Invasive Mold Infection of the Central Nervous System in Immunocompromised Children
Abstract
1. Introduction
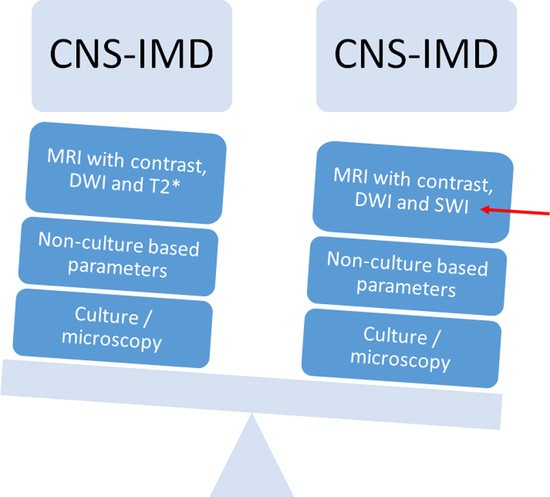
2. Materials and Methods
- (1)
- DWI and ADC were evaluated for infarction, lesions with central restricted diffusion, lesions with a rim of restricted diffusion, and others. Infarction was defined as territorial, cortical, and sub-cortical lesions without nodular or ring-like aspect. Non-infarction-DWI restrictions, with nodular or ring-like aspect, were classified as lesions with central or rim-like restricted diffusion. Others included patients with cerebritis and bleeding.
- (2)
- SWI/T2* were evaluated as follows:
- Parenchymal bleeding was defined as a hypointense parenchymal signal with “blooming”.
- Cluster of microbleeding was defined as a significantly hypointense cluster signal.
- Perivascular microbleeding was defined as a significantly hypointense perivascular signal.
- Ring-like was defined as a hypointense ring-like signal.
- Vascular blooming was defined as a vascular structure with signal loss and prominent “blooming” on SWI/T2*w sequences.
- (3)
- Focal or multifocal involvement as well as the type of enhancement on T1 images after contrast were evaluated.
3. Results
3.1. Patient Demographics and Outcome
3.2. Diffusion Imaging Aspects and Outcome
3.3. T2*, SWI Aspects Compared to Outcome
3.4. Parenchymal Abnormalities: Focal and Multifocal Involvement and Outcome
4. Discussion
4.1. Interpreting MRI Imaging of Invasive Mold Infection in Children—Diffusion Imaging
4.2. Interpreting MRI Imaging of Invasive Mold Infection in Children—T2*/ SWI Aspects
4.3. Interpreting MRI Imaging of Invasive Mold Infection in Children—Parenchymal Involvement and Outcome
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lauten, M.; Attarbaschi, A.; Cario, G.; Döring, M.; Moser, O.; Mücke, U.; Poyer, F.; Rieken, S.; Temme, C.; Voigt, S.; et al. Invasive mold disease of the central nervous system in children and adolescents with cancer or undergoing hematopoietic stem cell transplantation: Analysis of 29 contemporary patients. Pediatr. Blood Cancer 2019, 66, e27806. [Google Scholar] [CrossRef] [PubMed]
- Haßler, A.; Porto, L.; Lehrnbecher, T. Cerebral Fungal Infection in Pediatric Cancer Patients. Curr. Fungal Infect. Rep. 2015, 9, 6–14. [Google Scholar] [CrossRef]
- Wang, Y.; Roilides, E.; Qiao, L.; Zhao, F. Infant Central Nervous System Aspergillosis with First-episode of Intracranial Hemorrhage. Medicine 2017, 96, e8893. [Google Scholar] [CrossRef] [PubMed]
- Blazicevich-Carrillo, L.; Camacho, L.M.C.; Carrizosa, J.; Cornejo, W. Clinical and radiological findings in two cases of aspergillosis of the central nervous system in children. Rev. Neurol. 2003, 36, 632–635. [Google Scholar]
- Gupta, K.; Banerjee, A.; Saggar, K.; Ahluwalia, A.; Saggar, K. A prospective study of magnetic resonance imaging patterns of central nervous system infections in pediatric age group and young adults and their clinico-biochemical correlation. J. Pediatr. Neurosci. 2016, 11, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Sung, L.; Phillips, R.; Lehrnbecher, T. Time for paediatric febrile neutropenia guidelines—Children are not little adults. Eur. J. Cancer 2011, 47, 811–813. [Google Scholar] [CrossRef] [PubMed]
- Lehrnbecher, T.; Rath, P.M.; Attarbaschi, A.; Cario, G.; Döring, M.; Moser, O.; Mücke, U.; Poyer, F.; Rieken, S.; Temme, C.; et al. Galactomannan and PCR in the Central Nervous System to Detect Invasive Mold Disease—A Retrospective Analysis in Immunocompromised Children. Sci. Rep. 2019, 9, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tibanyenda, N.; De Bruin, S.H.; Haasnoot, C.A.; Van Der Marel, G.A.; Van Boom, J.H.; Hilbers, C.W. The effect of single base-pair mismatches on the duplex stability of d(T-A-T-T-A-A-T-A-T-C-A-A-G-T-T-G) · d(C-A-A-C-T-T-G-A-T-A-T-T-A-A-T-A). JBIC J. Biol. Inorg. Chem. 1984, 139, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Britt, R.H.; Enzmann, D.R.; Placone, R.C.; Obana, W.G.; Yeager, A.S. Experimental anaerobic brain abscess. J. Neurosurg. 1984, 60, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Antulov, R.; Dolic, K.; Fruehwald-Pallamar, J.; Miletic, D.; Thurnher, M.M. Differentiation of pyogenic and fungal brain abscesses with susceptibility-weighted MR sequences. Neuroradiology 2014, 56, 937–945. [Google Scholar] [CrossRef]
- Luthra, G.; Parihar, A.; Nath, K.; Jaiswal, S.; Prasad, K.; Husain, N.; Husain, M.; Singh, S.; Behari, S.; Gupta, R. Comparative Evaluation of Fungal, Tubercular, and Pyogenic Brain Abscesses with Conventional and Diffusion MR Imaging and Proton MR Spectroscopy. Am. J. Neuroradiol. 2007, 28, 1332–1338. [Google Scholar] [CrossRef]
- Aljuboori, Z.; Hruska, R.; Yaseen, A.; Arnold, F.; Wojda, B.; Nauta, H.J. Fungal brain abscess caused by “Black Mold” (Cladophialophora bantiana)—A case report of successful treatment with an emphasis on how fungal brain abscess may be different from bacterial brain abscess. Surg. Neurol. Int. 2017, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Mathur, M.; Johnson, C.E.; Sze, G. Fungal Infections of the Central Nervous System. Neuroimaging Clin. North Am. 2012, 22, 609–632. [Google Scholar] [CrossRef]
- Dietrich, U.; Hettmann, M.; Maschke, M.; Doerfler, A.; Schwechheimer, K.; Forsting, M. Cerebral aspergillosis: Comparison of radiological and neuropathologic findings in patients with bone marrow transplantation. Eur. Radiol. 2001, 11, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Marzolf, G.; Sabou, M.; Lannes, B.; Cotton, P.F.; Meyronet, D.; Galanaud, D.; Cottier, J.-P.; Grand, S.; Desal, H.; Kreutz, J.; et al. Magnetic Resonance Imaging of Cerebral Aspergillosis: Imaging and Pathological Correlations. PLoS ONE 2016, 11, e0152475. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Shrier, D.A.; Rubio, A.; Shan, Y.; Zoarski, G.H.; Yoshiura, T.; Iwanaga, S.; Nishimura, T.; Numaguchi, Y. Imaging findings in intracranial aspergillosis. Acad. Radiol. 2002, 9, 163–171. [Google Scholar] [CrossRef]


| Patients | Number |
|---|---|
| Female/Male | 5/14 |
| Age | Number |
| 2- < 5 yr. | 5 |
| 5- < 10 yr. | 3 |
| 10–18 yr. | 11 |
| Outcome | Number |
| No severe neurological sequelae | 7 (1 infarction) |
| Severe neurological sequela | 5 (3 infarctions) |
| Deceased | 7 (3 infarctions) |
| Pathogen | Number |
| Aspergillus fumigatus | 9 |
| Aspergillus fumigatus, Rhizopus arrhizus | 1 |
| Rhizomucor pusillus | 1 |
| Hyphae | 5 |
| Criteria of probable CNS-IMD without pathogen specified | 3 |
| Outcome | Infarction | Central Restricted Diffusion | Rim Restricted Diffusion | Others |
|---|---|---|---|---|
| Total number of patients | 7 | 10 | 3 | 2 |
| Died | 3 | 4 | 1 | |
| Severe neurological sequelae | 3 | 3 | 0 | 1 (cerebritis) |
| Favorable outcome | 1 (focal pericallosal vasculitis) | 3 | 2 | 1 (bleeding) |
| Outcome | Parenchymal Bleeding | Cluster of Microbleeds | Perivascular Microbleeding | Rim-Like | Vascular Blooming |
|---|---|---|---|---|---|
| Total number of patients | 5 | 4 | 3 | 9 | 2 |
| Died | 2 | 1 | 2 | 4 | 1 |
| Severe neurological sequelae | 0 | 2 | 1 | 2 | 0 |
| Favorable outcome | 3 | 1 | 0 | 3 | 1 |
| Outcome | Parenchymal Multifocal (Lobes) | Parenchymal 1 lobe | Enhancement Ring-Like | Enhancement Nodular | Enhancement Subependymal No Ventriculitis | Enhancement Diffuse Parenchymal |
|---|---|---|---|---|---|---|
| Total number of patients | 15 | 2 | 14 | 3 | 2 | 1 |
| Died | 7 | 0 | 5 | 1 | 1 | 0 |
| Severe Neurological sequelae | 2 | 1 | 3 | 1 | 1 | 1 |
| Favorable outcome | 6 | 1 | 6 | 1 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porto, L.; You, S.-J.; Attarbaschi, A.; Cario, G.; Döring, M.; Moser, O.; Mücke, U.; Poyer, F.; Temme, C.; Voigt, S.; et al. Invasive Mold Infection of the Central Nervous System in Immunocompromised Children. J. Fungi 2020, 6, 226. https://doi.org/10.3390/jof6040226
Porto L, You S-J, Attarbaschi A, Cario G, Döring M, Moser O, Mücke U, Poyer F, Temme C, Voigt S, et al. Invasive Mold Infection of the Central Nervous System in Immunocompromised Children. Journal of Fungi. 2020; 6(4):226. https://doi.org/10.3390/jof6040226
Chicago/Turabian StylePorto, Luciana, Se-Jong You, Andishe Attarbaschi, Gunnar Cario, Michaela Döring, Olga Moser, Urs Mücke, Fiona Poyer, Christian Temme, Sebastian Voigt, and et al. 2020. "Invasive Mold Infection of the Central Nervous System in Immunocompromised Children" Journal of Fungi 6, no. 4: 226. https://doi.org/10.3390/jof6040226
APA StylePorto, L., You, S.-J., Attarbaschi, A., Cario, G., Döring, M., Moser, O., Mücke, U., Poyer, F., Temme, C., Voigt, S., Groll, A. H., Lauten, M., Hattingen, E., & Lehrnbecher, T. (2020). Invasive Mold Infection of the Central Nervous System in Immunocompromised Children. Journal of Fungi, 6(4), 226. https://doi.org/10.3390/jof6040226