The Effects of Major Mushroom Bioactive Compounds on Mechanisms That Control Blood Glucose Level
Abstract
1. Introduction
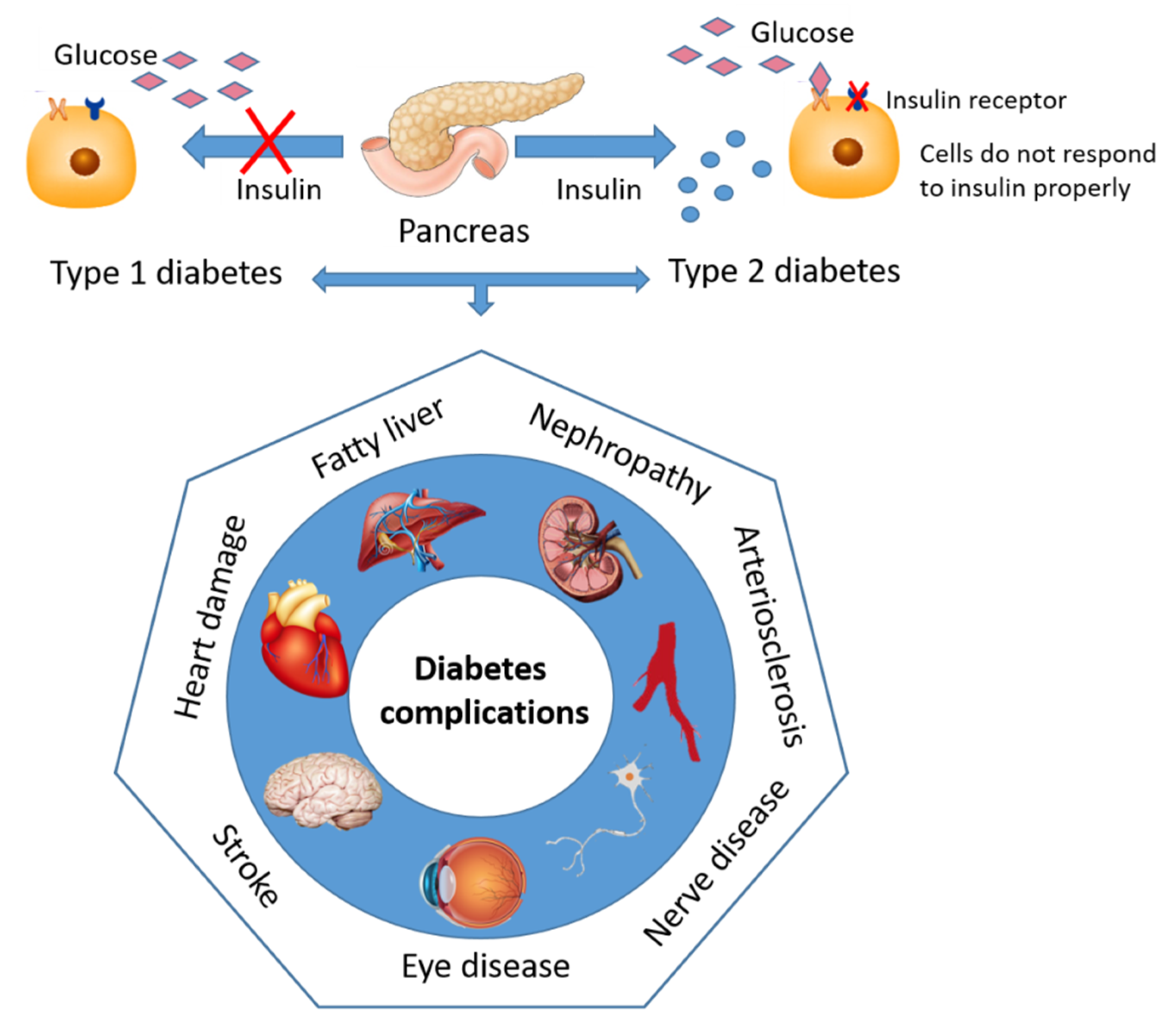
2. Diabetes—Sweet and Silent Killer but Not Unbeatable
Contemporary Drug Therapy for Diabetes and Major Adverse Effect
3. Major Bioactive Components of Mushrooms in the Treatment of Diabetes
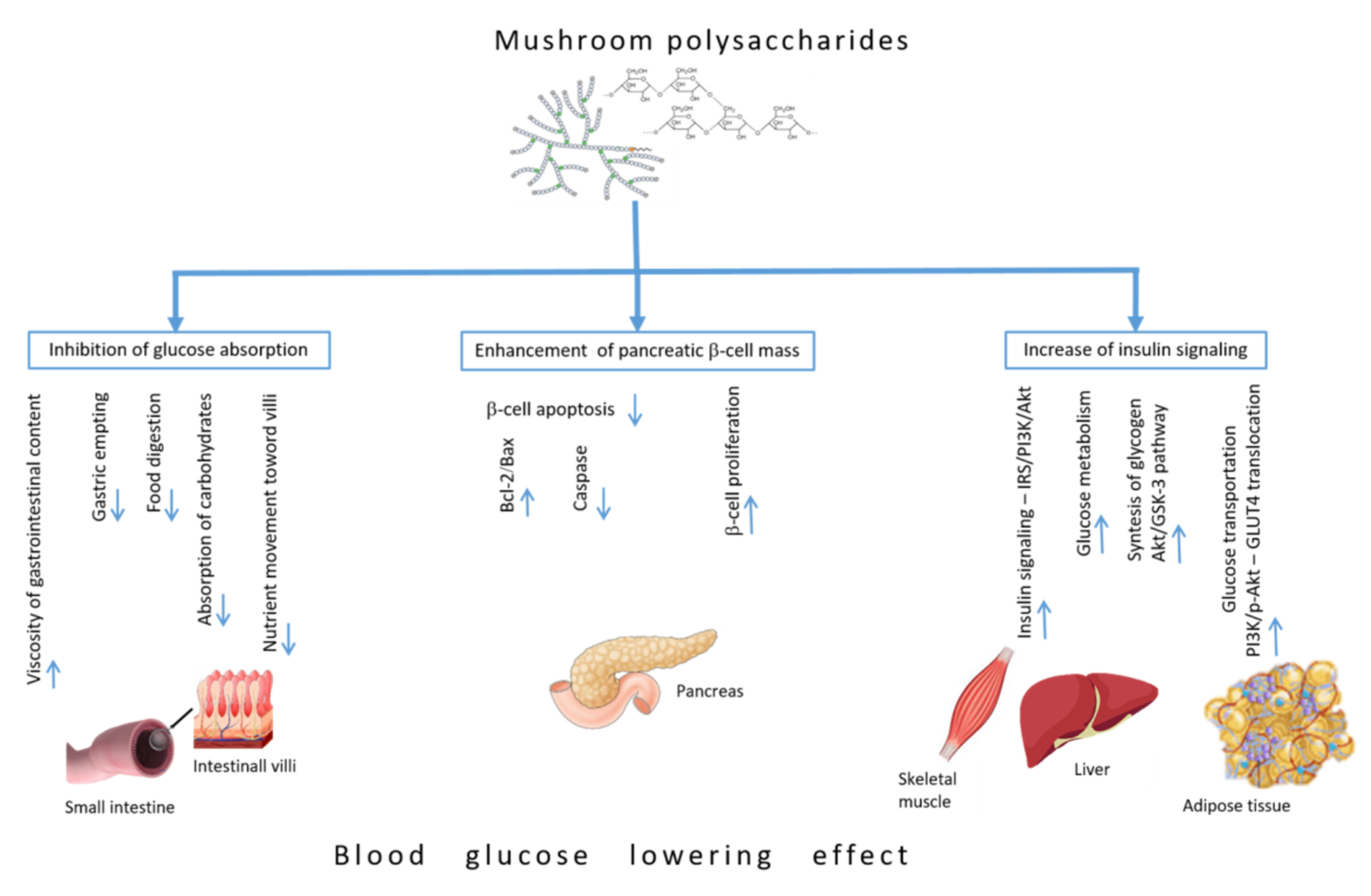
4. Blood Glucose-Lowering Mechanisms of Polysaccharides
4.1. Inhibiting Glucose Absorption Efficacy
4.2. Enhancement of Pancreatic β-Cell Mass
4.3. Increase of Insulin Signaling Pathways
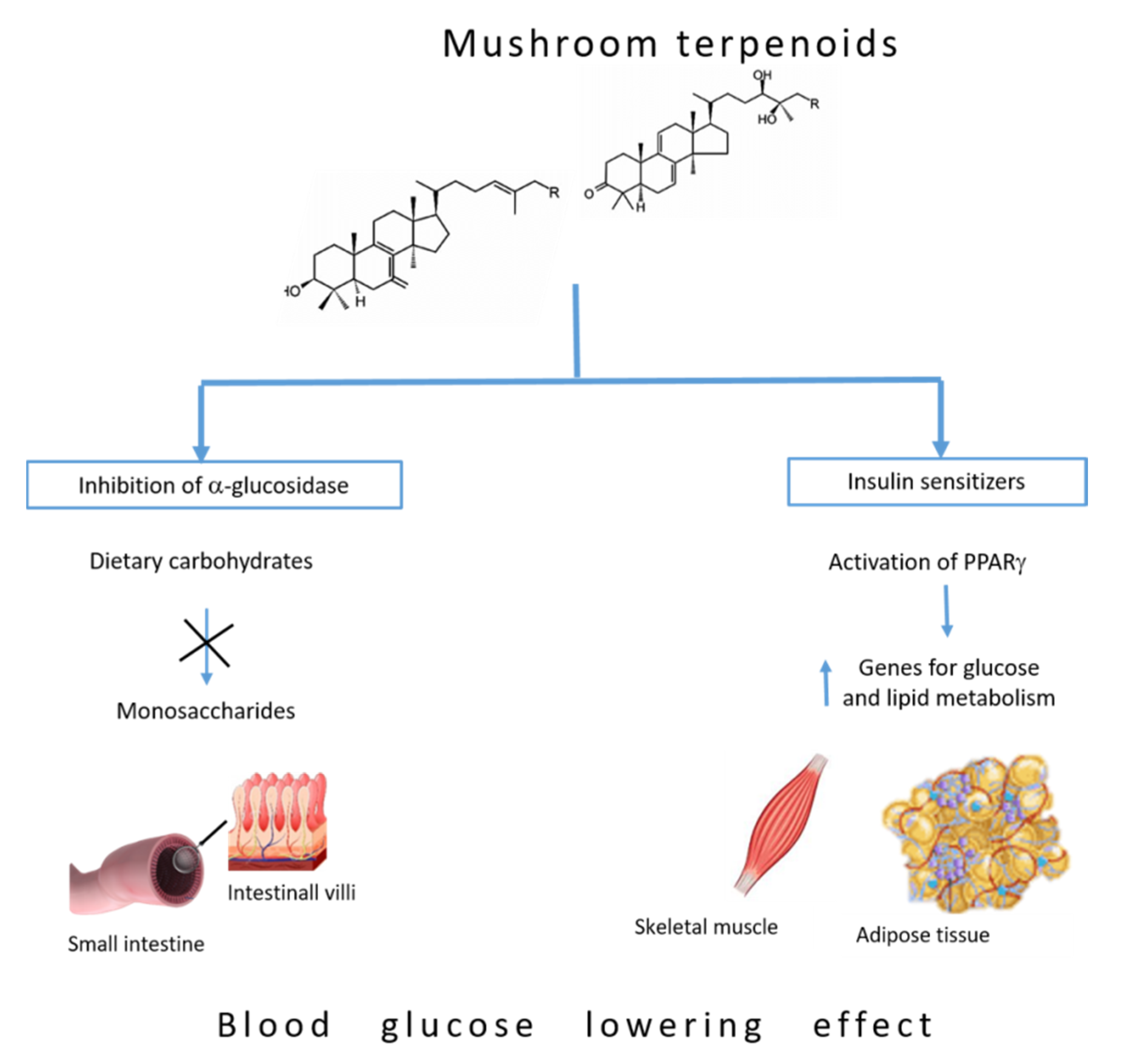
5. Blood Glucose-Lowering Mechanism of Terpenoids
5.1. Inhibition of α-Glucosidase
5.2. Insulin Sensitizers
6. Mushrooms as Drug Therapy for Diabetes—Overview of Clinical Trials
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global Trends in Diabetes Complications: A Review of Current Evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Zheng, H.C.; Bukuru, J.; Kimpe, N. Natural Medicines Used in the Traditional Chinese Medical System for Therapy of Diabetes Mellitus. J. Ethnopharmacol. 2004, 92, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.-C.; Wasser, S.P. Medicinal Mushrooms for Glycemic Control in Diabetes Mellitus: History, Current Status, Future Perspectives, and Unsolved Problems (Review). Int. J. Med. Mushrooms 2011, 13, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, J.A.; Mihailović, M.; Uskoković, A.S.; Grdović, N.; Dinić, S.; Poznanović, G.; Mujić, I.; Vidaković, M. Evaluation of the Antioxidant and Antiglycation Effects of Lactarius Deterrimus and Castanea Sativa Extracts on Hepatorenal Injury in Streptozotocin-Induced Diabetic Rats. Front. Pharmacol. 2017, 8, 793. [Google Scholar] [CrossRef]
- Idf Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019.
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B., III. Diabetes, Oxidative Stress, and Antioxidants: A Review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Schmeltz, L.; Metzger, B. Diabetes/Syndrome X; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Atkinson, M.A.; Eisenbarth, G.S. Type 1 diabetes: New perspectives on disease pathogenesis and treatment. Lancet 2001, 358, 221–229. [Google Scholar] [CrossRef]
- Skyler, J.S.; Bakris, G.L.; Bonifacio, E.; Darsow, T.; Eckel, R.H.; Groop, L.; Handelsman, Y.; Insel, R.A.; Mathieu, C.; McElvaine, A.T.; et al. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes 2017, 66, 241–255. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2011, 34, S62–S69. [Google Scholar] [CrossRef]
- Khawandanah, J. Double or Hybrid Diabetes: A Systematic Review on Disease Prevalence, Characteristics and Risk Factors. Nutr. Diabetes 2019, 9, 33. [Google Scholar] [CrossRef]
- Lowe, L.P.; Metzger, B.E.; Dyer, A.R.; Lowe, J.; McCance, D.R.; Lappin, T.R.J.; Trimble, E.R.; Coustan, D.R.; Hadden, D.R.; Hod, M.; et al. Hyperglycemia and Adverse Pregnancy Outcomes. Diabetes Care 2012, 35, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, S.; Ji, J.; Ge, A.; Chen, C.; Zhu, Y.; Xie, N.; Wang, Y. Risk Factors and Management of Gestational Diabetes. Cell Biophys. 2014, 71, 689–694. [Google Scholar]
- Fowler, M.J. Microvascular and Macrovascular Complications of Diabetes. Clin. Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef]
- Ceriello, A.; Davidson, J.; Hanefeld, M.; Leiter, L.; Monnier, L.; Owens, D.; Tajima, N.; Tuomilehto, J. Postprandial hyperglycaemia and cardiovascular complications of diabetes: An update. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.I.; Maser, R.E.; Mitchell, B.D.; Freeman, R. Diabetic Autonomic Neuropathy. Diabetes Care 2003, 26, 1553–1579. [Google Scholar] [CrossRef]
- Backholer, K.; Peeters, A.; Herman, W.H.; Shaw, J.E.; Liew, D.; Ademi, Z.; Magliano, D.J. Diabetes Prevention and Treatment Strategies. Diabetes Care 2013, 36, 2714–2719. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Buse, J.B.; Tuomilehto, J.; Fleming, G.A.; Ferrannini, E.; Gerstein, H.C.; Bennett, P.H.; Ramachandran, A.; Raz, I.; Rosenstock, J.; et al. Update and Next Steps for Real-World Translation of Interventions for Type 2 Diabetes Prevention: Reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care 2016, 39, 1186–1201. [Google Scholar] [CrossRef]
- Mohamed, S.A. Effect of lifestyle intervention on health behaviors, weight and blood glucose level among patients with diabetes mellitus. J. Nurs. Educ. Pr. 2014, 4, 75. [Google Scholar] [CrossRef]
- Agarwal, A.A.; Jadhav, P.R.; Deshmukh, Y.A. Prescribing pattern and efficacy of anti-diabetic drugs in maintaining optimal glycemic levels in diabetic patients. J. Basic Clin. Pharm. 2014, 5, 79–83. [Google Scholar] [CrossRef]
- Horakova, O.; Kroupova, P.; Bardova, K.; Buresova, J.; Janovska, P.; Kopecky, J.; Rossmeisl, M. Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.; Fleming, G.A.; Chen, K.; Bicsak, T.A. Metformin-Associated Lactic Acidosis: Current Perspectives on Causes and Risk. Metabolism 2016, 65, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Loubatières, A. The discovery of hypoglycemic sulfonamides and particularly of their action mechanism. Acta Diabetol. Lat. 1969, 6, 20–56. [Google Scholar] [PubMed]
- Proks, P.; Reimann, F.; Green, N.; Gribble, F.; Ashcroft, F. Sulfonylurea Stimulation of Insulin Secretion. Diabetes 2002, 51, S368–S376. [Google Scholar] [CrossRef] [PubMed]
- Sola, D.; Rossi, L.; Schianca, G.P.C.; Maffioli, P.; Bigliocca, M.; Mella, R.; Corlianò, F.; Fra, G.P.; Bartoli, E.; DeRosa, G. State of the art paper Sulfonylureas and their use in clinical practice. Arch. Med Sci. 2015, 4, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Hauner, H. The Mode of Action of Thiazolidinediones. Diabetes Metab. Res. Rev. 2002, 18, S10–S15. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Thiazolidinediones: The Forgotten Diabetes Medications. Curr. Diabetes Rep. 2019, 19, 1–13. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P. α-Glucosidase Inhibitors and Their Use in Clinical Practice. Arch. Med. Sci. 2012, 8, 899. [Google Scholar] [CrossRef]
- Nauck, M.A.; Vilsbøll, T.; Gallwitz, B.; Garber, A.; Madsbad, S. Incretin-Based Therapies. Diabetes Care 2009, 32, 223. [Google Scholar] [CrossRef]
- Egan, A.G.; Blind, E.; Dunder, K.; de Graeff, P.A.; Hummer, B.T.; Bourcier, T.; Rosebraugh, C. Pancreatic Safety of Incretin-Based Drugs-Fda and Ema Assessment. N. Engl. J. Med. 2014, 370, 794–797. [Google Scholar] [CrossRef]
- Elashoff, M.; Matveyenko, A.V.; Gier, B.; Elashoff, R.; Butler, P.C. Pancreatitis, Pancreatic, and Thyroid Cancer with Glucagon-Like Peptide-1-Based Therapies. Gastroenterology 2011, 141, 150–156. [Google Scholar] [CrossRef] [PubMed]
- De Silva, D.D.; Rapior, S.; Hyde, K.D.; Bahkali, A.H. Medicinal mushrooms in prevention and control of diabetes mellitus. Fungal Divers. 2012, 56, 1–29. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible Mushrooms: Improving Human Health and Promoting Quality Life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef] [PubMed]
- Han, N.S.; Ahmad, W.A.N.W.; Ishak, W.R.W. Quality Characteristics of Pleurotus Sajor-Caju Powder: Study on Nutritional Compositions, Functional Properties and Storage Stability. Sains Malays. 2016, 45, 1617–1623. [Google Scholar]
- Ho, L.-H.; Zulkifli, N.A.; Tan, T.-C. Edible Mushroom: Nutritional Properties, Potential Nutraceutical Values, and Its Utilisation in Food Product Development. In An Introduction to Mushroom; Passari, A.K., Sánchez, S., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Feeney, M.J.; Dwyer, J.; Hasler-Lewis, C.M.; Milner, J.A.; Noakes, M.; Rowe, S.; Wach, M.; Beelman, R.B.; Caldwell, J.; Cantorna, M.T.; et al. Mushrooms and Health Summit Proceedings. J. Nutr. 2014, 144, 1128S–1136S. [Google Scholar] [CrossRef]
- Deepak, K.R.; Malik, D. Diversity of Mushrooms and Their Metabolites of Nutraceutical and Therapeutic Significance. J. Mycol. 2016, 2016, 7654123. [Google Scholar]
- Zhong, J.J.; Tang, Y.J. Submerged Cultivation of Medicinal Mushrooms for Production of Valuable Bioactive Metabolites. In Biomanufacturing; Springer: Berlin, Germany, 2004. [Google Scholar]
- Zhong, J.J.; Xiao, J.H. Secondary Metabolites from Higher Fungi: Discovery, Bioactivity, and Bioproduction. In Biotechnology in China; Springer: Berlin, Germany, 2009. [Google Scholar]
- Subhadip, M.; Banerjee, D. Fungal Exopolysaccharide: Production, Composition and Applications. Microbiol. Insights 2013, 6, 1–16. [Google Scholar]
- Wagner, R.; Mitchell, D.A.; Sassaki, G.L.; Lopes de Almeida Amazonas, M.A. Links between Morphology and Physiology of Ganoderma lucidum in Submerged Culture for the Production of Exopolysaccharide. J. Biotechnol. 2004, 114, 153–164. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zhang, W.; Zhong, J.J. Performance Analyses of a Ph-Shift and Dot-Shift Integrated Fed-Batch Fermentation Process for the Production of Ganoderic Acid and Ganoderma Polysaccharides by Medicinal Mushroom Ganoderma lucidum. Bioresour. Technol. 2009, 100, 1852–1859. [Google Scholar] [CrossRef]
- Zhang, W.-X.; Zhong, J.-J. Effect of oxygen concentration in gas phase on sporulation and individual ganoderic acids accumulation in liquid static culture of Ganoderma lucidum. J. Biosci. Bioeng. 2010, 109, 37–40. [Google Scholar] [CrossRef]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Dilshad, S.M.R. Beta Glucan: A Valuable Functional Ingredient in Foods. Crit. Rev. Food Sci. Nutr. 2012, 52, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Seviour, R. Medicinal Importance of Fungal Beta-(1→3), (1→6)-Glucans. Mycol. Res. 2007, 111, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Mihailović, M.; Arambašić, J.; Uskoković, A.; Dinić, S.; Grdović, N.; Marković, J.; Mujić, I.; Šijački, D.A.; Poznanović, G.; Vidaković, M. Β-Glucan Administration to Diabetic Rats Reestablishes Redox Balance and Stimulates Cellular Pro-Survival Mechanisms. J. Funct. Foods 2013, 5, 267–278. [Google Scholar]
- Mihailović, M.; Arambašić, J.; Uskoković, A.; Dinić, S.; Grdović, N.; Marković, J.; Bauder, J.; Poznanović, G.; Vidaković, M. Β-Glucan Administration to Diabetic Rats Alleviates Oxidative Stress by Lowering Hyperglycaemia, Decreasing Non-Enzymatic Glycation and Protein O-Glcnacylation. J. Funct. Foods 2013, 5, 1226–1234. [Google Scholar] [CrossRef]
- Uskokovic, A.; Mihailovic, M.; Dinic, S.; Arambašić-Jovanović, J.D.; Grdovic, N.; Markovic, J.; Poznanovic, G.; Vidakovic, M. Administration of a β-glucan-enriched extract activates beneficial hepatic antioxidant and anti-inflammatory mechanisms in streptozotocin-induced diabetic rats. J. Funct. Foods 2013, 5, 1966–1974. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Jong, S. Fungal Cell Wall Glycans. Biopolym. Online 2002, 6. [Google Scholar] [CrossRef]
- Jong, S.C.; Birmingham, J.M. Medicinal Benefits of the Mushroom Ganoderma. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 1992. [Google Scholar]
- Hu, J.-L.; Nie, S.; Xie, M. Antidiabetic Mechanism of Dietary Polysaccharides Based on Their Gastrointestinal Functions. J. Agric. Food Chem. 2018, 66, 4781–4786. [Google Scholar] [CrossRef]
- Gray, A.M.; Flatt, P.R. Insulin-releasing and insulin-like activity of Agaricus campestris (mushroom). J. Endocrinol. 1998, 157, 259–266. [Google Scholar] [CrossRef]
- Yang, B.-K.; Kim, G.-N.; Jeong, Y.-T.; Jeong, H.; Mehta, P.; Song, C.-H. Hypoglycemic Effects of Exo-Biopolymers Produced by Five Different Medicinal Mushrooms in Stz-Induced Diabetic Rats. Mycobiology 2008, 36, 45–49. [Google Scholar] [CrossRef]
- Bonner-Weir, S. Life and Death of the Pancreatic β Cells. Trends Endocrinol. Metab. 2000, 11, 375–378. [Google Scholar] [CrossRef]
- Adeyemi, D.O.; Komolafe, O.A.; Adewole, O.S.; Obuotor, E.M.; Abiodun, A.A.; Adenowo, T.K. Histomorphological and Morphometric Studies of the Pancreatic Islet Cells of Diabetic Rats Treated with Extracts of Annona muricata. Folia Morphol. 2010, 69, 92–100. [Google Scholar]
- Vetere, A.; Wagner, B.K. Chemical Methods to Induce Beta-Cell Proliferation. Int. J. Endocrinol. 2012, 2012, 1–8. [Google Scholar]
- Mihailović, M.; Arambašić Jovanović, J.; Uskoković, A.; Grdović, N.; Dinić, S.; Vidović, S.; Poznanović, G.; Mujić, I.; Vidaković, M. Protective Effects of the Mushroom Lactarius Deterrimus Extract on Systemic Oxidative Stress and Pancreatic Islets in Streptozotocin-Induced Diabetic Rats. J. Diabetes Res. 2015, 2015, 576726. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Ren, C.; Lu, G.; Mu, Z.; Cui, W.; Gao, H.; Wang, Y. Anti-Diabetic Effect of Mulberry Leaf Polysaccharide by Inhibiting Pancreatic Islet Cell Apoptosis and Ameliorating Insulin Secretory Capacity in Diabetic Rats. Int. Immunopharmacol. 2004, 22, 248–257. [Google Scholar]
- Zhu, K.-X.; Nie, S.; Li, C.; Gong, D.; Xie, M. Ganoderma atrum polysaccharide improves aortic relaxation in diabetic rats via PI3K/Akt pathway. Carbohydr. Polym. 2014, 103, 520–527. [Google Scholar] [CrossRef]
- Zhu, K.; Nie, S.; Li, C.; Lin, S.; Xing, M.; Li, W.; Gong, D.; Xie, M. A Newly Identified Polysaccharide from Ganoderma Atrum Attenuates Hyperglycemia and Hyperlipidemia. Int. J. Biol. Macromol. 2013, 57, 142–150. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, B.; Yu, Y.; Chen, Q.; Huang, T.; Li, D. Ganoderma lucidum Polysaccharides Exert Anti-Hyperglycemic Effect on Streptozotocin-Induced Diabetic Rats through Affecting Β-Cells. Comb. Chem. High Throughput Screen. 2020, 15, 542–550. [Google Scholar] [CrossRef]
- Li, F.; Zhijian, Z.Y.; Zhong, Z. Antihyperglycemic Effect of Ganoderma lucidum Polysaccharides on Streptozotocin-Induced Diabetic Mice. Int. J. Mol. Sci. 2011, 12, 6135–6145. [Google Scholar]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The Pi3k/Akt Pathway in Obesity and Type 2 Diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Anti-Diabetic Effects and Mechanisms of Dietary Polysaccharides. Molecules 2019, 24, 2556. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, F.; Chen, Y.; Zhang, Y.; Hou, L.; Cao, X.; Wang, C. A Polysaccharide from Grifola Frondosa Relieves Insulin Resistance of Hepg2 Cell by Akt-Gsk-3 Pathway. Glycoconj. J. 2014, 31, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.X.; Nie, S.P.; Tan, L.H.; Li, C.; Gong, D.M.; Xie, M.Y. A Polysaccharide from Ganoderma Atrum Improves Liver Function in Type 2 Diabetic Rats Via Antioxidant Action and Short-Chain Fatty Acids Excretion. J. Agric. Food Chem. 2016, 64, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, C.; Li, S.; Li, W.; Yuan, G.; Pan, Y.; Chen, H. Anti-diabetic effects of Inonotus obliquus polysaccharides in streptozotocin-induced type 2 diabetic mice and potential mechanism via PI3K-Akt signal pathway. Biomed. Pharmacother. 2017, 95, 1669–1677. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, T.; Zhou, H.; Zhang, Y.; Jin, G.; Yang, Y. Antidiabetic Effect of Polysaccharides from Pleurotus Ostreatus in Streptozotocin-Induced Diabetic Rats. Int. J. Biol. Macromol. 2016, 83, 126–132. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Gabryelska, K.; Grabarczyk, M. Mushrooms of the Genus Ganoderma Used to Treat Diabetes and Insulin Resistance. Molecules 2019, 24, 4075. [Google Scholar] [CrossRef]
- Yin, M.C.; Lin, M.C.; Mong, M.C.; Lin, C.Y. Bioavailability, Distribution, and Antioxidative Effects of Selected Triterpenes in Mice. J. Agric. Food Chem. 2012, 60, 7697–7701. [Google Scholar] [CrossRef]
- Ma, H.-T.; Hsieh, J.-F.; Chen, S.-T. Anti-diabetic effects of Ganoderma lucidum. Phytochemistry 2015, 114, 109–113. [Google Scholar] [CrossRef]
- Nazaruk, J.; Borzymkluczyk, M. The role of triterpenes in the management of diabetes mellitus and its complications. Phytochem. Rev. 2015, 14, 675–690. [Google Scholar] [CrossRef]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Magalhães, P.O.; Silveira, D. α-Amylase Inhibitors: A Review of Raw Material and Isolated Compounds from Plant Source. J. Pharm. Pharm. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef]
- Welti, S.; Moreau, P.A.; Decock, C.; Danel, C.; Duhal, N.; Favel, A.; Courtecuisse, R. Oxygenated Lanostane-Type Triterpenes Profiling in Laccate Ganoderma Chemotaxonomy. Mycol. Prog. 2015, 14, 45. [Google Scholar] [CrossRef]
- Zhao, X.-R.; Huo, X.-K.; Dong, P.-P.; Wang, C.; Huang, S.-S.; Zhang, B.-J.; Zhang, H.-L.; Deng, S.; Liu, K.; Ma, X.-C. Inhibitory Effects of Highly Oxygenated Lanostane Derivatives from the Fungus Ganoderma lucidum on P-Glycoprotein and α-Glucosidase. J. Nat. Prod. 2015, 78, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Sarikurkcu, C.; Gunes, E.; Uysal, A.; Ceylan, R.; Uysal, S.; Gungor, H.; Aktumsek, A. Two Ganoderma species: Profiling of phenolic compounds by HPLC–DAD, antioxidant, antimicrobial and inhibitory activities on key enzymes linked to diabetes mellitus, Alzheimer’s disease and skin disorders. Food Funct. 2015, 6, 2794–2802. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Q.; Zhao, J.; Chen, L.-X.; Wang, S.-F.; Wang, Y.; Li, S.-P. Lanostane triterpenes from the mushroom Ganoderma resinaceum and their inhibitory activities against α-glucosidase. Phytochemistry 2018, 149, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Sri, F.; Shimizu, K.; Kondo, R. Ganoderol B: A Potent Α-Glucosidase Inhibitor Isolated from the Fruiting Body of Ganoderma lucidum. Phytomedicine 2011, 18, 1053–1055. [Google Scholar]
- Fatmawati, S.; Kondo, R.; Shimizu, K. Structure–activity relationships of lanostane-type triterpenoids from Ganoderma lingzhi as α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 5900–5903. [Google Scholar] [CrossRef] [PubMed]
- Satria, D.; Sonam, T.; Suhara, H.; Kaneko, S.; Shimizu, K. Mass Spectrometry-Based Untargeted Metabolomics and α-Glucosidase Inhibitory Activity of Lingzhi (Ganoderma lingzhi) During the Developmental Stages. Molecules 2019, 24, 2044. [Google Scholar] [CrossRef]
- Satria, D.; Amen, Y.; Niwa, Y.; Ashour, A.; Allam, A.E.; Shimizu, K. Lucidumol D, a new lanostane-type triterpene from fruiting bodies of Reishi (Ganoderma lingzhi). Nat. Prod. Res. 2018, 33, 189–195. [Google Scholar] [CrossRef]
- Ying, Y.-M.; Zhang, L.-Y.; Zhang, X.; Bai, H.-B.; Liang, D.-E.; Ma, L.-F.; Shan, W.-G.; Zhan, Z.-J. Terpenoids with alpha-glucosidase inhibitory activity from the submerged culture of Inonotus obliquus. Phytochemistry 2014, 108, 171–176. [Google Scholar] [CrossRef]
- Michalik, L.; Wahli, W. Involvement of PPAR nuclear receptors in tissue injury and wound repair. J. Clin. Investig. 2006, 116, 598–606. [Google Scholar] [CrossRef]
- Ferré, P. The Biology of Peroxisome Proliferator-Activated Receptors: Relationship with Lipid Metabolism and Insulin Sensitivity. Diabetes 2004, 53, S43–S50. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.M.; Moore, L.B.; Smith-Oliver, T.A.; Wilkison, W.O.; Willson, T.M.; Kliewer, S.A. An Antidiabetic Thiazolidinedione Is a High Affinity Ligand for Peroxisome Proliferator-Activated Receptor Gamma (Ppar Gamma). J. Biol. Chem. 1995, 270, 12953–12956. [Google Scholar] [CrossRef] [PubMed]
- Willson, T.M.; Cobb, J.E.; Cowan, D.J.; Wiethe, R.W.; Correa, I.D.; Prakash, S.R.; Beck, K.D.; Moore, L.B.; Kliewer, S.A.; Lehmann, J.M. The Structure-Activity Relationship between Peroxisome Proliferator-Activated Receptor Gamma Agonism and the Antihyperglycemic Activity of Thiazolidinediones. J. Med. Chem. 1996, 39, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, T.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The Peroxisome Proliferator-Activated Receptor: A Family of Nuclear Receptors Role in Various Diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–340. [Google Scholar]
- Sato, M.; Tai, T.; Nunoura, Y.; Yajima, Y.; Kawashima, S.; Tanaka, K. Dehydrotrametenolic Acid Induces Preadipocyte Differentiation and Sensitizes Animal Models of Noninsulin-Dependent Diabetes Mellitus to Insulin. Biol. Pharm. Bull. 2002, 25, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.S.; Martins, A.; Vasconcelos, M.H.; Morales, P.; Ferreira, I.C. Functional Foods Based on Extracts or Compounds Derived from Mushrooms. Trends Food Sci. Technol. 2017, 66, 48–62. [Google Scholar] [CrossRef]
- Hsu, C.H.; Liao, Y.L.; Lin, S.C.; Hwang, K.C.; Chou, P. The Mushroom Agaricus Blazei Murill in Combination with Metformin and Gliclazide Improves Insulin Resistance in Type 2 Diabetes: A Randomized, Double-Blinded, and Placebo-Controlled Clinical Trial. J. Altern. Complement. Med. 2007, 13, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lan, J.; Dai, X.; Ye, J.; Zhou, S. A Phase I/II Study of Ling Zhi Mushroom Ganoderma lucidum (W.Curt.:Fr.)Lloyd (Aphyllophoromycetideae) Extract in Patients with Type II Diabetes Mellitus. Int. J. Med. Mushrooms 2004, 6, 8–40. [Google Scholar] [CrossRef]
- Klupp, N.L.; Kiat, H.; Bensoussan, A.; Steiner, G.Z.; Chang, D.H. A double-blind, randomised, placebo-controlled trial of Ganoderma lucidum for the treatment of cardiovascular risk factors of metabolic syndrome. Sci. Rep. 2016, 6, 29540. [Google Scholar] [CrossRef]
- Wang, C.W.; Tschen, J.S.M.; Sheu, W.H.H. Ganoderma Lucidum on Metabolic Control in Type 2 Diabetes Subjects: A Double-Blinded Placebo Control Study. J. Intern. Med. Taiwan 2008, 19, 15–60. [Google Scholar]
- Jayasuriya, W.B.N.; Wanigatunge, C.A.; Fernando, G.H.; Abeytunga, D.T.U.; Suresh, T.S. Hypoglycaemic Activity of Culinary Pleurotus Ostreatus and P. Cystidiosus Mushrooms in Healthy Volunteers and Type 2 Diabetic Patients on Diet Control and the Possible Mechanisms of Action. Phytother. Res. 2015, 29, 303–309. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aramabašić Jovanović, J.; Mihailović, M.; Uskoković, A.; Grdović, N.; Dinić, S.; Vidaković, M. The Effects of Major Mushroom Bioactive Compounds on Mechanisms That Control Blood Glucose Level. J. Fungi 2021, 7, 58. https://doi.org/10.3390/jof7010058
Aramabašić Jovanović J, Mihailović M, Uskoković A, Grdović N, Dinić S, Vidaković M. The Effects of Major Mushroom Bioactive Compounds on Mechanisms That Control Blood Glucose Level. Journal of Fungi. 2021; 7(1):58. https://doi.org/10.3390/jof7010058
Chicago/Turabian StyleAramabašić Jovanović, Jelena, Mirjana Mihailović, Aleksandra Uskoković, Nevena Grdović, Svetlana Dinić, and Melita Vidaković. 2021. "The Effects of Major Mushroom Bioactive Compounds on Mechanisms That Control Blood Glucose Level" Journal of Fungi 7, no. 1: 58. https://doi.org/10.3390/jof7010058
APA StyleAramabašić Jovanović, J., Mihailović, M., Uskoković, A., Grdović, N., Dinić, S., & Vidaković, M. (2021). The Effects of Major Mushroom Bioactive Compounds on Mechanisms That Control Blood Glucose Level. Journal of Fungi, 7(1), 58. https://doi.org/10.3390/jof7010058





