Investigation of the Antifungal and Anti-Aflatoxigenic Potential of Plant-Based Essential Oils against Aspergillus flavus in Peanuts
Abstract
1. Introduction
2. Materials and Methods
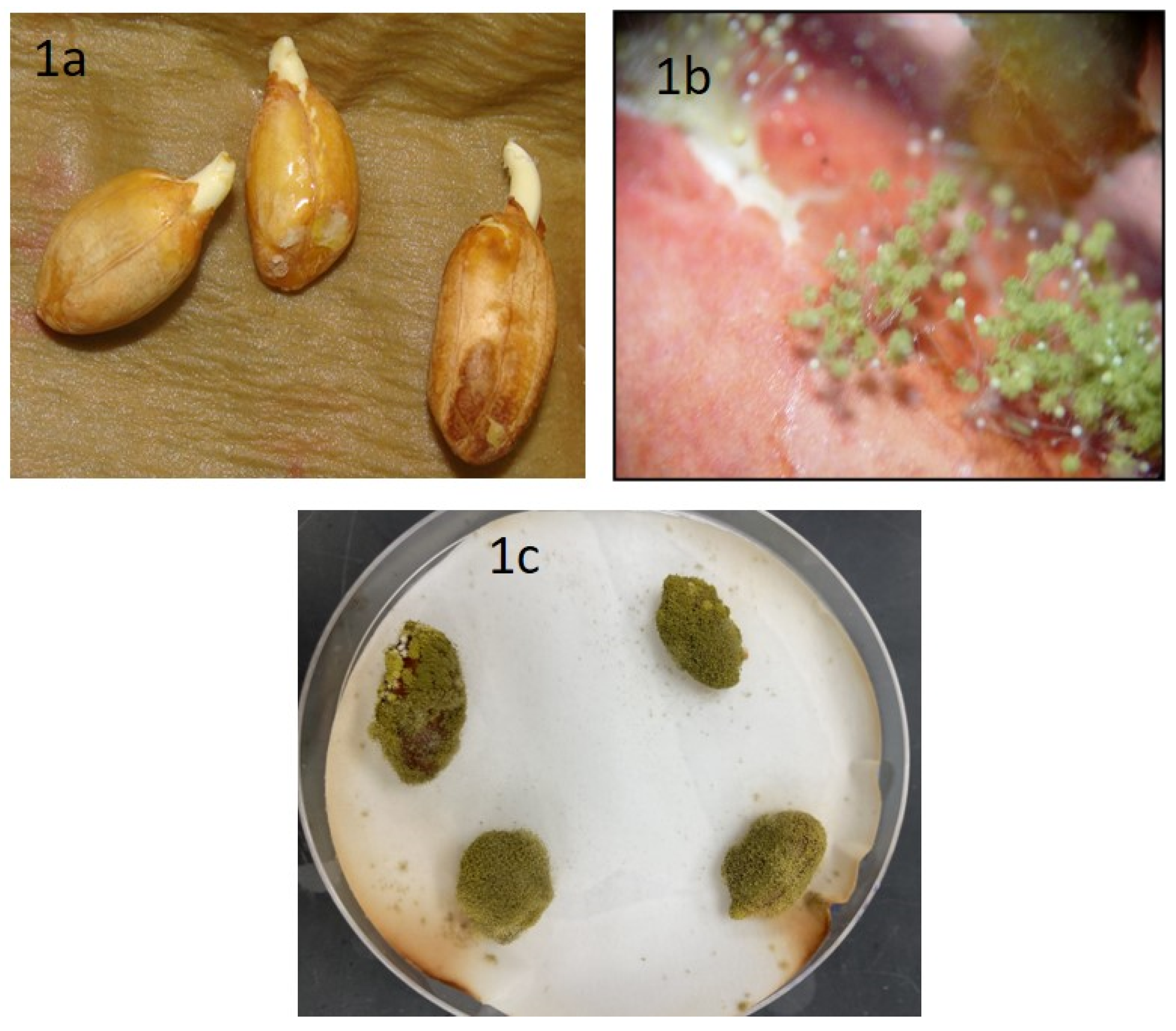
2.1. Isolation of A. flavus from Peanuts
2.2. Selection of Plant-Based Essential Oils
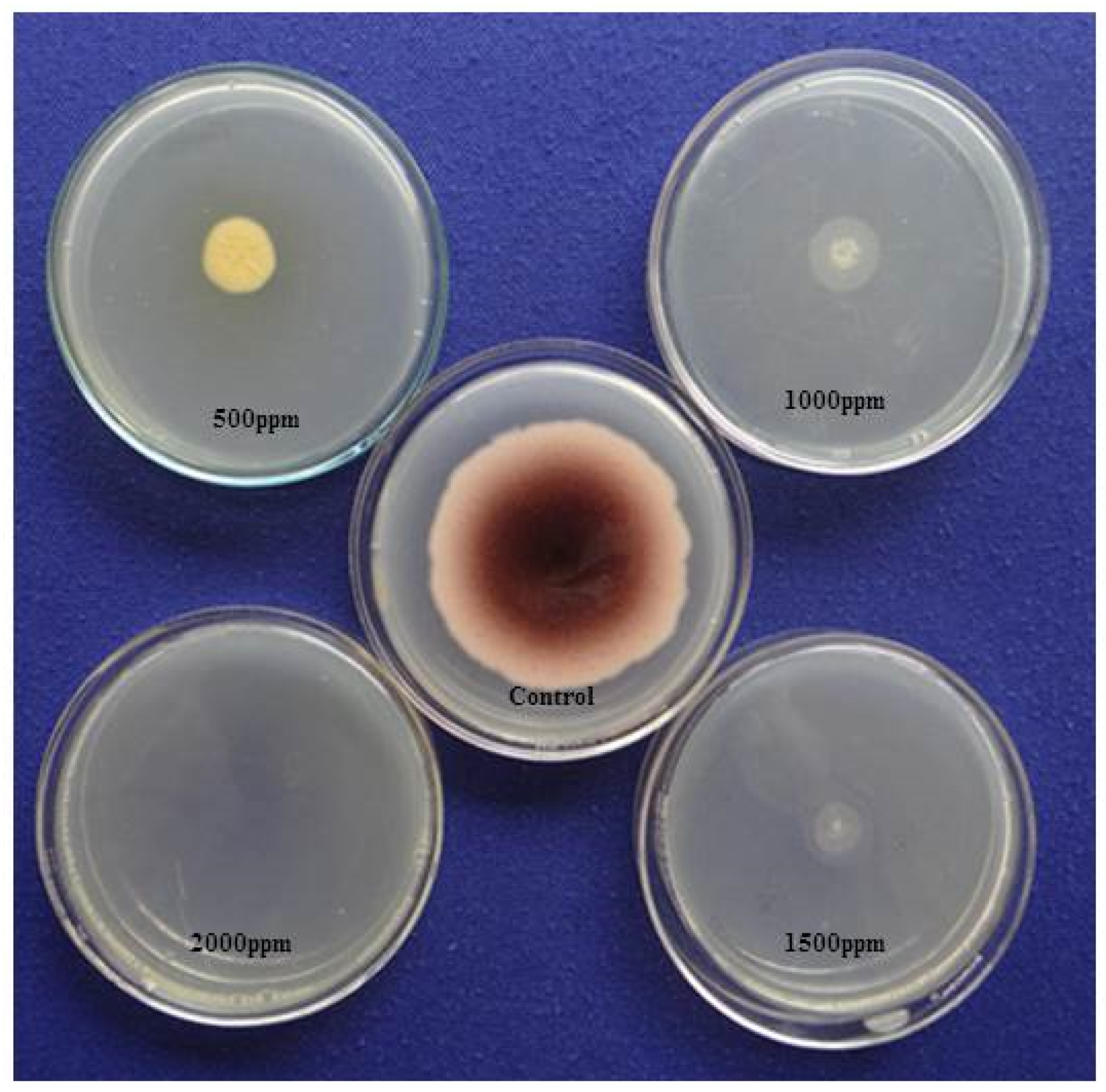
2.3. Antifungal Activity Assay of Essential Oils
2.4. Gas Chromatographic-Mass Spectrometry of Clove Oil
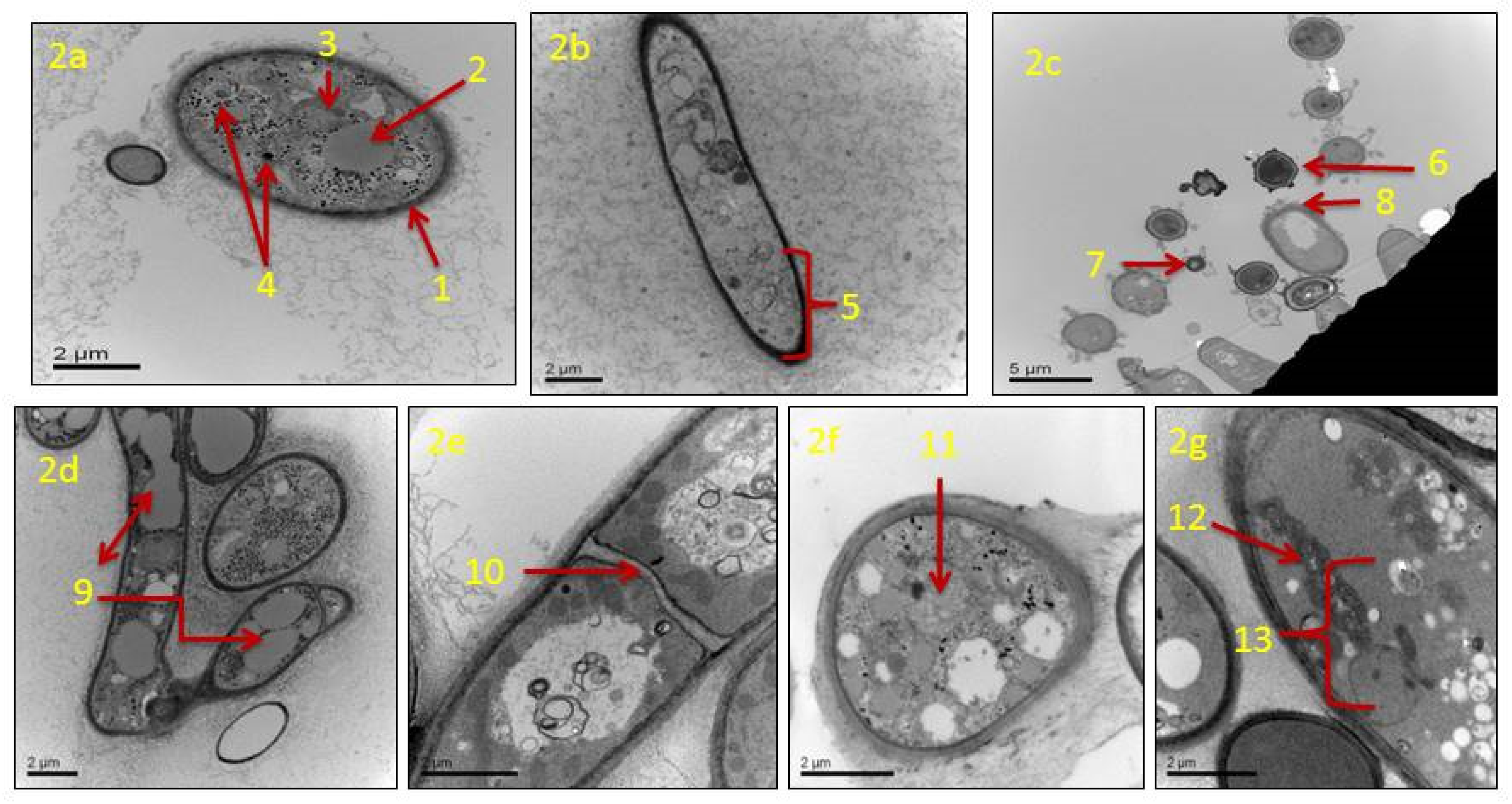
2.5. Transmission Electron Microscopy
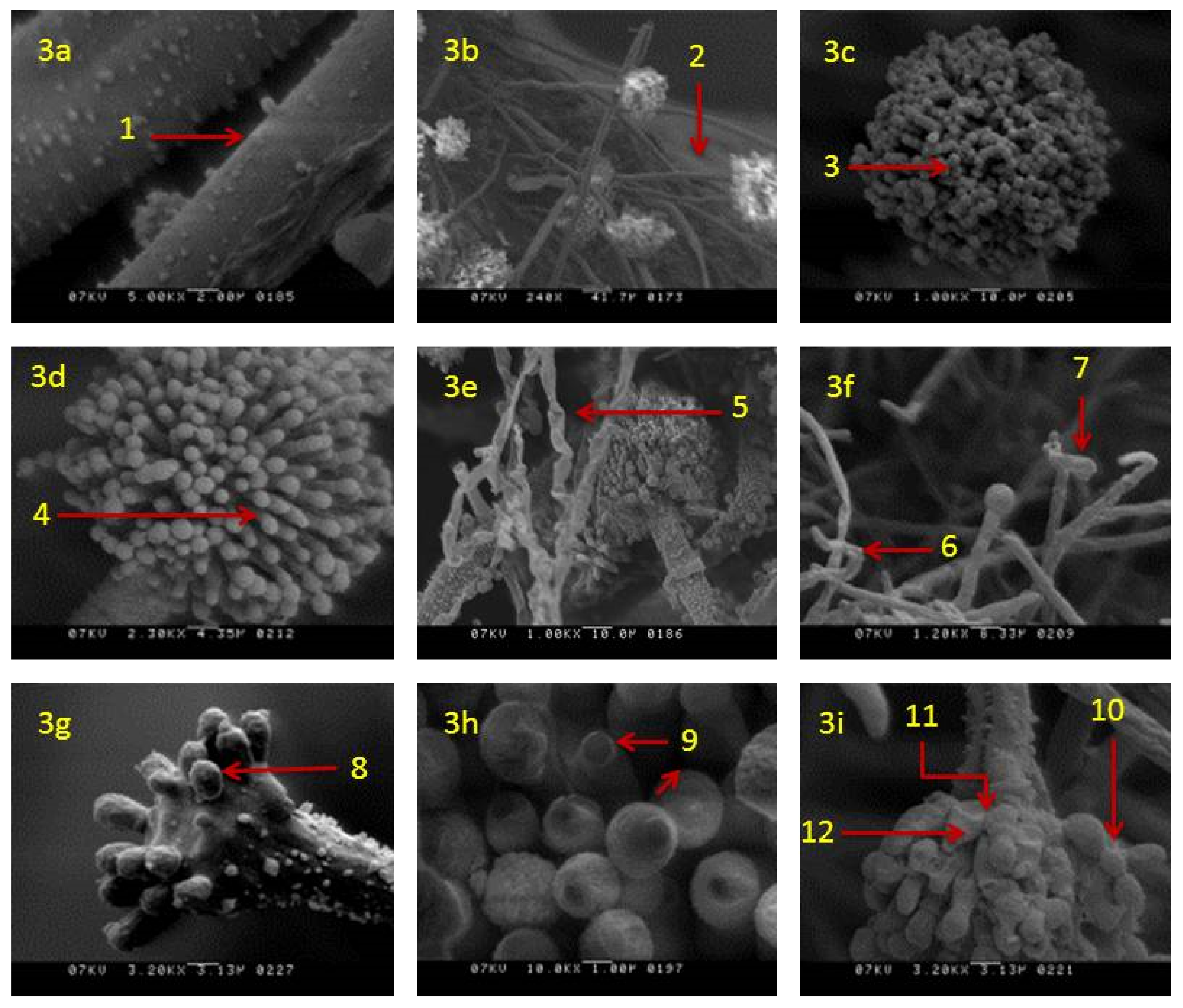
2.6. Scanning Electron Microscopy
2.7. Detection of Aflatoxin by Qualitative Methods
2.7.1. Ammonia Vapor Method
2.7.2. Coconut Milk Agar Method
2.7.3. Quantification of Aflatoxin by High-Performance Liquid Chromatography
2.8. Statistical Analysis
3. Results
3.1. Isolation of A. flavus from Peanuts
3.2. Antifungal Activity Assay of Essential Oils
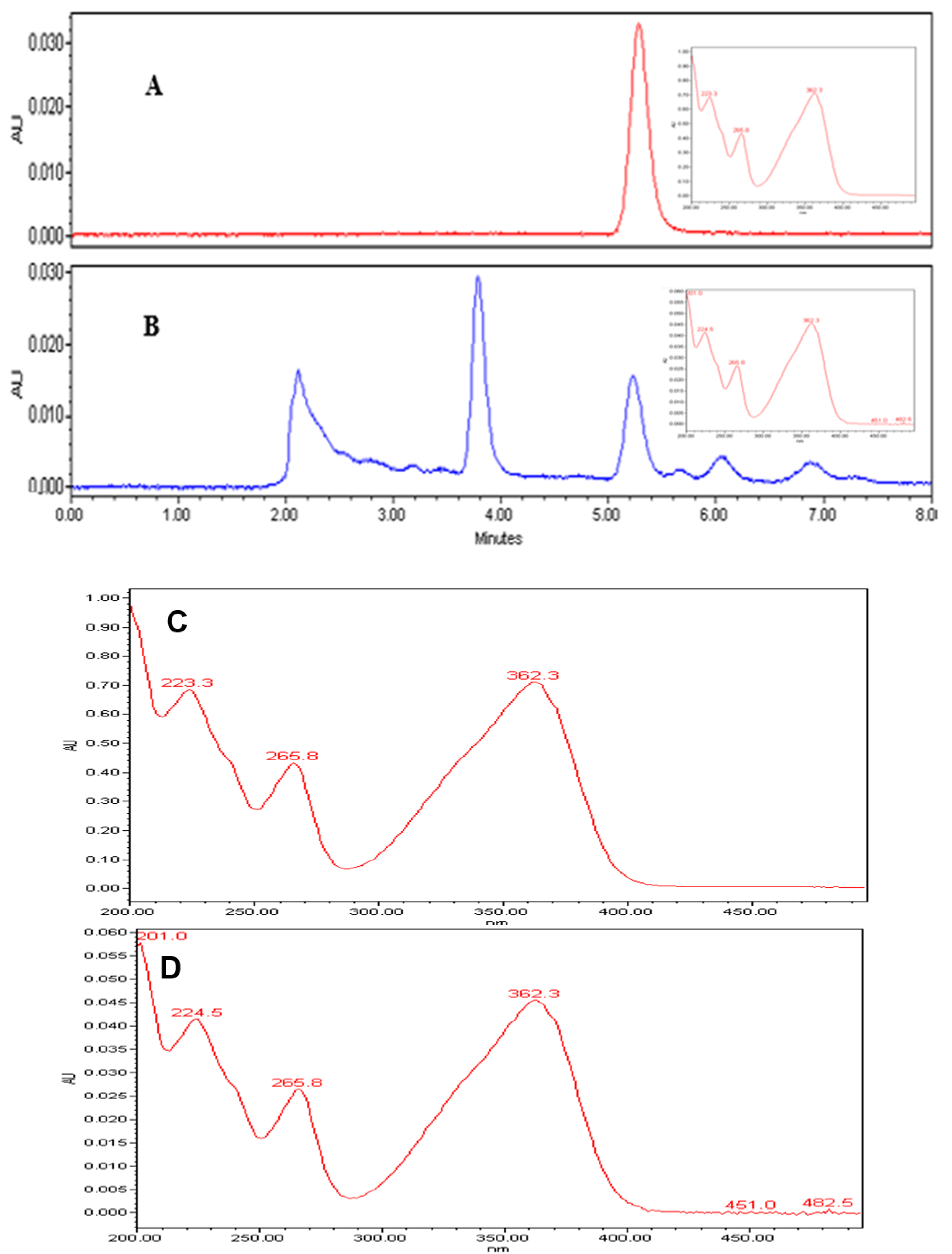
3.3. Major Active Compounds of Clove Oil
3.4. Effects of Clove Oil on Morphology and Ultrastructure of A. flavus Fungus
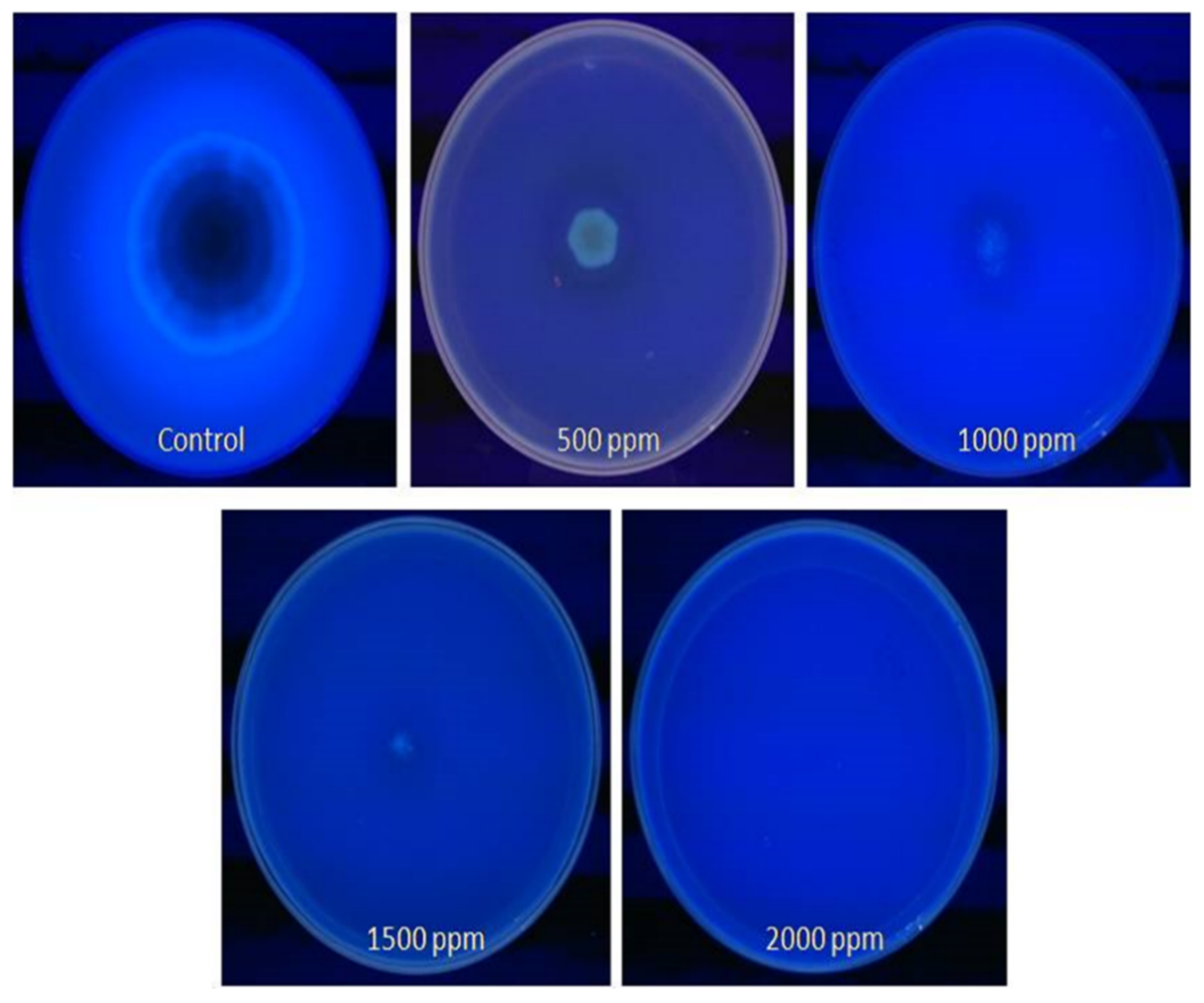
3.5. Detection of Fungus Aflatoxin
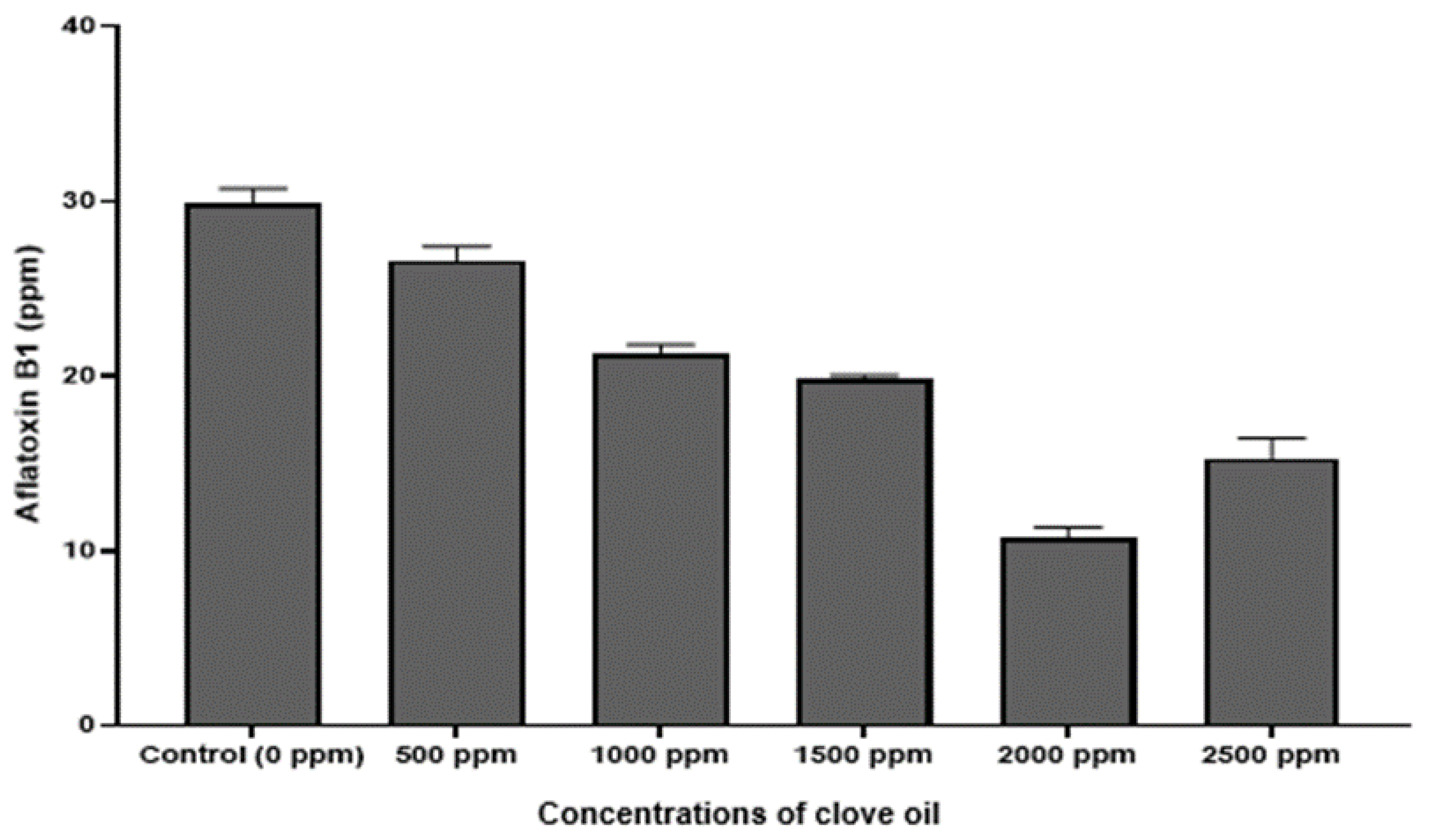
3.6. Quantification of Aflatoxin (AFB1)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mycotoxins. Available online: https://www.who.int/news-room/fact-sheets/detail/mycotoxins (accessed on 20 June 2020).
- Cullen, J.M.; Newberne, P.M. Acute hepatotoxicity of aflatoxins. In The Toxicology of Aflatoxins; Elsevier: Amsterdam, The Netherlands, 1994; pp. 3–26. [Google Scholar]
- World Health Organization; International Agency for Research on Cancer. Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. In IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; World Health Organization: Geneva, Switzerland, 1993; Volume 56. [Google Scholar]
- N’dede, C.B.; Jolly, C.M.; Vodouhe, S.D.; Jolly, P.E. Economic risks of aflatoxin contamination in marketing of peanut in benin. Econ. Res. Int. 2012, 1–12. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Mitchell, N.J.; Bowers, E.; Hurburgh, C.; Wu, F. Potential economic losses to the US corn industry from aflatoxin contamination. Food Addit. Contam. Part A 2016, 33, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Faustinelli, P.C.; Wang, X.M.; Palencia, E.R.; Arias, R.S. Genome sequences of eight Aspergillus flavus spp. and one A. parasiticus sp., isolated from peanut seeds in Georgia: Table 1. Genome Announc. 2016, 4, e00278-16. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Rajendran, S.; Srivastava, A.; Sharma, S.; Kundu, B. Antifungal activities of selected essential oils against Fusarium oxysporum f. sp. lycopersici 1322, with emphasis on Syzygium aromaticum essential oil. J. Biosci. Bioeng. 2017, 123, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Lawley, R. Aflatoxins. Food Safety Watch. 2013. Available online: http://www.foodsafetywatch.org/factsheets/aflatoxins/ (accessed on 21 August 2020).
- Pitt, J.I.; Dyer, S.K.; McCammon, S. Systemic invasion of developing peanut plants by Aspergillus flavus. Lett. Appl. Microbiol. 1991, 13, 16–20. [Google Scholar] [CrossRef]
- Achar, P.N.; Hermetz, K.; Rao, S.; Apkarian, R.; Taylor, J. Microscopic studies on the Aspergillus flavus infected kernels of commercial peanuts in Georgia. Ecotoxicol. Environ. Saf. 2009, 72, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Suszkiw, J.; Dorner, J.W.; Lamb, M.C. Protecting peanuts from aflatoxins. Environ. Health Perspect. 2004, 112, A987. [Google Scholar]
- Daferera, D.J.; Ziogas, B.N.; Polissiou, M.G. The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. michiganensis. Crop Prot. 2003, 22, 39–44. [Google Scholar] [CrossRef]
- Basilico, M.Z.; Basilico, J.C. Inhibitory effects of some spice essential oils on Aspergillus ochraceus NRRL 3174 growth and ochratoxin A production. Lett. Appl. Microbiol. 1999, 29, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.; Salleh, B.; Saad, B.; Abbas, H.; Abel, C.; Shier, W. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev. 2010, 29, 3–26. [Google Scholar] [CrossRef]
- Dorner, J.W. Management and prevention of mycotoxins in peanuts. Food Addit. Contam. Part A 2008, 25, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Dorner, J.W. Biological control of aflatoxin contamination of crops. J. Toxicol. Toxin Rev. 2004, 23, 425–450. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Mycotoxins in Australia: Biocontrol of aflatoxin in peanuts. Mycopathologia 2006, 162, 233–243. [Google Scholar] [CrossRef]
- Čvek, D.; Markov, K.; Frece, J.; Dragičević, T.; Majica, M.; Delaš, F. Growth inhibition of Aspergillus ochraceus ZMPBF 318 and Penicillium expansum ZMPBF 565 by four essential oils. Arch. Ind. Hyg. Toxicol. 2010, 61, 191–196. [Google Scholar] [CrossRef]
- Passone, M.A.; Girardi, N.S.; Etcheverry, M. Evaluation of the control ability of five essential oils against Aspergillus section Nigri growth and ochratoxin A accumulation in peanut meal extract agar conditioned at different water activities levels. Int. J. Food Microbiol. 2012, 159, 198–206. [Google Scholar] [CrossRef]
- Císarová, M.; Tančinová, D.; Medo, J.; Kačániová, M. The in vitro effect of selected essential oils on the growth and mycotoxin production of Aspergillus species. J. Environ. Sci. Health Part B 2016, 51, 668–674. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.; Ying, G.; Yang, M.; Nian, Y.; Wei, F.; Kong, W. Antifungal evaluation of plant essential oils and their major components against toxigenic fungi. Ind. Crops Prod. 2018, 120, 180–186. [Google Scholar] [CrossRef]
- US Department of Agriculture. New Peanut Variety Resistant to Nematodes, Virus. ScienceDaily. 2008. Available online: www.sciencedaily.com/releases/2008/05/080521101458.htm (accessed on 11 June 2020).
- Holbrook, C.C.; Timper, P.; Culbreath, A.K.; Kvien, C.K. Registration of ‘Tifguard’ peanut. J. Plant Regist. 2008, 2, 92–94. [Google Scholar] [CrossRef]
- Lopez, A.; Alzamora, S.M.; Guerrero, S. Natural antimicrobials from plants. In Minimally Processed Fruits and Vegetables. Fundamentals Aspects and Applications; Alzamora, S.M., Tapia, M.S., Lopez-Malo, A., Eds.; Aspen Publishers: Gaithersburg, MD, USA, 2000; pp. 237–264. [Google Scholar]
- Raper, K.B.; Fennell, D.I. The Genus Aspergillus; Williams & Wilkins: Baltimore, MD, USA; E. & S. Livingstone: Edinburgh, UK, 1965. [Google Scholar]
- Prindle, R.F.; Wright, E.S. Phenolic compounds. In Disinfection, Sterilization and Preservation; Lawrence, C.A., Block, S.S., Eds.; Lea & Febiger: Pennsylvania, PA, USA, 1977; pp. 115–118. [Google Scholar]
- Klich, M.A. Identification of Common Aspergillus Species; CBS: Utrecht, The Netherlands, 2002; ISBN 9070351463. [Google Scholar]
- Gqaleni, N.; Smith, J.E.; Lacey, J.; Gettinby, G. Effects of temperature, water activity, and incubation time on production of aflatoxins and cyclopiazonic acid by an isolate of Aspergillus flavus in surface agar culture. Appl. Environ. Microbiol. 1997, 63, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Machida, S. A rapid identification method for aflatoxin-producing strains of Aspergillus flavus and A. parasiticus by ammonia vapor. Mycoscience 1999, 40, 205–208. [Google Scholar] [CrossRef]
- Davis, N.D.; Iyer, S.K.; Diener, U.L. Improved method of screening for aflatoxin with a coconut agar medium. Appl. Environ. Microbiol. 1987, 53, 1593–1595. [Google Scholar] [CrossRef] [PubMed]
- Vosough, M.; Bayat, M.; Salemi, A. Matrix-free analysis of aflatoxins in pistachio nuts using parallel factor modeling of liquid chromatography diode-array detection data. Anal. Chim. Acta 2010, 663, 11–18. [Google Scholar] [CrossRef]
- Reddy, K.R.N.; Reddy, C.S.; Muralidharan, K. Potential of botanicals and biocontrol agents on growth and aflatoxin production by Aspergillus flavus infecting rice grains. Food Control 2009, 20, 173–178. [Google Scholar] [CrossRef]
- Soliman, K.; Badeaa, R. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem. Toxicol. 2002, 40, 1669–1675. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Razzaghi-Abyaneh, M.; Shams-Ghahfarokhi, M.; Rezaee, M.-B.; Jaimand, K.; Alinezhad, S.; Saberi, R.; Yoshinari, T. Chemical composition and antiaflatoxigenic activity of Carum carvi L., Thymus vulgaris and Citrus aurantifolia essential oils. Food Control 2009, 20, 1018–1024. [Google Scholar] [CrossRef]
- Sreenivasa, M.Y.; Dass, R.S.; Charith Raj, A.P.; Nagendra Prasad, M.N.; Achar, P.N.; Janardhana, G.R. Assessment of the growth inhibiting effect of some plant essential oils on different Fusarium species isolated from sorghum and maize grains. J. Plant Dis. Prot. 2011, 118, 208–213. [Google Scholar] [CrossRef]
- Prasad, N.; Bhat, S. Antifungal activity of essential oils against Phomopsis azadirachtae—The causative agent of die-back disease of neem. J. Agric. Technol. 2010, 6, 127–133. [Google Scholar]
- Adukwu, E.C.; Bowles, M.; Edwards-Jones, V.; Bone, H. Antimicrobial activity, cytotoxicity and chemical analysis of lemongrass essential oil (Cymbopogon flexuosus) and pure citral. Appl. Microbiol. Biotechnol. 2016, 100, 9619–9627. [Google Scholar] [CrossRef] [PubMed]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Najar, B.; Bertelloni, F.; Pistelli, L.; Mancianti, F.; Nardoni, S. Chemical composition and in vitro antimicrobial efficacy of sixteen essential oils against Escherichia coli and Aspergillus fumigatus isolated from poultry. Vet. Sci. 2018, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Elcocks, E.R.; Spencer-Phillips, P.T.N.; Adukwu, E.C. Rapid bactericidal effect of cinnamon bark essential oil against Pseudomonas aeruginosa. J. Appl. Microbiol. 2020, 128, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Purkait, S.; Bhattacharya, A.; Bag, A.; Chattopadhyay, R.R. Synergistic antibacterial, antifungal and antioxidant efficacy of cinnamon and clove essential oils in combination. Arch. Microbiol. 2020, 202, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Tullio, V.; Nostro, A.; Mandras, N.; Dugo, P.; Banche, G.; Cannatelli, M.A.; Cuffini, A.M.; Alonzo, V.; Carlone, N.A. Antifungal activity of essential oils against filamentous fungi determined by broth microdilution and vapour contact methods. J. Appl. Microbiol. 2007, 102, 1544–1550. [Google Scholar] [CrossRef]
- Hu, F.; Tu, X.F.; Thakur, K.; Hu, F.; Li, X.L.; Zhang, Y.S.; Zhang, J.G.; Wei, Z.J. Comparison of antifungal activity of essential oils from different plants against three fungi. Food Chem. Toxicol. 2019, 134, 110821. [Google Scholar] [CrossRef]
- Pinto, E.; Vale-Silva, L.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2009, 58, 1454–1462. [Google Scholar] [CrossRef]
- Khosravi, A.R.; Minooeianhaghighi, M.H.; Shokri, H.; Emami, S.A.; Alavi, S.M.; Asili, J. The potential inhibitory effect of Cuminum cyminum, Ziziphora clinopodioides and Nigella sativa essential oils on the growth of Aspergillus fumigatus and Aspergillus flavus. Braz. J. Microbiol. 2011, 42, 216–224. [Google Scholar] [CrossRef]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Chen, Y.; Wang, Y. The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS ONE 2012, 7, e30147. [Google Scholar] [CrossRef]
- Wanders, R.J.A.; Waterham, H.R.; Ferdinandusse, S. Metabolic interplay between peroxisomes and other subcellular organelles including mitochondria and the endoplasmic reticulum. Front. Cell Dev. Biol. 2016, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ben Miri, Y.; Belasli, A.; Djenane, D.; Ariño, A. Prevention by essential oils of the occurrence and growth of Aspergillus flavus and aflatoxin B1 production in food systems: Review. Aflatoxin B1 Occur. Detect. Toxicol. Eff. 2020, 1, 1–17. [Google Scholar] [CrossRef]
- Zambonelli, A.; d’Aulerio, A.Z.; Bianchi, A.; Albasini, A. Effects of essential oils on phytopathogenic fungi in vitro. J. Phytopathol. 1996, 144, 491–494. [Google Scholar] [CrossRef]
- De Billerbeck, V.G.; Roques, C.G.; Bessière, J.M.; Fonvieille, J.L.; Dargent, R. Effects of Cymbopogon nardus (L.) W. Watson essential oil on the growth and morphogenesis of Aspergillus niger. Can. J. Microbiol. 2001, 47, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Helal, G.A.; Sarhan, M.M.; Abu Shahla, A.N.K.; Abou El-Khair, E.K. Effects of Cymbopogon citratus L. essential oil on the growth, lipid content and morphogenesis of Aspergillus niger ML2-strain. J. Basic Microbiol. 2006, 46, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Xing, F.; Selvaraj, J.N.; Wang, Y.; Zhao, Y.; Zhou, L.; Liu, X.; Liu, Y. Inhibitory effect of essential oils on Aspergillus ochraceus growth and ochratoxin a production. PLoS ONE 2014, 9, e108285. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Gemeda, N.; Woldeamanuel, Y.; Asrat, D.; Debella, A. Effect of essential oils on Aspergillus spore germination, growth and mycotoxin production: A potential source of botanical food preservative. Asian Pac. J. Trop. Biomed. 2014, 4, S373–S381. [Google Scholar] [CrossRef]
- Abbas, H.K.; Zablotowicz, R.M.; Weaver, M.A.; Horn, B.W.; Xie, W.; Shier, W.T. Comparison of cultural and analytical methods for determination of aflatoxin production by Mississippi Delta Aspergillus isolates. Can. J. Microbiol. 2004, 50, 193–199. [Google Scholar] [CrossRef]
- Naik, M.K.; Guru Prasad, G.R.; Jadhav, H.P.; Hashem, A.; Abd_allah, E.F.; Sayyed, R.Z. Differentiation of toxigenic and atoxigenic Aspergillus flavus: Polyphasic approach, a new dimension. Indian J. Exp. Biol. 2018, 56, 892–898. [Google Scholar]
- Kushiro, M.; Hatabayashi, H.; Yabe, K.; Loladze, A. Detection of aflatoxigenic and atoxigenic Mexican Aspergillus strains by the dichlorvos–ammonia (DV–AM) method. Toxins 2018, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elgawad, M.M.; Omer, E.A. Effect of essential oils of some medicinal plants on phytonematodes. Anz. Schädlingskd. Pflanzenschutz Umweltschutz 1995, 68, 82–84. [Google Scholar] [CrossRef]
- Mondali, N.K.; Mojumdar, A.; Chattterji, S.K.; Banerjee, A.; Datta, J.K.; Gupta, S. Antifungal activities and chemical characterization of Neem leaf extracts on the growth of some selected fungal species in vitro culture medium. J. Appl. Sci. Environ. Manag. 2009, 13, 49–53. [Google Scholar]
- Latif, M.A.; Saleh, A.K.M.; Khan, M.A.I.; Rahman, H.; Hossain, M.A. Efficacy of some plant extracts in controlling seed-borne fungal infections of mustard. Bangladesh J. Microbiol. 1970, 23, 168–170. [Google Scholar] [CrossRef]
- Sitara, U.; Niaz, I.; Naseem, J.; Sultana, N. Antifungal effect of essential oils on in vitro growth of pathogenic fungi. Pak. J. Bot. 2008, 40, 409–414. [Google Scholar]
- Mabrouk, S.S.; El-Shayeb, N.M.A. Inhibition of aflatoxin formation by some spices. Z. Lebensm. Unters. Forsch. 1980, 171, 344–347. [Google Scholar] [CrossRef]
- Chenault, K.D.; Burns, J.A.; Melouk, H.A.; Payton, M.E. Hydrolase activity in transgenic peanut. Peanut Sci. 2002, 29, 89–95. [Google Scholar] [CrossRef]
| Essential Oils | Percentage (%) of Mycelial Inhibition of A. flavus from Contaminated Peanuts Concentrations (ppm) of Essential Oils | |||||
|---|---|---|---|---|---|---|
| 125 | 250 | 500 | 1000 | 2000 | 4000 | |
| Clove | 82.75 ± 2.12 a | 93.63 ± 0.00 a | 100 a | 100 a | 100 a | 100 a |
| Thyme | 74.62 ± 1.75 b | 79.12 ± 0.00 b | 88.87 ± 3.12 b | 100 a | 100 a | 100 a |
| Lavender | 15.25 ± 2.29 f | 29.62 ± 1.23 f | 48.75 ± 2.46 e | 51.25 ± 1.92 f | 64.37 ± 2.11 d | 88.00 ± 2.53 |
| Cumin | 15.62 ± 1.65 f | 28.37 ± 3.86 f | 43.75 ± 1.29 f | 51.62 ± 2.28 f | 65.87 ± 3.86 d | 91.25 ± 2.38 b |
| Spearmint | 24.37 ± 1.36 e | 33.75 ± 3.05 e | 48.62 ± 2.28 e | 58.37 ± 2.40 e | 85.32 ± 2.84 c | 100 a |
| Cardamom | 27.12 ± 2.78 d | 40.37 ± 2.75 d | 47.75 ± 3.62 e | 60.00 ± 2.65 e | 81.25 ± 3.59 c | 100 a |
| Ginger | 0.00 i | 0.00 i | 7.75 ± 1.11 i | 14.62 ± 1.14 | 36.12 ± 2.83 | 57.00 ± 2.50 d |
| Lemongrass | 33.37 ± 1.56 c | 56.25 ± 2.83 c | 84.87 ± 3.39 c | 92.75 ± 3.52 b | 100 a | 100 a |
| Cinnamon | 31.62 ± 3.21 c | 77.87 ± 3.42 b | 85.25 ± 2.74 bc | 91.00 ± 2.50 b | 100 a | 100 a |
| Peppermint | 21.25 ± 2.47 ef | 27.87 ± 1.76 fg | 29.62 ± 2.06 h | 48.37 ± 2.87 fg | 54.75 ± 3.47 f | 86.87 ± 1.73 c |
| Citronella | 25.50 ± 1.27 e | 31.50 ± 2.18 f | 53.75 ± 1.86 d | 85.62 ± 2.64 c | 94.00 ± 1.68 b | 100 a |
| Black pepper | 10.50 ± 1.11 g | 25.50 ± 2.33 g | 41.62 ± 2.39 f | 50.37 ± 3.71 f | 63.87 ± 1.28 d | 91.62 ± 2.89 b |
| Cedarwood | 16.62 ± 2.12 f | 24.50 ± 1.78 g | 31.25 ± 1.07 g | 45.87 ± 2.73 g | 60.87 ± 1.69 e | 94.12 ± 3.68 b |
| Orange | 2.37 ± 1.25 h | 17.50 ± 2.10 h | 27.25 ± 2.25 h | 33.37 ± 1.69 h | 59.62 ± 2.78 g | 84.62 ± 2.45 c |
| Eucalyptus | 23.50 ± 3.21 e | 35.37 ± 1.95 e | 48.87 ± 3.01 e | 73.37 ± 2.28 d | 82.62 ± 3.42 c | 100 a |
| Clove Essential Oil | ||
|---|---|---|
| Components | RI | Area (%) |
| Eugenol | 1392 | 83.25 |
| E-caryophyllene | 1424 | 13.36 |
| α-humulene | 1579 | 2.18 |
| α-cubebene | 1349 | 0.34 |
| Caryophyllene oxide | 1587 | 0.27 |
| Siporlepcenihene | 1452 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achar, P.N.; Quyen, P.; Adukwu, E.C.; Sharma, A.; Msimanga, H.Z.; Nagaraja, H.; Sreenivasa, M.Y. Investigation of the Antifungal and Anti-Aflatoxigenic Potential of Plant-Based Essential Oils against Aspergillus flavus in Peanuts. J. Fungi 2020, 6, 383. https://doi.org/10.3390/jof6040383
Achar PN, Quyen P, Adukwu EC, Sharma A, Msimanga HZ, Nagaraja H, Sreenivasa MY. Investigation of the Antifungal and Anti-Aflatoxigenic Potential of Plant-Based Essential Oils against Aspergillus flavus in Peanuts. Journal of Fungi. 2020; 6(4):383. https://doi.org/10.3390/jof6040383
Chicago/Turabian StyleAchar, Premila Narayana, Pham Quyen, Emmanuel C. Adukwu, Abhishek Sharma, Huggins Zephaniah Msimanga, Hanumanthu Nagaraja, and Marikunte Yanjarappa Sreenivasa. 2020. "Investigation of the Antifungal and Anti-Aflatoxigenic Potential of Plant-Based Essential Oils against Aspergillus flavus in Peanuts" Journal of Fungi 6, no. 4: 383. https://doi.org/10.3390/jof6040383
APA StyleAchar, P. N., Quyen, P., Adukwu, E. C., Sharma, A., Msimanga, H. Z., Nagaraja, H., & Sreenivasa, M. Y. (2020). Investigation of the Antifungal and Anti-Aflatoxigenic Potential of Plant-Based Essential Oils against Aspergillus flavus in Peanuts. Journal of Fungi, 6(4), 383. https://doi.org/10.3390/jof6040383







