Biological Control of Fusarium oxysporum f. sp. cubense Tropical Race 4 Using Natively Isolated Bacillus spp. YN0904 and YN1419
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. The Culture Media Was Used as Follows
2.1.2. Pathogen
2.1.3. Banana Cultivar
2.2. Isolation and Screening of Antagonistic Endophytic Bacteria against FWB
2.2.1. Isolation of Endophytic Bacteria
- (i).
- Washing the collected pseudostems with sterile water, then sterilizing with 75% ethanol by spraying after drying naturally;
- (ii).
- The 2–3 cm middle tissue of pseudostems was extracted with a sterile blade, soaked in 75% ethanol for 30 s–1 min, then transferred in 0.1% mercuric chloride for 30 s–1 min, rinsed 2–3 times with sterile water, and ground into sap with a sterile pestle and mortar;
- (iii).
- Streaking the sap on the NA medium and culturing it at 37 °C for 48 h. Then, single colonies with different morphologies were selected for purification. The isolated strains were stored at −80 °C with 50% glycerin.
2.2.2. Screening of Antagonistic Endophytic Bacteria against TR4
2.3. Effect of Antagonistic Strains on Morphology of TR4 Hyphae
2.4. Identification of Antagonistic Strains
2.4.1. Morphological Observation
2.4.2. Physiological and Biochemical Characteristics
2.4.3. Molecular Identification of Antagonistic Strains
2.5. Biological Characteristics of Antagonistic Strains
2.5.1. Effect of Temperature on Growth of Antagonistic Strains
2.5.2. Effect of pH Value on Growth of Antagonistic Strains
2.6. Greenhouse Pot Experiment
2.6.1. Pot Experiment Plantlets
2.6.2. Preparation of Fermentation Broth
2.6.3. Preparing the TR4 Spore Suspension
2.6.4. Pot Experiment
2.6.5. Investigation of TR4 Biocontrol Effects
2.6.6. Evaluation of Growth-Promoting Effects of Biocontrol Bacillus Strains on Banana Plantlets
2.7. PCR Amplification of Biocontrol and Plant Growth-Promotion Related Genes in YN0904 and YN1419
2.8. Data Analysis
3. Results
3.1. Isolation and Screening of Antagonistic Endophytic Bacteria
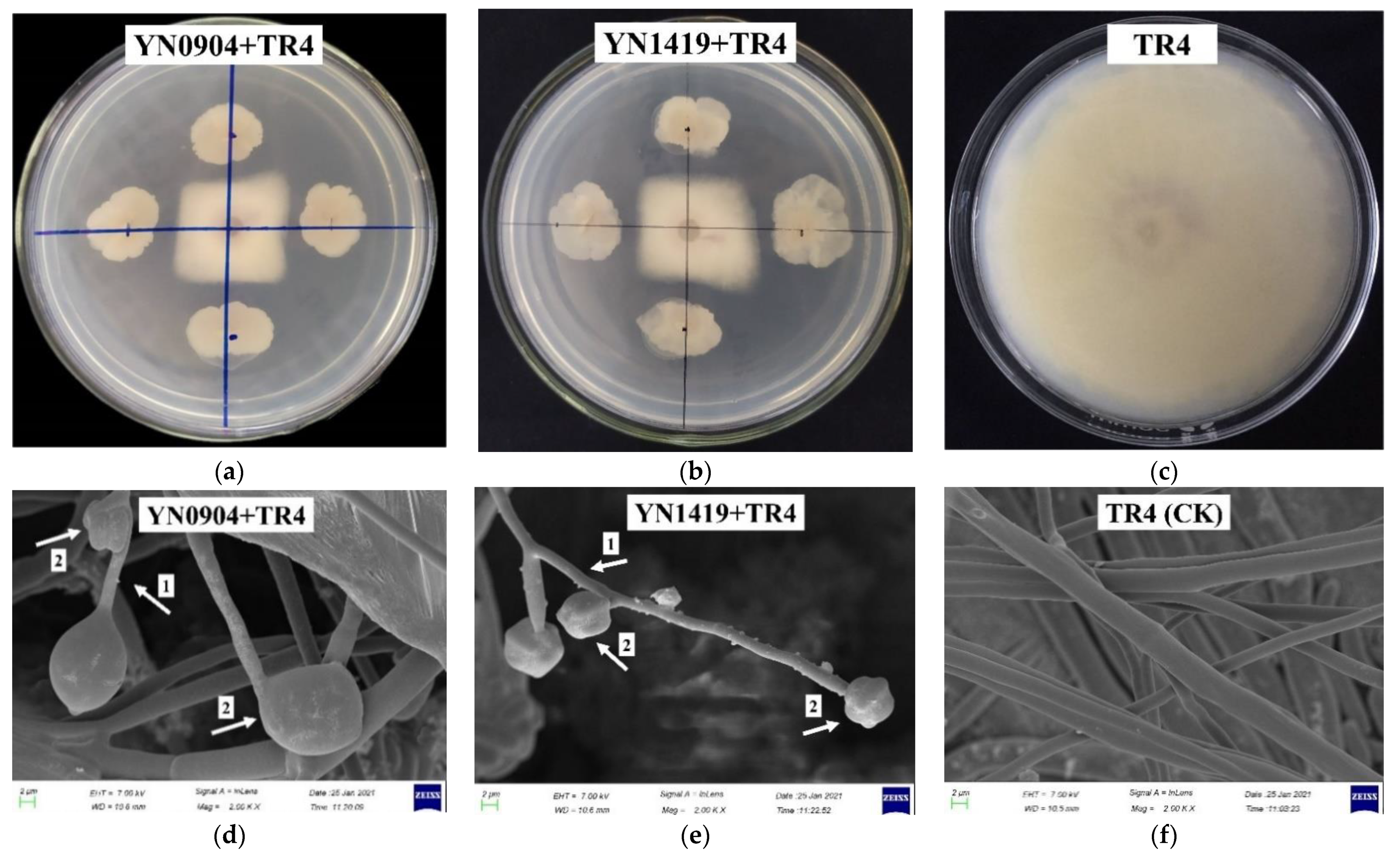
3.2. Effectiveness of Antagonistic Endophytic Bacteria In Vitro
3.3. Morphological Characteristics of Antagonistic Strains
3.4. Physiological and Biochemical Characteristics of Antagonistic Strains
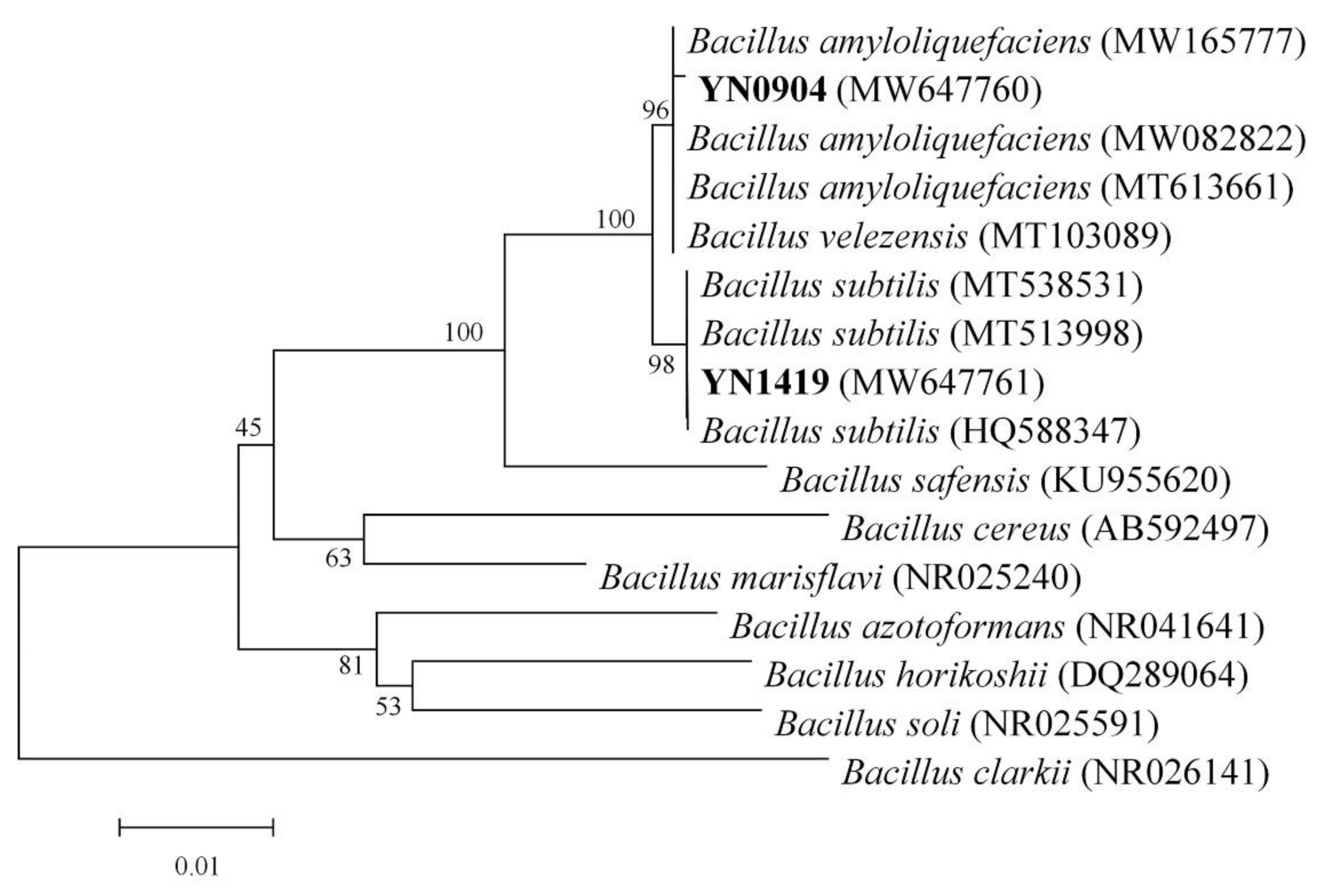
3.5. Molecular Identification of Antagonistic Bacteria
3.6. YN0904 and YN1419 Had Good Biocontrol Effects on TR4 in Pot Experiment
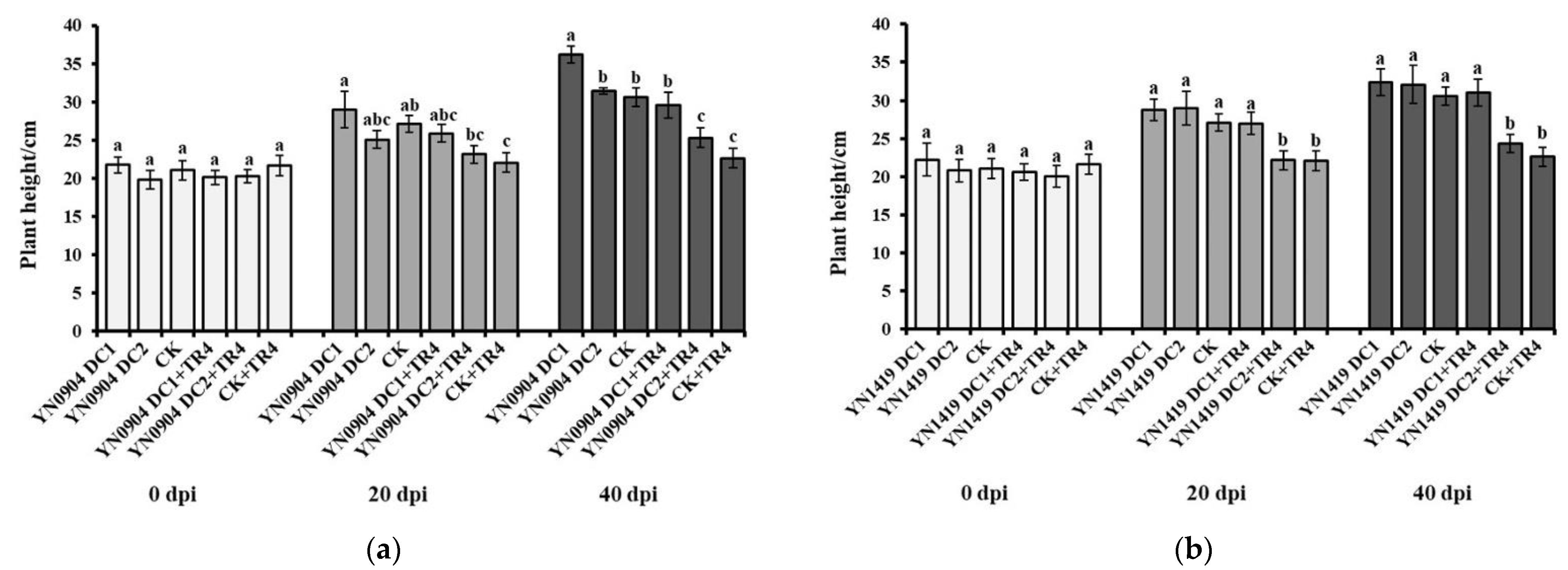
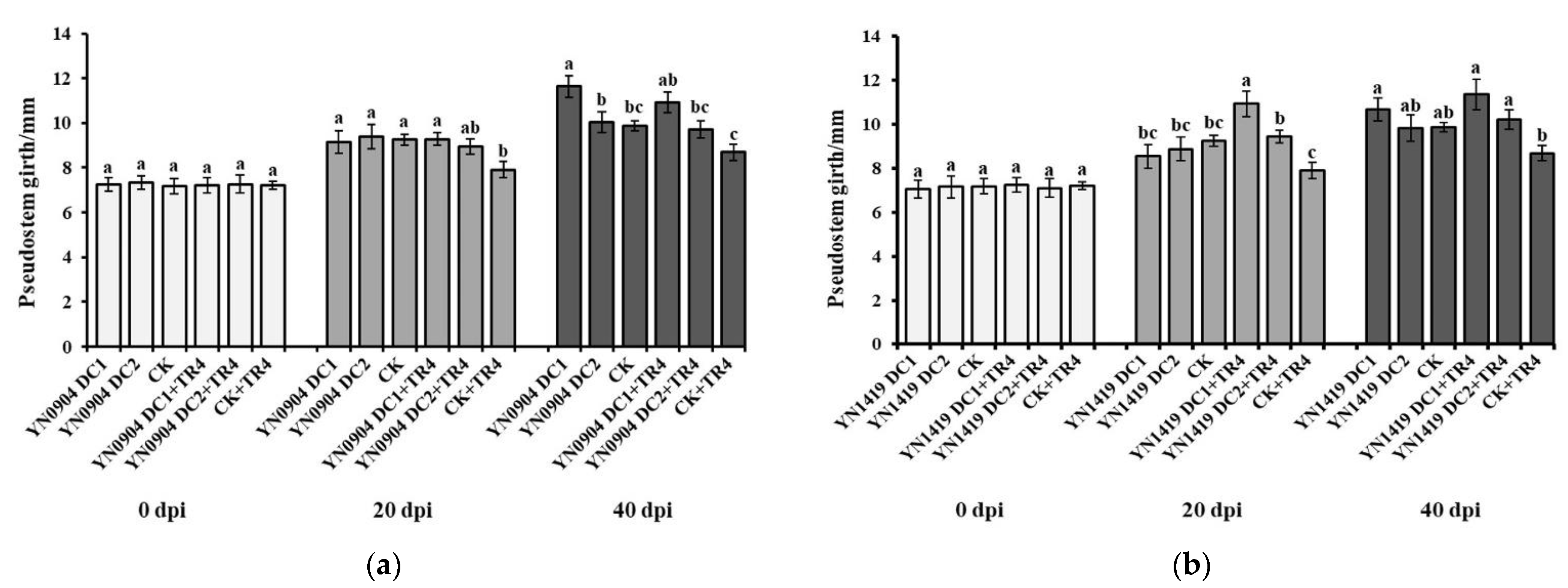
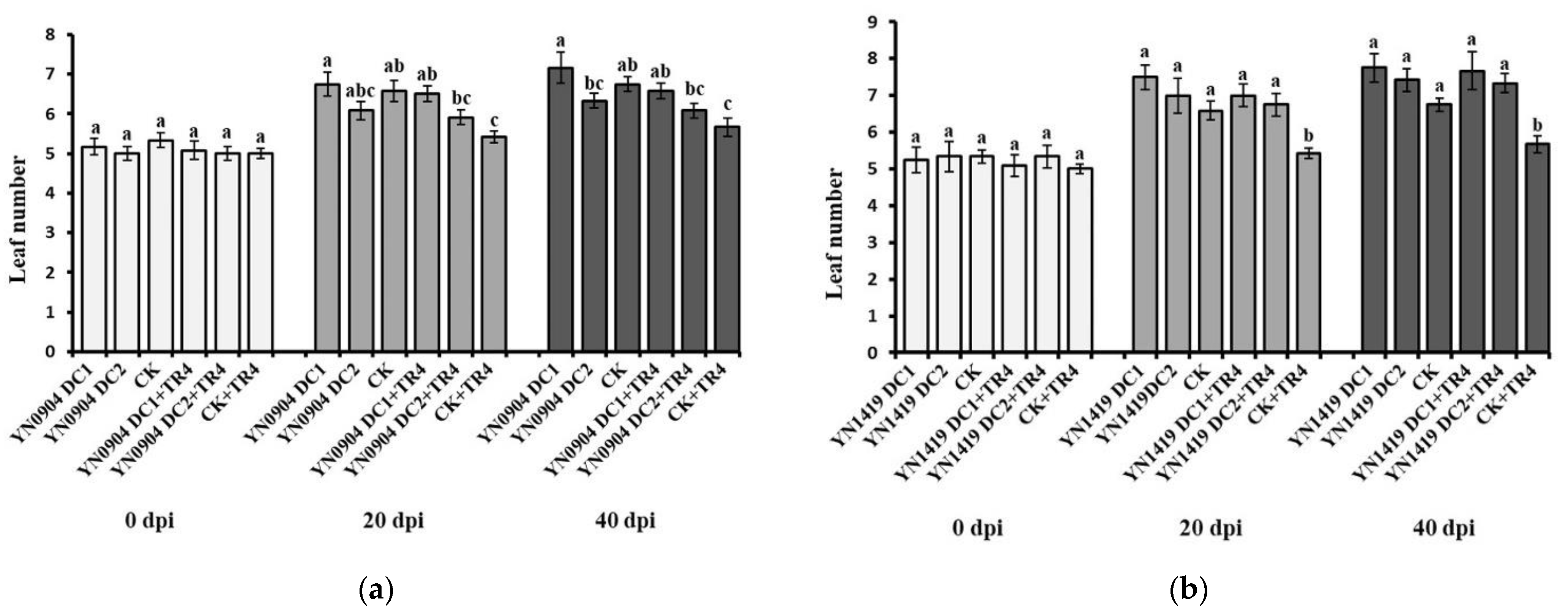
3.7. Growth-Promoting Effect of Antagonistic Bacteria on Banana Plantlets
3.8. PCR Amplification Results of Biocontrol and Plant Growth-Promotion Related Genes in YN0904 and YN1419
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bubici, G.; Kaushal, M.; Prigigallo, M.I.; Gomez-Lama Cabanas, C.; Mercado-Blanco, J. Biological Control Agents against Fusarium Wilt of Banana. Front. Microbiol. 2019, 10, 616. [Google Scholar] [CrossRef] [Green Version]
- Denoeud, F.; Carreel, F.; Aury, J.M.; Baurens, F.C.; Al, E.; D’Hont, A. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 2012, 488, 213–217. [Google Scholar]
- Li, H.-P.; Li, Y.-F.; Nie, Y.-F. Research status of occurrence and control of Fusarium wilt of banana. J. South China Agric. Univ. 2019, 40, 128–136. [Google Scholar]
- Butler, D. Fungus threatens top banana. Nature 2013, 504, 195–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.-M.; Dita, M.; Wu, W.; Hu, G.B.; Xie, J.H.; Ge, X.J. Resistance sources to Fusarium oxysporum f. sp. cubense tropical race 4 in banana wild relatives. Plant Pathol. 2015, 64, 1061–1067. [Google Scholar] [CrossRef]
- Zuo, C.-W.; Deng, G.M.; Li, B.; Huo, H.Q.; Yi, G.J. Germplasm screening of Musa spp. for resistance to Fusarium oxysporum f. sp. cubense tropical race 4 (Foc TR4). Eur. J. Plant Pathol. 2018, 151, 723–734. [Google Scholar] [CrossRef]
- Jorge, P.F.; Fu, S.L. Biological Indices for Soil Quality Evaluation: Perspectives and Limitations. Land Degrad. Dev. 2013, 27, 14–25. [Google Scholar]
- Ortiz, R. Conventional Banana and Plantain Breeding. Acta Hortic. 2013, 986, 177–194. [Google Scholar] [CrossRef]
- Somu, R.; Thammaiah, N.; Swamy, G.S.K.; Kulkarni, M.S.; Devappa, V. In vitro evaluation of fungicides against Fusarium oxysporum f. sp. cubense. Int. J. Plant Prot. 2014, 7, 221–224. [Google Scholar]
- Niwas, R.; Chand, G.; Azad, C.S. In vitro efficacy of fungicides and bioagents against Fusarium oxysporium f. sp. cubense. Ann. Plant Prot. Sci. 2020, 28, 47. [Google Scholar] [CrossRef]
- Monteiro, F.P.; Ferreira, L.C.; Silva, J.L.; Pacheco, L.P.; Souza, P.E. Influence of Plant Extracts and Essential Oils against Panama Disease (Fusarium oxysporum f. sp. cubense) in Banana Seedlings. J. Agric. Sci. 2013, 5, 63. [Google Scholar] [CrossRef]
- Huang, Z.; Liuyang, R.; Dong, C.; Lei, Y.; Zhang, A.; Lin, Y. Polymeric quaternary ammonium salt activity against Fusarium oxysporum f. sp. cubense race 4: Synthesis, structure-activity relationship and mode of action. React. Funct. Polym. 2017, 114, 13–22. [Google Scholar] [CrossRef]
- Wang, B.; Li, R.; Ruan, Y.; Ou, Y.; Zhao, Y.; Shen, Q. Pineapple–banana rotation reduced the amount of Fusarium oxysporum more than maize–banana rotation mainly through modulating fungal communities. Soil Biol. Biochem. 2015, 86, 77–86. [Google Scholar] [CrossRef]
- Zhang, H.; Mallik, A.; Zeng, R.S. Control of Panama Disease of Banana by Rotating and Intercropping with Chinese Chive (Allium Tuberosum Rottler): Role of Plant Volatiles. J. Chem. Ecol. 2013, 39, 243–252. [Google Scholar] [CrossRef]
- Khan, B.; Akash, Z.; Asad, S.; Javed, N.; Atif, R.M. Antagonistic potential of Trichoderma harzianum against Fusarium oxysporum f. sp. cubense associated with Panama Wilt of banana. Pak. J. Phytopathol. 2017, 29, 111–116. [Google Scholar] [CrossRef]
- Zacky, F.A.; Ting, A.S.Y. Biocontrol of Fusarium oxysporum f. sp. cubense tropical race 4 by formulated cells and cell-free extracts of Streptomyces griseusin sterile soil environment. Biocontrol Sci. Technol. 2015, 25, 685–696. [Google Scholar] [CrossRef]
- Li, X.-J.; Li, K.; Zhou, D.B.; Zhang, M.Y.; Qi, D.F.; Jing, T.; Zang, X.P.; Qi, C.L.; Wang, W.; Xie, J.H. Biological control of banana wilt disease caused by Fusarium oxyspoum f. sp. cubense using Streptomyces sp. H4. Biol. Control 2021, 155, 104524. [Google Scholar] [CrossRef]
- Qi, D.-F.; Zou, L.P.; Zhou, D.B.; Chen, Y.F.; Gao, Z.F.; Feng, R.J.; Zhang, M.Y.; Li, K.; Xie, J.H.; Wang, W. Taxonomy and Broad-Spectrum Antifungal Activity of Streptomyces sp. SCA3-4 Isolated from Rhizosphere Soil of Opuntia stricta. Front. Microbiol. 2019, 10, 1390. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhao, Y.K.; Zhou, D.B.; Qi, D.F.; Li, K.; Tang, W.; Chen, Y.F.; Jing, T.; Zang, X.P.; Xie, J.H.; et al. A Newly Isolated Streptomyces sp. YYS-7 With a Broad-Spectrum Antifungal Activity Improves the Banana Plant Resistance to Fusarium oxysporum f. sp. cubense Tropical Race 4. Front. Microbiol. 2020, 11, 1712. [Google Scholar] [CrossRef]
- Nel, B.; Steinberg, C.; Labuschagne, N.; Viljoen, A. The potential of nonpathogenic Fusarium oxysporum and other biological control organisms for suppressing fusarium wilt of banana. Plant Pathol. 2006, 55, 217–223. [Google Scholar] [CrossRef]
- Li, S.; He, P.; Fan, H.C.; Liu, L.N.; Yin, K.S.; Yang, B.M.; Li, Y.P.; Huang, S.M.; Li, X.D.; Zheng, S.J. A Real-Time Fluorescent Reverse Transcription Quantitative PCR Assay for Rapid Detection of Genetic Markers’ Expression Associated with Fusarium Wilt of Banana Biocontrol Activities in Bacillus. J. Fungi 2021, 7, 353. [Google Scholar] [CrossRef] [PubMed]
- Ongena, M.; Jourdan, E.; Adam, A.; Paquot, M.; Brans, A.; Joris, B.; Arpigny, J.L.; Thonart, P. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ. Microbiol. 2007, 9, 1084–1090. [Google Scholar] [CrossRef]
- Sansinenea, E.; Ortiz, A. Secondary metabolites of soil Bacillus spp. Biotechnol. Lett. 2011, 33, 1523–1538. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Gardener, M.S. Identification and Characterization of Novel Genetic Markers Associated with Biological Control Activities in Bacillus subtilis. Phytopathology 2006, 96, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, J.; Kim, J.; Shea, P.J.; Oh, B. IAA production by Bacillus sp. JH 2-2 promotes Indian mustard growth in the presence of hexavalent chromium. J. Basic Microbiol. 2015, 55, 652–658. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, T.L.; Wang, Y.Z.; Zhang, D.; Zheng, S.J. Identification and evaluation of resistance to Fusarium oxysporum f. sp. cubense tropical race 4 in Musa acuminata Pahang. Euphytica 2018, 214, 106. [Google Scholar] [CrossRef]
- Fang, Z.D. Research Methodology of Plant Diseases, 3rd ed.; China Agriculture Press: Beijing, China, 1998. [Google Scholar]
- Kumari, S.; Chetty, D.; Ramdhani, N.; Bux, F. Phenol degrading ability of Rhodococcus pyrinidivorans and Pseudomonas aeruginosa isolated from activated sludge plants in South Africa. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2013, 48, 947–953. [Google Scholar] [CrossRef]
- Xu, S.-T.; Bai, T.T.; Zhang, L.; Fan, H.C.; Yang, P.W.; Yin, K.S.; Zeng, L.; Li, X.D.; Guo, Z.X.; Yang, B.M.; et al. Evaluation of Different Banana Varieties on Fusarium Wilt TR4 Resistance by Phenotypic Symptom and Real-time Quantitative PCR. Southwest China J. Agric. Sci. 2017, 30, 1997–2002. [Google Scholar]
- Chen, A.; Sun, J.M.; Matthews, A.; Armas-Egas, L.; Chen, N.; Hamill, S.; Mintoff, S.; Tran-Nguyen, L.T.T.; Batley, J.; Aitken, E.A.B. Assessing Variations in Host Resistance to Fusarium oxysporum f sp. cubense Race 4 in Musa Species, With a Focus on the Subtropical Race 4. Front. Microbiol. 2019, 10, 1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izquierdo-Garcia, L.F.; Carmona, S.L.; Zuluaga, P.; Rodriguez, G.; Dita, M.; Betancourt, M.; Soto-Suarez, M. Efficacy of Disinfectants against Fusarium oxysporum f. sp. cubense Tropical Race 4 Isolated from La Guajira, Colombia. J. Fungi 2021, 7, 297. [Google Scholar] [CrossRef]
- Ryan, R.P.; Germaine, K.; Franks, A.; Ryan, D.J.; Dowling, D.N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008, 278, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tontou, R.; Gaggia, F.; Baffoni, L.; Devescovi, G.; Venturi, V.; Giovanardi, D.; Stefani, E. Molecular characterisation of an endophyte showing a strong antagonistic activity against Pseudomonas syringae pv. actinidiae. Plant Soil 2015, 405, 97–106. [Google Scholar] [CrossRef]
- Cheng, X.-K.; Ji, X.X.; Ge, Y.Z.; Li, J.J.; Qi, W.Z.; Qiao, K. Characterization of antagonistic Bacillus methylotrophicus isolated from rhizosphere and its biocontrol effects on maize stalk rot. Phytopathology 2019, 109, 571–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elshaghabee, F.; Namita, R.; Gulhane, R.D.; Chetan, S.; Harsh, P. Bacillus As Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Wang, M.; Zheng, Y.; Luo, J.M.; Yang, X.R.; Wang, X.L. Quantitative changes of plant defense enzymes and phytohormone in biocontrol of cucumber Fusarium wilt by Bacillus subtilis B579. World J. Microbiol. Biotechnol. 2010, 26, 675–684. [Google Scholar] [CrossRef]
- Ömür, B.; Lai, D.; Xu, H.H.; Siragusa, M.; Çalışkan, M.; Carimi, F.; Silva, J.A.T.D.; Tör, M. A Proteomic Approach Provides New Insights into the Control of Soil-Borne Plant Pathogens by Bacillus Species. PLoS ONE 2013, 8, e53182. [Google Scholar]
- Yu, X.; Ai, C.X.; Li, X.; Zhou, G.F. The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur. J. Soil Biol. 2011, 47, 138–145. [Google Scholar] [CrossRef]
- Wang, B.; Yuan, J.; Zhang, J.; Shen, Z.Z.; Zhang, M.X.; Li, R.; Ruan, Y.Z.; Shen, Q.R. Effects of novel bioorganic fertilizer produced by Bacillus amyloliquefaciens W19 on antagonism of Fusarium wilt of banana. Biol. Fertil. Soils 2013, 49, 435–446. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Ruan, Y. Effects of Bio-organic Fertilizers Produced by Four Bacillus amyloliquefaciens Strains on Banana Fusarium Wilt Disease. Compos. Sci. Util. 2015, 23, 185–198. [Google Scholar] [CrossRef]
- Chao, X.; Penton, C.R.; Shen, Z.Z.; Zhang, R.F.; Huang, Q.W.; Li, R.; Ruan, Y.Z.; Shen, Q.R. Manipulating the banana rhizosphere microbiome for biological control of Panama disease. Sci. Rep. 2015, 5, 11124. [Google Scholar]
- Zhu, S.; Liu, X.B.; Cai, J.M.; Chen, Y.P.; Huang, G.X. Isolation of Endophytic Bacterial BEB33 and Its Bio-control Evaluation against Banana Fusarium Wilt. Chin. J. Trop. Crop. 2014, 35, 1177. [Google Scholar]
- Soares, M.A.; Li, H.; Bergen, M.; Silva, J.M.D.; Kowalski, K.P.; White, J.F. Functional role of an endophytic Bacillus amyloliquefaciens in enhancing growth and disease protection of invasive English ivy (Hedera helix L.). Plant Soil 2016, 405, 107–123. [Google Scholar] [CrossRef]
- Asaturova, A.; Razina, A.; Dyatlova, O.; Esaulenko, E. Biological protection of spring wheat from root rot in the forest-steppe zone of Eastern Siberia. BIO Web Conf. 2020, 21, 00034. [Google Scholar]
- Estep, M.C.; Gowda, B.S.; Huang, K.; Timko, M.P.; Bennetzen, J.L. Genomic Characterization for Parasitic Weeds of the Genus Striga by Sample Sequence Analysis. Plant Genome 2012, 5, 30–41. [Google Scholar] [CrossRef]
- Aranda, F.J.; Teruel, J.A.; Ortiz, A. Further aspects on the hemolytic activity of the antibiotic lipopeptide iturin A. Biochim. Biophys. Acta 2005, 1713, 51–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koumoutsi, A.; Chen, X.; Henne, A.; Liesegang, H.; Hitzeroth, G.; Franke, P.; Vater, J.; Borriss, R. Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J. Bacteriol. 2004, 186, 1084–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Shao, J.H.; Li, B.; Yan, X.; Shen, Q.R.; Zhang, R.F. Contribution of bacillomycin D in Bacillus amyloliquefaciens SQR9 to antifungal activity and biofilm formation. Appl. Environ. Microbiol. 2013, 79, 808–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sang, J.; Yang, Y.; Chen, Y.P.; Cai, J.M.; Liu, C.M.; Huang, G.X. Antibacterial activity analysis of lipopeptide and polyketide compounds produced by endophytic bacteria Bacillus amyloliquefaciens BEB17. Acta Phytopathol. Sin. 2018, 48, 402–412. [Google Scholar]
- Huang, T.; Geng, H.; Miyyapuram, V.R.; Sit, C.S.; Vederas, J.C.; Nakano, M.M. Isolation of a variant of subtilosin a with hemolytic activity. J. Bacteriol. 2009, 191, 5690–5696. [Google Scholar] [CrossRef] [Green Version]


| Group | Treatment |
|---|---|
| I | Inoculation of fermentation broth + spore suspension of TR4 (DC1 + TR4) |
| II | Inoculation of 10 times diluted fermentation broth + spore suspension of TR4 (DC2 + TR4) |
| III | Inoculation of blank LB liquid medium+ spore suspension of TR4 (CK + TR4) |
| IV | Inoculation of fermentation broth only (DC1) |
| V | Inoculation of 10 times diluted fermentation broth only (DC2) |
| VI | Inoculation of blank LB liquid medium only (CK) |
| Strains | Diameter of TR4 Colony (cm) | Inhibition Rate (%) |
|---|---|---|
| YN0904 | 1.8 ± 0.17 b | 79.6 ± 0.11 ** |
| YN1419 | 1.7 ± 0.17 c | 81.3 ± 0.22 ** |
| CK | 9.0 ± 0.03 a | / |
| Item Tested | Reaction | Item Tested | Reaction | ||
|---|---|---|---|---|---|
| YN0904 | YN1419 | YN0904 | YN1419 | ||
| Gram stain | + | + | D-Sorbitol | + | + |
| Sucrose | + | + | D-Mannitol | + | + |
| α-D-Glucose | + | + | 1% NaCl | + | + |
| D-Maltose | + | + | 4% NaCl | + | + |
| Fructose | + | + | 8% NaCl | + | + |
| Mannose | + | + | Amylose hydrolysis | + | + |
| Treatment | Disease Index | Control Effect (%) | ||
|---|---|---|---|---|
| Corm | Leaf | Corm | Leaf | |
| YN0904 DC1 + TR4 | 14.58 ± 2.08 c | 12.50 ± 3.61 c | 74.26 ± 2.27 a | 82.58 ± 4.61 a |
| YN0904 DC2 + TR4 | 18.75 ± 3.61 c | 20.83 ± 2.08 bc | 67.22 ± 4.33 a | 70.71 ± 2.02 ab |
| YN1419 DC1 + TR4 | 16.67 ± 2.08 c | 10.42 ± 4.17 c | 70.09 ± 4.41 a | 85.61 ± 5.30 a |
| YN1419 DC2 + TR4 | 37.50 ± 6.25 b | 31.25 ± 6.25 b | 32.87 ± 11.46 b | 56.30 ± 7.34 b |
| CK + TR4 | 56.25 ± 3.61 a | 70.83 ± 2.08 a | ||
| Category | Metabolites | Synthesis Gene | YN0904 | YN1419 |
|---|---|---|---|---|
| Non-ribosomal peptide synthetases (NRPS) | Surfactin | srfAA | − | − |
| Fengycin | fenD | + | − | |
| Iturin | ituC | + | − | |
| bacillomycine D | bamD | + | − | |
| yngG | yngG | + | − | |
| yngJ | yndJ | + | − | |
| Bacillibactin | dhb | + | − | |
| Polytide synthetases (PKS) | Difficidin | dfn | + | − |
| Bacillaene | bae | + | − | |
| Macrolactin | mln | − | − | |
| Bacilysin | bac | + | − | |
| Ribosomal peptide synthetases (RPS) | Subtilosin | sboA | − | + |
| Auxin | ysnE | + | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, H.; Li, S.; Zeng, L.; He, P.; Xu, S.; Bai, T.; Huang, Y.; Guo, Z.; Zheng, S.-J. Biological Control of Fusarium oxysporum f. sp. cubense Tropical Race 4 Using Natively Isolated Bacillus spp. YN0904 and YN1419. J. Fungi 2021, 7, 795. https://doi.org/10.3390/jof7100795
Fan H, Li S, Zeng L, He P, Xu S, Bai T, Huang Y, Guo Z, Zheng S-J. Biological Control of Fusarium oxysporum f. sp. cubense Tropical Race 4 Using Natively Isolated Bacillus spp. YN0904 and YN1419. Journal of Fungi. 2021; 7(10):795. https://doi.org/10.3390/jof7100795
Chicago/Turabian StyleFan, Huacai, Shu Li, Li Zeng, Ping He, Shengtao Xu, Tingting Bai, Yuling Huang, Zhixiang Guo, and Si-Jun Zheng. 2021. "Biological Control of Fusarium oxysporum f. sp. cubense Tropical Race 4 Using Natively Isolated Bacillus spp. YN0904 and YN1419" Journal of Fungi 7, no. 10: 795. https://doi.org/10.3390/jof7100795
APA StyleFan, H., Li, S., Zeng, L., He, P., Xu, S., Bai, T., Huang, Y., Guo, Z., & Zheng, S.-J. (2021). Biological Control of Fusarium oxysporum f. sp. cubense Tropical Race 4 Using Natively Isolated Bacillus spp. YN0904 and YN1419. Journal of Fungi, 7(10), 795. https://doi.org/10.3390/jof7100795






