoxicity of
Penicillium sp.,
Aspergillus sp. or
Stachybotrys sp. genera, mostly recovered from dwellings with infiltrations and humid environments [
38,
39,
40,
41,
42], from occupational settings [
43,
44], or even from protection devices used in high fungal load settings such as the waste sorting industry [
45,
46,
47]. One study evaluating the concentration of airborne fungi in rooms of asthma patients concluded that the home environment was a potential source of exposure to molds and a risk factor for asthma patients [
33]. A previous study revealed that 47% of the evaluated airborne fungi, collected from humid apartments in Scotland, displayed cytotoxicity in vivo [
38]. Other in vitro studies refer to the cytotoxicity of building materials as related to their contamination by molds and mycotoxins [
48].
Regarding the cytotoxicity of the
Aspergillus genera, a study by Gniadek et al. reported a low cytotoxicity effect of airborne
Aspergillus section
Flavi recovered from hospital rooms and tracheostomy tubes [
49]. A previous study comparing the cytotoxicity of indoor molds, by means of the MTT assay, concluded that IC50 for
Aspergillus section
Fumigati spores was higher than for
Aspergillus section
Nigri spores [
40], whereas several other studies describe that
Aspergillus section
Fumigati present the highest cytotoxicity among
Aspergillus species [
39,
42], including one study in a hospital environment [
50].
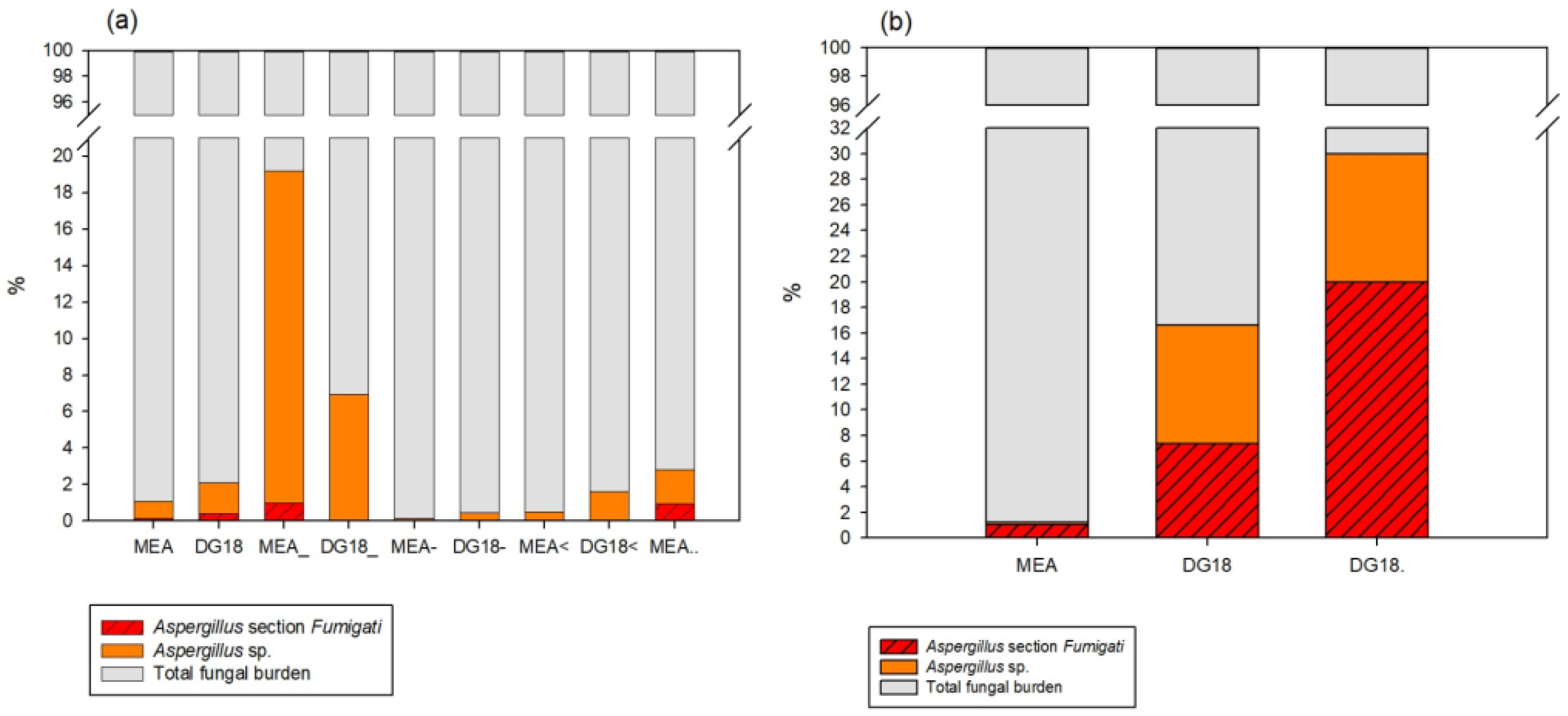
Aspergillus section
Fumigati was more prevalent in DG18 (compared to MEA), thus, supporting the use of more than one culture media in HCE assessments. Indeed, DG18 restricts the size of fungal colonies with higher growth rates that often hinder
Aspergillus sp. [
51]. Therefore, a more accurate characterization of the contamination by this
Aspergillus section should be considered [
52].
The high prevalence of section
Fumigati obtained by air impaction also highlights the risk of exposure by inhalation of airborne spores [
3]. It is known that, when suspended in the air, the 2–3 μm conidia of this
Aspergillus section can reach deeply inside the respiratory system (alveoli or the sinuses) after inhalation [
53]. Previous work on health care units evaluated the air quality of indoor hospital environments, namely, in adult and new-born intensive care units, as well as surrounding areas such as corridors and hallways, concluding that fungal spores’ contamination was within limits (750 CFU.m
−3) according to current norms [
54]. Other studies, however, refer to fungal contamination in hospital room’s frequently exceeding limits [
5,
27,
28,
55,
56,
57]. In the enlarged project where the isolates from this study were recovered, the quantification limit complied with the Portuguese legislation in most of the HCE assessed (I/O < 1), which is the cut off to avoid fungal species identification. However, a deeper analysis enabled the identification of harmful fungal species (including section
Fumigati among others), which are indicators for corrective measure implementation in the same Portuguese legal framework [
27].
Besides fungal quantification suggested in most guidelines and legislation, the identification of toxigenic fungal species is also important for risk assessment [
27,
28]. Performing regular fungal assessments, targeting for
Aspergillus section
Fumigati, may help to unveil contamination sources at HCE [
5,
58]. Moreover, the sampling approach should comprise both active (air) and passive sampling methods and be adjusted to the identified contamination sources, contextual information, and variability of the exposure [
52].
The quality guarantee of the HCE is aligned with the Sustainable Development Goals (SDGs), to ensure healthy lives and promote well-being for all at all ages (Goal 3) [
59]. Studies held in European hospitals [
60] reported that nosocomial infections significantly increase morbidity and mortality rates, with most of these infections being transmitted by airborne pathogens [
61]. These specific indoor environments also present a high risk of cross infection between staff and patients. Additionally, fungal contamination in the air and on hospital surfaces has been associated with the number of fungal infections in hospitalized immunocompromised patients [
9,
34]. Monitoring and control of microbial contamination in HCE is, therefore, mandatory as it is crucial to prevent and control hospital-acquired infections [
62,
63]. Notably, several
Aspergillus section
Fumigati isolates from the environmental sampling were also able to grow in at least one azole, including isolates from air samples able to grow in two or more different azoles. This might be particularly critical in the HCE, where patients, visitors, and staff might be exposed, and in particular more susceptible populations, such as immunocompromised individuals [
10,
15,
35]. Azole resistance must be confirmed in future studies, through antifungal susceptibility testing, for a more precise characterization of the relation between cytotoxicity and azole resistance of
Aspergillus section
Fumigati isolates collected in the environment of healthcare facilities. Unfortunately, the section
Fumigati was classified based on macroscopic and microscopic characteristics and because of that it was impossible to identify the species among the section. However, in previous studies held by the same team also with environmental isolates from different indoor environments, results revealed a good correlation between phenotypic and molecular identification [
19,
64]. Further studies should comprehend molecular identification of
Aspergillus isolates and cytotoxicity analyses.