Cellular and Molecular Response of Macrophages THP-1 during Co-Culture with Inactive Trichophyton rubrum Conidia
Abstract
1. Introduction
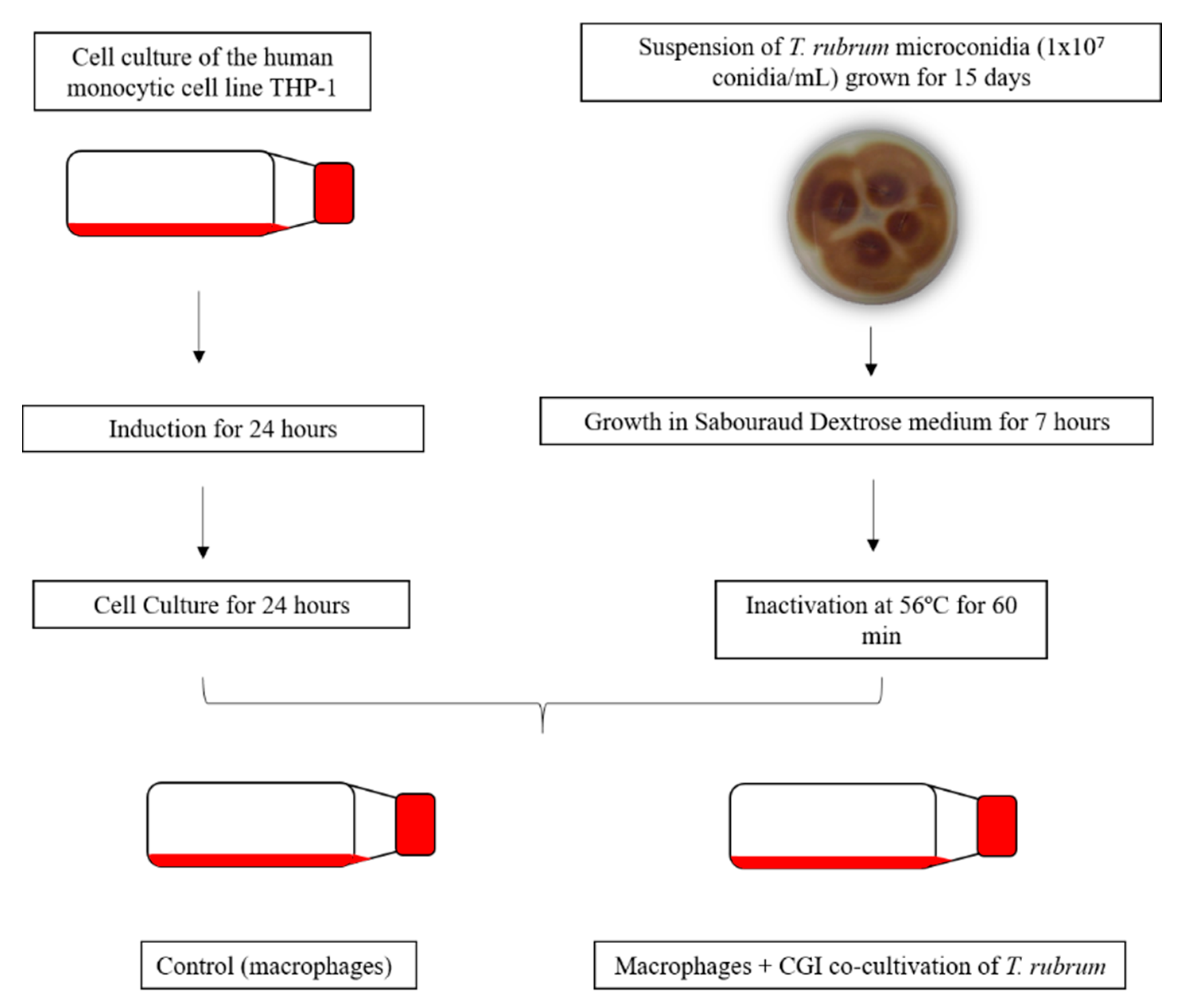
2. Materials and Methods
2.1. Trichophyton Rubrum Strain, Media and Growth Conditions
2.2. Human THP-1-Derived Macrophages, Media and Growth Conditions
2.3. Co-Culture Assay Conditions
2.4. Cell Viability
2.5. Cytokine Quantification
2.6. Determination of Reactive Oxygen Species
2.7. RNA Isolation and Integrity Analysis
2.8. MiSeq
2.9. Sequence Data Analysis
2.10. qRT-PCR Validation
2.11. In Silico Analysis
3. Results
3.1. Quantification of Interleukins in Co-Cultures of Trichophyton Rubrum with Human THP-1 Macrophages
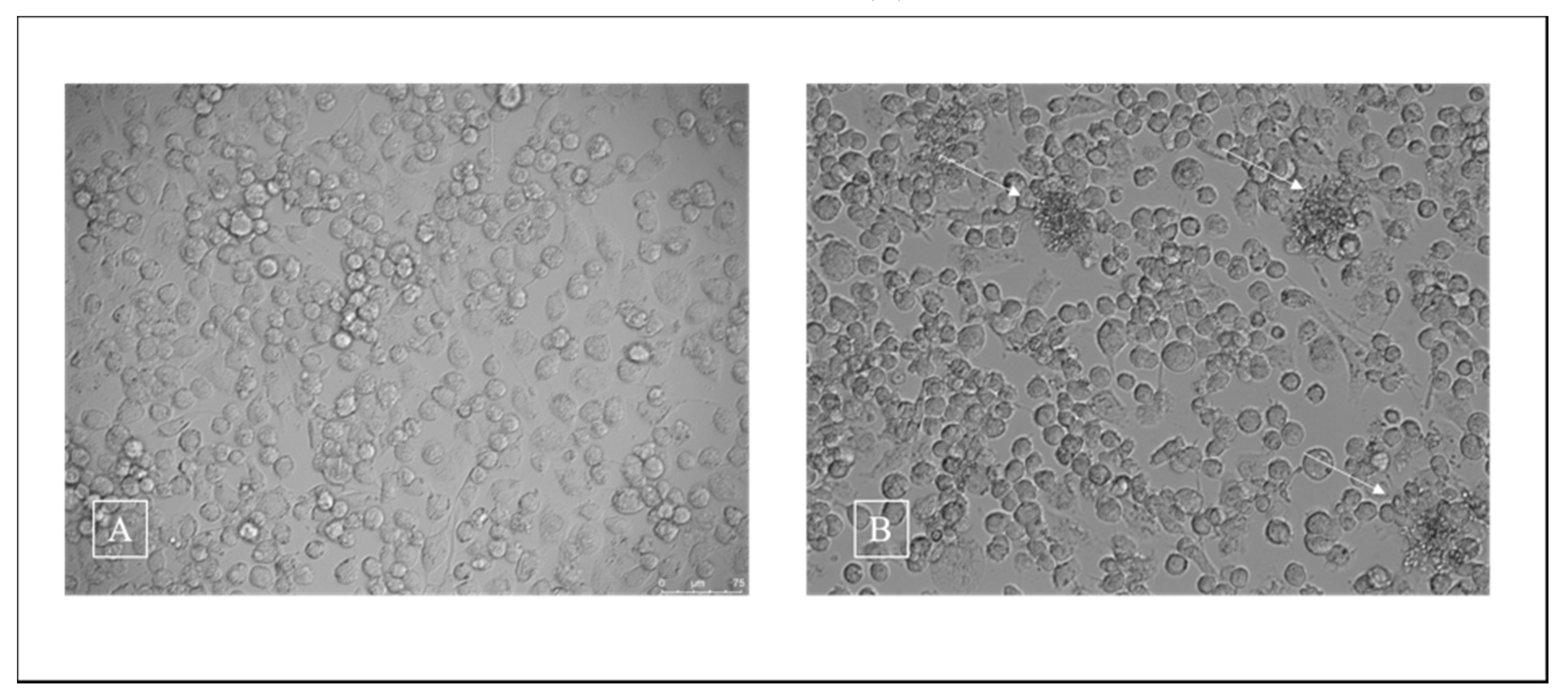
3.2. Cell Viability
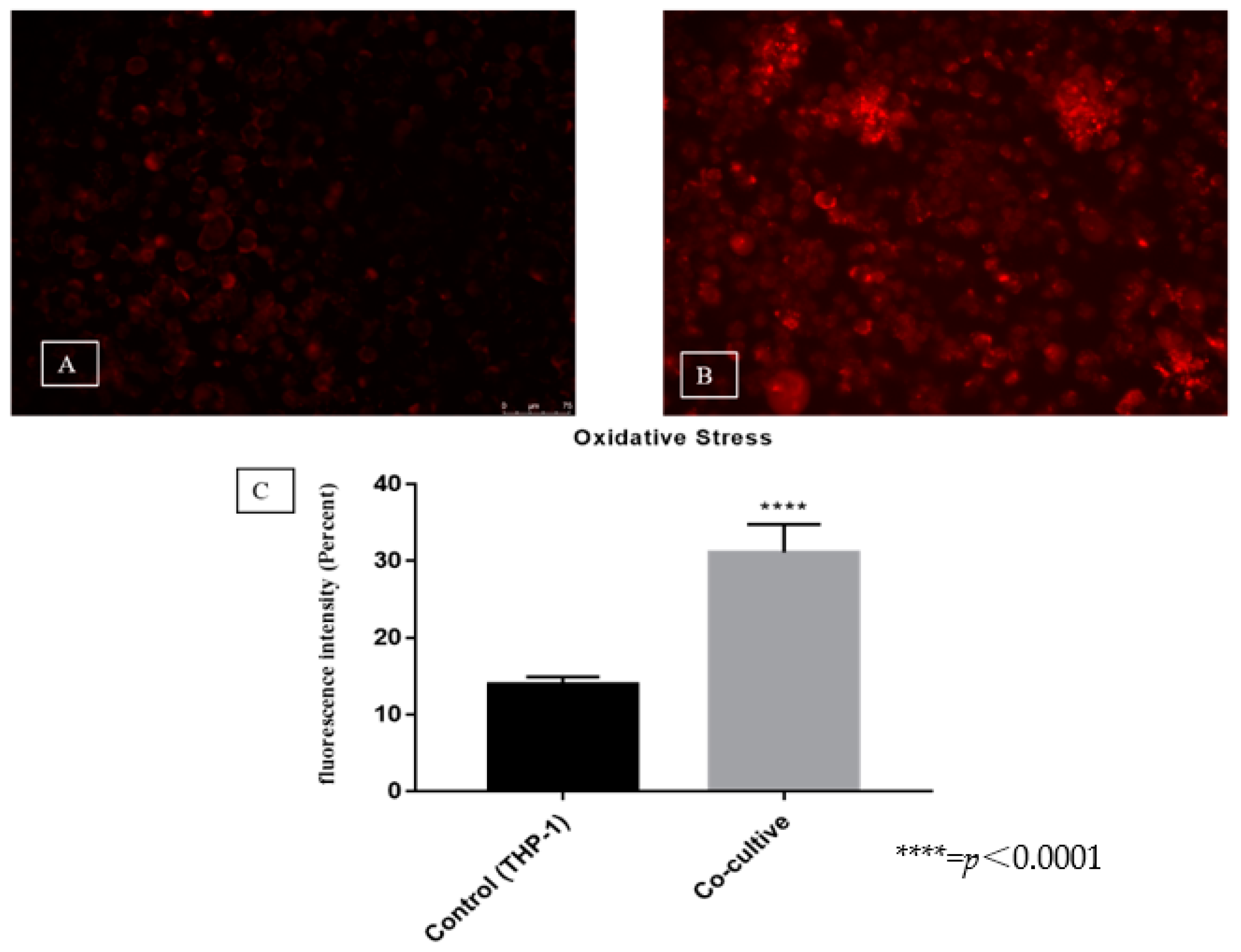
3.3. Evaluation of Reactive Oxygen Species Production
3.4. Differentially Expressed MicroRNAs
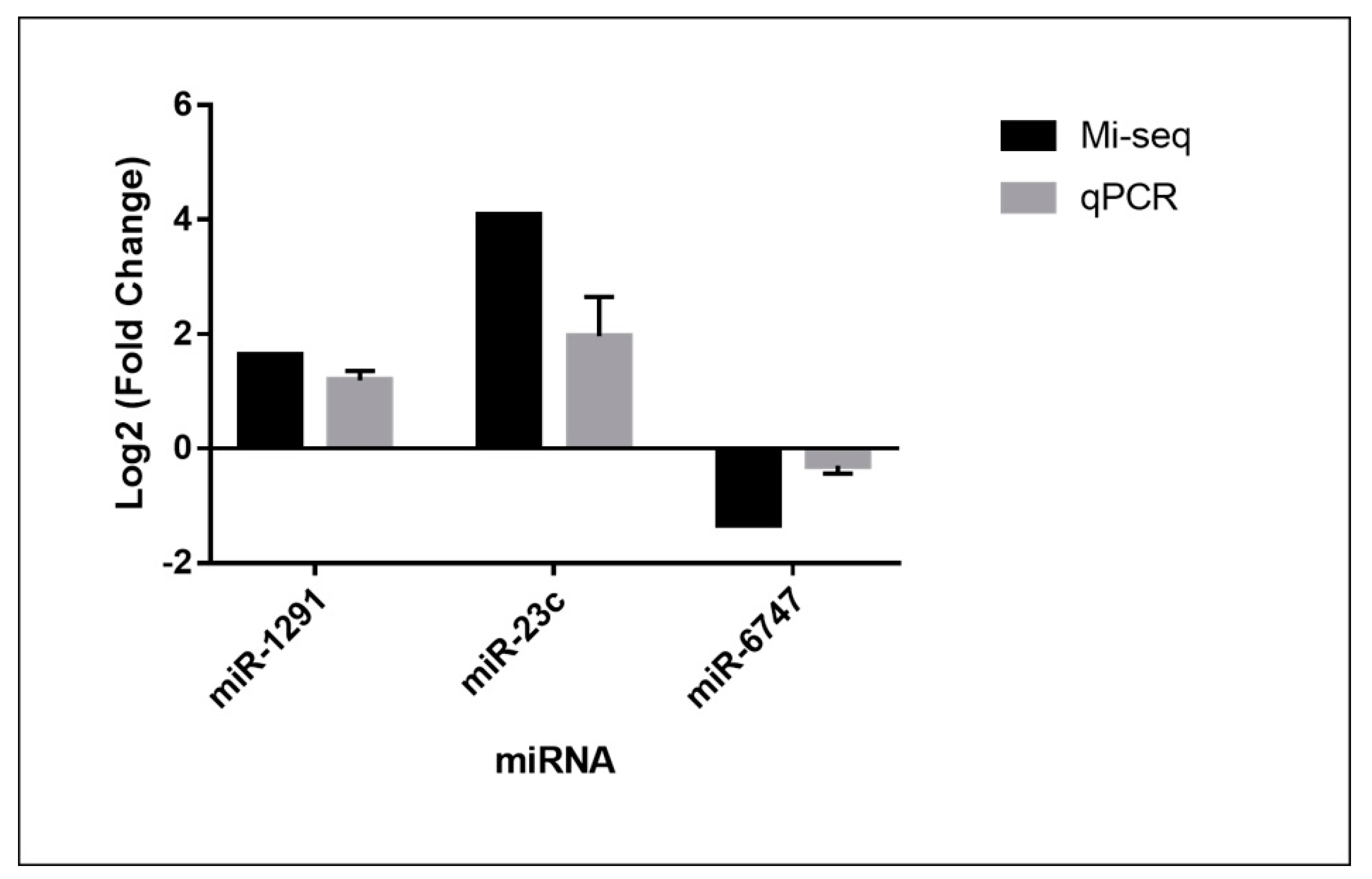
3.5. Validation of MicroRNAs by RT-PCR
3.6. In Silico Analysis of Significantly Modulated MicroRNAs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hube, B.; Hay, R.; Brasch, J.; Veraldi, S.; Schaller, M. Dermatomycoses and inflammation: The adaptive balance between growth, damage, and survival. J. Mycol. Med. 2015, 25, e44–e58. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.E.F.; Neves, R.P.; Delgado, M.M.; Lima-Neto, R.G.; Morais, V.M.S.; Coêlho, M.R.C.D. Dermatophytosis in patients with human immunodeficiency virus infection: Clinical aspects and etiologic agents. Acta Trop. 2015, 150, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Kershenovich, R.; Sherman, S.; Reiter, O.; Huss, S.R.; Didkovsky, E.; Mimouni, D.; Hodak, E.; Segal, R. A Unique Clinicopathological Manifestation of Fungal Infection: A Case Series of Deep Dermatophytosis in Immunosuppressed Patients. Am. J. Clin. Dermatol. 2017, 18, 697–704. [Google Scholar] [CrossRef]
- Kim, S.H.; Jo, I.H.; Kang, J.; Joo, S.Y.; Choi, J.H. Dermatophyte abscesses caused by Trichophyton rubrum in a patient without pre-existing superficial dermatophytosis: A case report. BMC Infect. Dis. 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Faway, É.; Cambier, L.; Mignon, B.; Poumay, Y.; De Rouvroit, C.L. Modeling dermatophytosis in reconstructed human epidermis: A new tool to study infection mechanisms and to test antifungal agents. Med. Mycol. 2017, 55, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Madhvi, A.; Mishra, H.; Leisching, G.R.; Mahlobo, P.Z.; Baker, B. Comparison of human monocyte derived macrophages and THP1-like macrophages as in vitro models for M. tuberculosis infection. Comp. Immunol. Microbiol. Infect. Dis. 2019, 67, 101355. [Google Scholar] [CrossRef]
- Criado, P.R.; de Oliveira, C.B.; Dantas, K.C.; Takiguti, F.A.; Benini, L.V.; Vasconcellos, C. Micoses superficiais e os elementos da resposta imune. An. Bras. Dermatol. 2011, 86, 726–731. [Google Scholar] [CrossRef]
- Calderon, R.A.; Hay, R.J. Fungicidal activity of human neutrophils and monocytes on dermatophyte fungi, Trichophyton quinckeanum and Trichophyton rubrum. Immunology 1987, 61, 289–295. [Google Scholar] [PubMed]
- Mignon, B.; Tabart, J.; Baldo, A.; Mathy, A.; Losson, B.; Vermout, S. Immunization and dermatophytes. Curr. Opin. Infect. Dis. 2008, 21, 134–140. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Raisch, J.; Darfeuille-Michaud, A.; Nguyen, H.T.T. Role of microRNAs in the immune system, inflammation and cancer. World J. Gastroenterol. 2013, 19, 2985–2996. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Saba, R.; Sorensen, D.L.; Booth, S.A. MicroRNA-146a: A dominant, negative regulator of the innate immune response. Front. Immunol. 2014, 5, 578. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Li, Y.; Li, M.; Deng, G.; Wu, X.; Zeng, J.; Hao, X.; Wang, X.; Liu, J.; Cho, W.C.S.; et al. MicroRNA-124 negatively regulates TLR signaling in alveolar macrophages in response to mycobacterial infection. Mol. Immunol. 2014, 62, 150–158. [Google Scholar] [CrossRef]
- Cantelli, B.A.M.; Bitencourt, T.A.; Komoto, T.T.; Beleboni, R.O.; Marins, M.; Fachin, A.L. Caffeic acid and licochalcone A interfere with the glyoxylate cycle of Trichophyton rubrum. Biomed. Pharmacother. 2017, 96, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Petrucelli, M.; Peronni, K.; Sanches, P.; Komoto, T.; Matsuda, J.; Silva, W.; Beleboni, R.; Martinez-Rossi, N.; Marins, M.; Fachin, A. Dual RNA-Seq Analysis of Trichophyton rubrum and HaCat Keratinocyte Co-Culture Highlights Important Genes for Fungal-Host Interaction. Genes 2018, 9, 362. [Google Scholar] [CrossRef]
- Chai, L.Y.A.; Kullberg, B.J.; Vonk, A.G.; Warris, A.; Cambi, A.; Latgé, J.P.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Netea, M.G. Modulation of toll-like receptor 2 (TLR2) and TLR4 responses by Aspergillus fumigatus. Infect. Immun. 2009, 77, 2184–2192. [Google Scholar] [CrossRef]
- Bener, G.; Félix, A.J.; de Diego, C.S.; Fabregat, I.P.; Ciudad, C.J.; Noé, V. Silencing of CD47 and SIRPα by Polypurine reverse Hoogsteen hairpins to promote MCF-7 breast cancer cells death by PMA-differentiated THP-1 cells. BMC Immunol. 2016, 17, 32. [Google Scholar] [CrossRef]
- Santiago, K.; Bomfim, G.F.; Criado, P.R.; Almeida, S.R. Monocyte-Derived Dendritic Cells from Patients with Dermatophytosis Restrict the Growth of Trichophyton rubrum and Induce CD4-T Cell Activation. 2014, 9, 1–8. PLoS ONE 2014, 9, e110879. [Google Scholar] [CrossRef]
- Das Gupta, M.; Fliesser, M.; Springer, J.; Breitschopf, T.; Schlossnagel, H.; Schmitt, A.L.; Kurzai, O.; Hünniger, K.; Einsele, H.; Löffler, J. Aspergillus fumigatus induces microRNA-132 in human monocytes and dendritic cells. Int. J. Med. Microbiol. 2014, 304, 592–596. [Google Scholar] [CrossRef]
- Rizzi, E.; Amaral, J.H.; Guimarães, D.A.; Conde-Tella, S.O.; Pinheiro, L.C.; Gerlach, R.F.; Castro, M.M.; Tanus-Santos, J.E. Nitrite treatment downregulates vascular MMP-2 activity and inhibits vascular remodeling in hypertension independently of its antihypertensive effects. Free Radic. Biol. Med. 2019, 130, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Gee, H.E.; Buffa, F.M.; Camps, C.; Ramachandran, A.; Leek, R.; Taylor, M.; Patil, M.; Sheldon, H.; Betts, G.; Homer, J.; et al. The small-nucleolar RNAs commonly used for microRNA normalisation correlate with tumour pathology and prognosis. Br. J. Cancer 2011, 104, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Bouazzi, D.; Fabricius, S.; Jemec, G.B.; Lindhardt Saunte, D.M. Invasive dermatophytosis mimicking vasculitis. Med. Mycol. Case Rep. 2019, 26, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, Y.; Ota, K.; Sato, K.; Nara, S.; Yaguchi, T.; Nakano, H.; Sawamura, D. Deep pseudocystic dermatophytosis caused by trichophyton rubrum in a patient with myasthenia gravis. Acta Derm. Venereol. 2013, 93, 358–359. [Google Scholar] [CrossRef][Green Version]
- Balci, D.D.; Cetin, M. Widespread, chronic, and fluconazole-resistant Trichophyton rubrum infection in an immunocompetent patient. Mycoses 2008, 51, 546–548. [Google Scholar] [CrossRef]
- Romani, L.; Puccetti, P.; Bistoni, F. Interleukin-12 in infectious diseases. Clin. Microbiol. Rev. 1997, 10, 611–636. [Google Scholar] [CrossRef]
- Yoshikawa, F.S.Y.; Ferreira, L.G.; de Almeida, S.R. IL-1 signaling inhibits trichophyton rubrum conidia development and modulates the IL-17 response in vivo. Virulence 2015, 6, 449–457. [Google Scholar] [CrossRef]
- Granucci, F.; Feau, S.; Angeli, V.; Trottein, F.; Ricciardi-Castagnoli, P. Early IL-2 Production by Mouse Dendritic Cells Is the Result of Microbial-Induced Priming. J. Immunol. 2003, 170, 5075–5081. [Google Scholar] [CrossRef]
- Sancho, D.; Reis e Sousa, C. Signaling by Myeloid C-Type Lectin Receptors in Immunity and Homeostasis. Annu. Rev. Immunol. 2012, 30, 491–529. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, C.; Ren, S. Role of IL-2 in cancer immunotherapy. Oncoimmunology 2016, 5, e1163462. [Google Scholar] [CrossRef] [PubMed]
- Regev-Shoshani, G.; Shoseyov, O.; Bilkis, I.; Kerem, Z. Glycosylation of resveratrol protects it from enzymic oxidation. Biochem. J. 2003, 374, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, S.H. Mitochondrial reactive oxygen species regulate fungal protease-induced inflammatory responses. Toxicology 2017, 378, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, L.D.; Santos, P.C.; de Paula, T.P.; Rachid, M.A.; Cisalpino, P.S.; Souza, D.G.; Santos, D.A. IFN-γ impairs Trichophyton rubrum proliferation in a murine model of dermatophytosis through the production of IL-1β and reactive oxygen species. Med. Mycol. 2014, 52, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Tóth, E.J.; Boros, É.; Hoffmann, A.; Szebenyi, C.; Homa, M.; Nagy, G.; Vágvölgyi, C.; Nagy, I.; Papp, T. Interaction of THP-1 monocytes with conidia and hyphae of different Curvularia strains. Front. Immunol. 2017, 8, 1369. [Google Scholar] [CrossRef]
- Quinn, E.M.; Wang, J.H.; O’Callaghan, G.; Redmond, H.P. MicroRNA-146a Is Upregulated by and Negatively Regulates TLR2 Signaling. PLoS ONE 2013, 8, e62232. [Google Scholar] [CrossRef]
- Smyth, L.A.; Boardman, D.A.; Tung, S.L.; Lechler, R.; Lombardi, G. MicroRNAs affect dendritic cell function and phenotype. Immunology 2015, 144, 197–205. [Google Scholar] [CrossRef]
- Drury, R.E.; O’Connor, D.; Pollard, A.J. The clinical application of MicroRNAs in infectious disease. Front. Immunol. 2017, 8, 1182. [Google Scholar] [CrossRef]
- Agustinho, D.P.; de Oliveira, M.A.; Tavares, A.H.; Derengowski, L.; Stolz, V.; Guilhelmelli, F.; Mortari, M.R.; Kuchler, K.; Silva-Pereira, I. Dectin-1 is required for miR155 upregulation in murine macrophages in response to Candida albicans. Virulence 2017, 8, 41–52. [Google Scholar] [CrossRef]
- Li, X.; Zeng, L.; Cao, C.; Lu, C.; Lian, W.; Han, J.; Zhang, X.; Zhang, J.; Tang, T.; Li, M. Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy. Exp. Cell Res. 2017, 350, 327–335. [Google Scholar] [CrossRef]
- Parada, H.; Veríssimo, C.; Brandão, J.; Nunes, B.; Boavida, J.; Duarte, R.; Peerally, Z.; Oliveira, R.; Rosado, L.; Sabino, R. Dermatomycosis in lower limbs of diabetic patients followed by podiatry consultation. Rev. Iberoam. Micol. 2013, 30, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.N.; Umapathy, D.; Anandharaj, A.; Ravichandran, J.; Sasikumar, C.S.; Chandra, S.K.R.; Kesavan, R.; Kunka Mohanram, R. miR-23c regulates wound healing by targeting stromal cell-derived factor-1α (SDF-1α/CXCL12) among patients with diabetic foot ulcer. Microvasc. Res. 2020, 127, 103924. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.; Hall, S.R.; Perrella, M.A. Role of haem oxygenase-1 in microbial host defence. Cell. Microbiol. 2009, 11, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Ohta, K.; Naruse, T.; Kato, H.; Fukui, A.; Shigeishi, H.; Nishi, H.; Tobiume, K.; Takechi, M. Candida albicans β-glucan-containing particles increase ho-1 expression in oral keratinocytes via a reactive oxygen species/p38 mitogen-activated protein kinase/Nrf2 pathway. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef] [PubMed]
- Lipke, P.N. What we do not know about fungal cell adhesion molecules. J. Fungi 2018, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.E.; Bitencourt, T.A.; Marins, M.; Fachin, A.L. In silico characterization of tandem repeats in Trichophyton rubrum and related dermatophytes provides new insights into their role in pathogenesis. Database (Oxford) 2017, 2017, bax035. [Google Scholar] [CrossRef]
- Bitencourt, T.A.; Macedo, C.; Franco, M.E.; Assis, A.F.; Komoto, T.T.; Stehling, E.G.; Beleboni, R.O.; Malavazi, I.; Marins, M.; Fachin, A.L. Transcription profile of Trichophyton rubrum conidia grown on keratin reveals the induction of an adhesin-like protein gene with a tandem repeat pattern. BMC Genom. 2016, 17, 249. [Google Scholar] [CrossRef]
- Levdansky, E.; Sharon, H.; Osherov, N. Coding fungal tandem repeats as generators of fungal diversity. Fungal Biol. Rev. 2008, 22, 85–96. [Google Scholar] [CrossRef]
- Wang, C.; St Leger, R.J. The MAD1 adhesin of Metarhizium anisopliae links adhesion with blastospore production and virulence to insects, and the MAD2 adhesin enables attachment to plants. Eukaryot. Cell 2007, 6, 808–816. [Google Scholar] [CrossRef]
- de Abreu, M.H.; Bitencourt, T.A.; Franco, M.E.; Moreli, I.S.; Cantelli, B.A.M.; Komoto, T.T.; Marins, M.; Fachin, A.L. Expression of genes containing tandem repeat patterns involved in the fungal-host interaction and in the response to antifungals in Trichophyton rubrum. Mycoses 2020, 63, 610–616. [Google Scholar] [CrossRef]

| Expression | microRNA | Log2 Fold Change | p-Value | Expression | microRNA | Log2 Fold Change | p-Value |
|---|---|---|---|---|---|---|---|
| Upregulated | hsa-miR-1244 | 4.58 | 0.012 | Downregulated | hsa-let-7g-5p | −0.04 | 0.017 |
| hsa-miR-3202 | 4.37 | 0.006 | hsa-let-7i-5p | −0.04 | 0.010 | ||
| hsa-miR-4751 | 4.28 | 0.006 | hsa-miR-148a-3p | −0.06 | 0.000 | ||
| hsa-miR-23c | 4.07 | 0.018 | hsa-miR-450b-5p | −0.16 | 0.042 | ||
| hsa-miR-3680-5p | 3.78 | 0.026 | hsa-miR-19a-3p | −0.29 | 0.000 | ||
| hsa-miR-3129-3p | 3.77 | 0.027 | hsa-miR-10395-3p | −0.34 | 0.041 | ||
| hsa-miR-153-5p | 3.72 | 0.029 | hsa-miR-582-5p | −0.37 | 0.021 | ||
| hsa-miR-5696 | 3.70 | 0.029 | hsa-miR-331-3p | −0.39 | 0.000 | ||
| hsa-miR-4523 | 3.43 | 0.049 | hsa-miR-210-3p | −0.51 | 0.009 | ||
| hsa-miR-3691-3p | 3.42 | 0.050 | hsa-miR-195-5p | −0.52 | 0.037 | ||
| hsa-miR-4507 | 3.42 | 0.050 | hsa-miR-218-5p | −0.67 | 0.050 | ||
| hsa-miR-1292-5p | 2.57 | 0.019 | hsa-miR-34b-3p | −0.67 | 0.036 | ||
| hsa-miR-16-1-3p | 2.37 | 0.009 | hsa-miR-3135b | −0.69 | 0.042 | ||
| hsa-miR-6810-5p | 2.21 | 0.027 | hsa-miR-1246 | −0.85 | 0.033 | ||
| hsa-miR-1291 | 1.62 | 0.037 | hsa-miR-96-5p | −0.86 | 0.030 | ||
| hsa-miR-378h | 1.49 | 0.035 | hsa-miR-769-3p | −0.88 | 0.010 | ||
| hsa-miR-4767 | 1.46 | 0.003 | hsa-miR-33b-3p | −0.89 | 0.046 | ||
| hsa-miR-9901 | 1.46 | 0.018 | hsa-miR-6793-3p | −1.05 | 0.021 | ||
| hsa-miR-885-5p | 1.04 | 0.032 | hsa-miR-6747-3p | −1.33 | 0.033 | ||
| hsa-miR-3188 | 0.97 | 0.041 | hsa-miR-1908-3p | −1.37 | 0.006 | ||
| hsa-miR-590-5p | 0.84 | 0.041 | hsa-miR-548f-3p | −1.40 | 0.000 | ||
| hsa-miR-627-3p | 0.82 | 0.016 | hsa-miR-3619-3p | −1.49 | 0.033 | ||
| hsa-miR-133b | 0.77 | 0.007 | hsa-miR-3187-3p | −1.58 | 0.048 | ||
| hsa-miR-133a-3p | 0.74 | 0.004 | hsa-miR-4786-5p | −1.78 | 0.040 | ||
| hsa-miR-212-5p | 0.69 | 0.000 | hsa-miR-3189-3p | −1.79 | 0.033 | ||
| hsa-miR-1343-3p | 0.65 | 0.028 | hsa-miR-4485-3p | −2.17 | 0.000 | ||
| hsa-miR-224-5p | 0.65 | 0.035 | hsa-miR-10395-5p | −2.22 | 0.000 | ||
| hsa-miR-106a-5p | 0.57 | 0.044 | hsa-miR-548ai | −3.65 | 0.023 | ||
| hsa-miR-193a-3p | 0.46 | 0.003 | hsa-miR-3663-3p | −3.66 | 0.019 | ||
| hsa-miR-501-3p | 0.42 | 0.009 | hsa-miR-5187-3p | −3.87 | 0.024 | ||
| hsa-miR-6511a-3p | 0.34 | 0.043 | hsa-miR-3682-3p | −3.90 | 0.012 | ||
| hsa-miR-1976 | 0.34 | 0.044 | hsa-miR-6876-5p | −4.11 | 0.005 | ||
| hsa-miR-1249-3p | 0.32 | 0.003 | hsa-miR-144-3p | −4.28 | 0.010 | ||
| hsa-miR-23a-5p | 0.28 | 0.001 | |||||
| hsa-miR-29b-3p | 0.27 | 0.001 | |||||
| hsa-miR-100-5p | 0.26 | 0.017 | |||||
| hsa-miR-124-3p | 0.26 | 0.026 | |||||
| hsa-miR-181c-3p | 0.25 | 0.034 | |||||
| hsa-miR-326 | 0.24 | 0.004 | |||||
| hsa-miR-2116-3p | 0.24 | 0.004 | |||||
| hsa-miR-1301-3p | 0.22 | 0.007 | |||||
| hsa-miR-378c | 0.17 | 0.001 | |||||
| hsa-miR-378d | 0.16 | 0.000 | |||||
| hsa-miR-378a-3p | 0.15 | 0.000 | |||||
| hsa-miR-941 | 0.14 | 0.000 | |||||
| hsa-miR-221-3p | 0.11 | 0.001 | |||||
| hsa-miR-30e-3p | 0.08 | 0.008 | |||||
| hsa-miR-30a-3p | 0.08 | 0.007 |
| miRNA | Target Genes | Pathways Involved |
|---|---|---|
| hsa-miR-1244 | MAPK1-CSNK1A1-ACER2-AVPR1A1-TDGF1P3-HSP90AA1-SMAD7-ABHD2-RAB10-TMEM161B-HSBP1-UQCRB-AKR1B10 | Beta cells activation; cytokine- and chemokine-mediated inflammation; integrity signaling; T cell activation; immune system; stimulus response |
| hsa-miR-3202 | VAMP3-PLCG2-CACNB1-SDK1-UBE25-TRAF6-MYH2-ARRB2-SMARCD1-TNFSF15-FPR1-SESN2-HSPA6-CCL16-SXT7-NR1H2-PKM-MAG-JUNB-UBE4B-RNF185 | 5HT1, 5HT2, 5HT3, 5HT4 receptors; Beta cells activation; β1, β2 and β3 adrenergic receptors, release of corticotropin, histamine; ubiquitin; P38 MAPK; WNT; immune system; stimulus response |
| hsa-miR-1291 | SPINT3-CHRNB2-ERN1-TAPBP-LIMD1- HO-1 | Stimulus response |
| miRNA | Target Genes | Pathways Involved |
|---|---|---|
| hsa-miR-6747 | PRKX-GNB5-SYK-ITPR2-IL6R-SPIB-FOXO3-SPIC-SRF-UBE2B-TXR-HMGB1-GBP4-TLR10-SLFN13-FPR1-TLR7-BMPR1A-UGGT2-SIGLEC9-DENNDSB-GSR-PAQR7-SGTB-F2-SSR1-RBM43-FCAR-CCS-KCNMB1- | 5HT1, 5HT2, 5HT3, 5HT4 receptors; β cell activation; interleukin, corticotropin release; ubiquitin; P38 MAPK; immune system; stimulus response |
| hsa-miR-3682-3p | MYLK-UBA6-TXLNG-SRRT-IL7R | Cytokine-mediated inflammation; ubiquitin; immune system; stimulus response |
| hsa-miR-144-3p | CPS1-RAC1-MAP3K4-GNG12-UBE2A-FZD6-SMARCAS-SMAD4-FBXW7-ARIDIA-ARID1B-MYCN-WNT7A-MAP3K4-PTGS2-GNG12-DAB2-ACSL4-PTGS2-DENNDSB-HSPA13-YOD1-TGFB1-IRS1-LEFTY1 | Arginine biosynthesis; β cell activation; stress response; T cell activation; cytokine receptor-mediated signaling; ubiquitin; WNT; P38 MAPK; Toll receptor; MYO signaling, histamine H1 and H2; immune response; stimulus response |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez Segura, G.; Cantelli, B.A.; Peronni, K.; Rodrigo Sanches, P.; Komoto, T.T.; Rizzi, E.; Beleboni, R.O.; da Silva Junior, W.A.; Martinez-Rossi, N.M.; Marins, M.; et al. Cellular and Molecular Response of Macrophages THP-1 during Co-Culture with Inactive Trichophyton rubrum Conidia. J. Fungi 2020, 6, 363. https://doi.org/10.3390/jof6040363
Gonzalez Segura G, Cantelli BA, Peronni K, Rodrigo Sanches P, Komoto TT, Rizzi E, Beleboni RO, da Silva Junior WA, Martinez-Rossi NM, Marins M, et al. Cellular and Molecular Response of Macrophages THP-1 during Co-Culture with Inactive Trichophyton rubrum Conidia. Journal of Fungi. 2020; 6(4):363. https://doi.org/10.3390/jof6040363
Chicago/Turabian StyleGonzalez Segura, Gabriela, Bruna Aline Cantelli, Kamila Peronni, Pablo Rodrigo Sanches, Tatiana Takahasi Komoto, Elen Rizzi, Rene Oliveira Beleboni, Wilson Araújo da Silva Junior, Nilce Maria Martinez-Rossi, Mozart Marins, and et al. 2020. "Cellular and Molecular Response of Macrophages THP-1 during Co-Culture with Inactive Trichophyton rubrum Conidia" Journal of Fungi 6, no. 4: 363. https://doi.org/10.3390/jof6040363
APA StyleGonzalez Segura, G., Cantelli, B. A., Peronni, K., Rodrigo Sanches, P., Komoto, T. T., Rizzi, E., Beleboni, R. O., da Silva Junior, W. A., Martinez-Rossi, N. M., Marins, M., & Fachin, A. L. (2020). Cellular and Molecular Response of Macrophages THP-1 during Co-Culture with Inactive Trichophyton rubrum Conidia. Journal of Fungi, 6(4), 363. https://doi.org/10.3390/jof6040363





