Self-Inhibitory Activity of Trichoderma Soluble Metabolites and Their Antifungal Effects on Fusarium oxysporum
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Strains and Culture Conditions
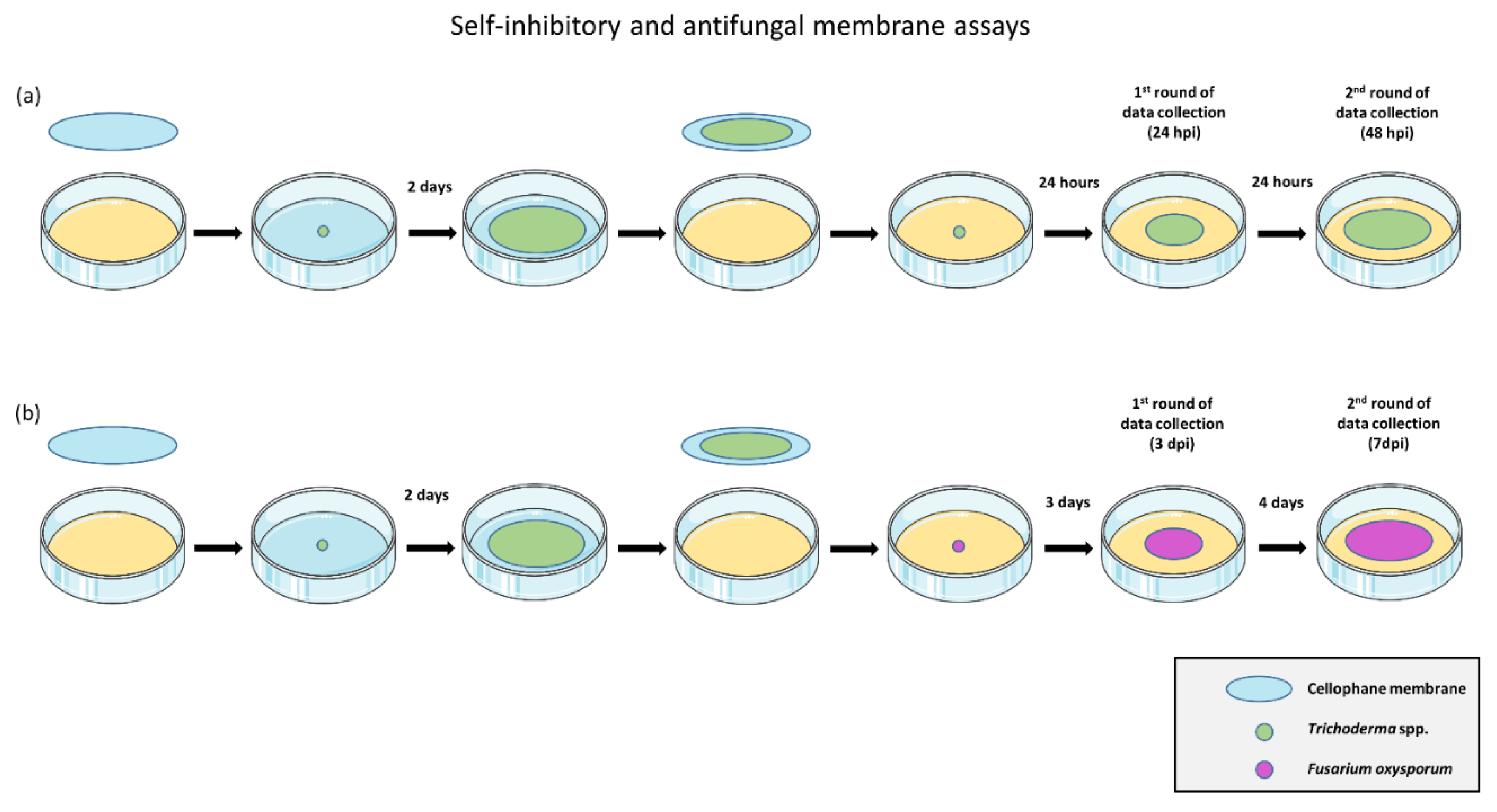
2.2. In Vitro Evaluation of Antifungal and Self-Inhibitory Activity of Soluble Metabolites Produced by Trichoderma spp.
2.3. Data Treatment and Statistical Analysis
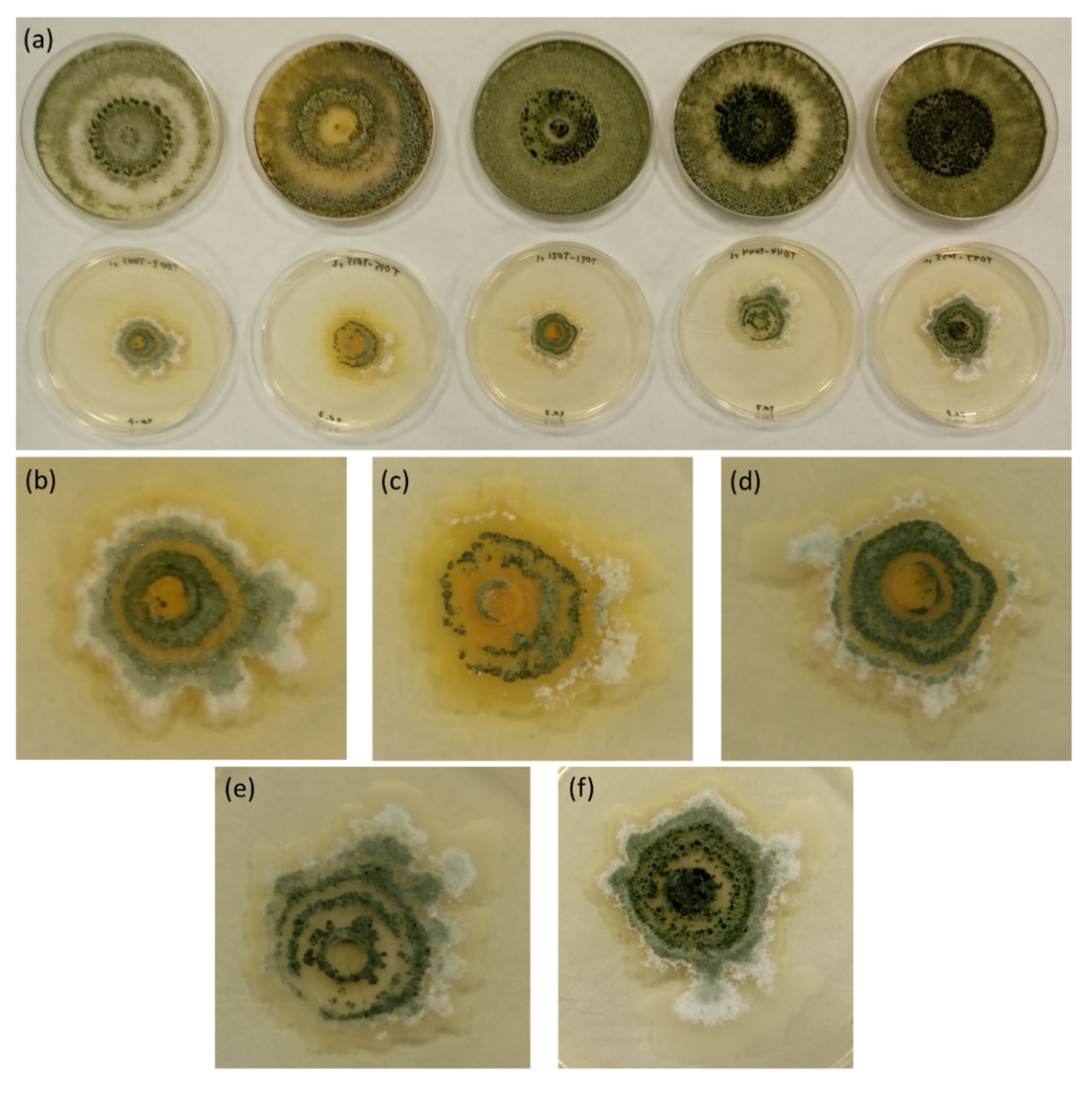
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mazzoleni, S.; Cartenì, F.; Bonanomi, G.; Senatore, M.; Termolino, P.; Giannino, F.; Incerti, G.; Rietkerk, M.; Lanzotti, V.; Chiusano, M.L. Inhibitory effects of extracellular self-DNA: A general biological process? New Phytol. 2015, 206, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.A. Talking to themselves: Autoregulation and quorum sensing in fungi. Eukaryot. Cell 2006, 5, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Gillot, G.; Decourcelle, N.; Dauer, G.; Barbier, G.; Coton, E.; Delmail, D.; Mounier, J. 1-Octanol, a self-inhibitor of spore germination in Penicillium camemberti. Food Microbiol. 2016, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Polyanskaya, L.M.; Tolstikhina, T.E.; Kochkina, G.A.; Ivanushkina, N.E.; Zvyagintsev, D.G. Regularities in the germination of conidia of phytopathogenic fungi. Microbiology 2004, 73, 383–388. [Google Scholar] [CrossRef]
- Chitarra, G.S.; Abee, T.; Rombouts, F.M.; Posthumus, M.A.; Dijksterhuis, J. Germination of Penicillium paneum conidia is regulated by 1-octen-3-ol, a volatile self-inhibitor. Appl. Environ. Microbiol. 2004, 70, 2823–2829. [Google Scholar] [CrossRef]
- Bottone, E.J.; Nagarsheth, N.; Chiu, K. Evidence of self-inhibition by filamentous fungi accounts for unidirectional hyphal growth in colonies. Can. J. Microbiol. 1998, 44, 390–393. [Google Scholar] [CrossRef]
- Mayo, S.; Gutiérrez, S.; Malmierca, M.G.; Lorenzana, A.; Campelo, M.P.; Hermosa, R.; Casquero, P.A. Influence of Rhizoctonia solani and Trichoderma spp. in growth of bean (Phaseolus vulgaris L.) and in the induction of plant defense-related genes. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Zachow, C.; Berg, C.; Müller, H.; Monk, J.; Berg, G. Endemic plants harbour specific Trichoderma communities with an exceptional potential for biocontrol of phytopathogens. J. Biotechnol. 2016, 235, 162–170. [Google Scholar] [CrossRef]
- Szczałba, M.; Kopta, T.; Gąstoł, M.; Sękara, A. Comprehensive insight into arbuscular mycorrhizal fungi, Trichoderma spp. and plant multilevel interactions with emphasis on biostimulation of horticultural crops. J. Appl. Microbiol. 2019, 127, 630–647. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016, 92, fiw036. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Rai, P.; Srivastava, A.K.; Kumar, S. Trichoderma for climate resilient agriculture. World J. Microbiol. Biotechnol. 2017, 33, 155. [Google Scholar] [CrossRef]
- de Lamo, F.J.; Takken, F.L.W. Biocontrol by Fusarium oxysporum Using Endophyte-Mediated Resistance. Front. Plant Sci. 2020, 11, 37. [Google Scholar] [CrossRef]
- de Borba, M.C.; Garcés-Fiallos, F.R.; Stadnik, M.J. Reactions of black bean seedlings and adult plants to infection by Fusarium oxysporum f. sp. phaseoli. Crop Prot. 2017, 96, 221–227. [Google Scholar] [CrossRef]
- Li, N.; Alfiky, A.; Wang, W.; Islam, M.; Nourollahi, K.; Liu, X.; Kang, S. Volatile Compound-Mediated Recognition and Inhibition Between Trichoderma Biocontrol Agents and Fusarium oxysporum. Front. Microbiol. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Mutawila, C.; Vinale, F.; Halleen, F.; Lorito, M.; Mostert, L. Isolation, production and in vitro effects of the major secondary metabolite produced by Trichoderma species used for the control of grapevine trunk diseases. Plant Pathol. 2016, 65, 104–113. [Google Scholar] [CrossRef]
- Gotor-Vila, A.; Teixidó, N.; Di Francesco, A.; Usall, J.; Ugolini, L.; Torres, R.; Mari, M. Antifungal effect of volatile organic compounds produced by Bacillus amyloliquefaciens CPA-8 against fruit pathogen decays of cherry. Food Microbiol. 2017, 64, 219–225. [Google Scholar] [CrossRef]
- Mayo-Prieto, S.; Campelo, M.P.; Lorenzana, A.; Rodríguez-González, A.; Reinoso, B.; Gutiérrez, S.; Casquero, P.A. Antifungal activity and bean growth promotion of Trichoderma strains isolated from seed vs soil. Eur. J. Plant Pathol. 2020. [Google Scholar] [CrossRef]
- Carvalho, D.D.C.; Lobo Júnior, M.; Martins, I.; Inglis, P.W.; Mello, S.C.M. Biological control of Fusarium oxysporum f. sp. phaseoli by Trichoderma harzianum and its use for common bean seed treatment. Trop. Plant Pathol. 2014, 39, 384–391. [Google Scholar] [CrossRef]
- John, R.P.; Tyagi, R.D.; Prévost, D.; Brar, S.K.; Pouleur, S.; Surampalli, R.Y. Mycoparasitic Trichoderma viride as a biocontrol agent against Fusarium oxysporum f. sp. adzuki and Pythium arrhenomanes and as a growth promoter of soybean. Crop Prot. 2010, 29, 1452–1459. [Google Scholar] [CrossRef]
- Zotti, M.; De Filippis, F.; Cesarano, G.; Ercolini, D.; Tesei, G.; Allegrezza, M.; Giannino, F.; Mazzoleni, S.; Bonanomi, G. One ring to rule them all: An ecosystem engineer fungus fosters plant and microbial diversity in a Mediterranean grassland. New Phytol. 2020. [Google Scholar] [CrossRef]
- Mucha, J.; Zadworny, M.; Werner, A. Cytoskeleton and mitochondrial morphology of saprotrophs and the pathogen Heterobasidion annosum in the presence of Suillus bovinus metabolites. Mycol. Res. 2009, 113, 981–990. [Google Scholar] [CrossRef]
- Mucha, J. Changes in hyphal morphology and activity of phenoloxidases during interactions between selected ectomycorrhizal fungi and two species of Trichoderma. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2011, 100, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Carrero-Carrón, I.; Trapero-Casas, J.L.; Olivares-García, C.; Monte, E.; Hermosa, R.; Jiménez-Díaz, R.M. Trichoderma asperellum is effective for biocontrol of Verticillium wilt in olive caused by the defoliating pathotype of Verticillium dahliae. Crop Prot. 2016, 88. [Google Scholar] [CrossRef]
- Jensen, B.D.; Knorr, K.; Nicolaisen, M. In vitro competition between Fusarium graminearum and Epicoccum nigrum on media and wheat grains. Eur. J. Plant Pathol. 2016, 146, 657–670. [Google Scholar] [CrossRef]
- Taghdi, Y.; Hermosa, R.; Domínguez, S.; Rubio, M.B.; Essalmani, H.; Nicolás, C.; Monte, E. Effectiveness of composts and Trichoderma strains for control of Fusarium wilt of tomato. Phytopathol. Mediterr. 2015, 54, 232–240. [Google Scholar] [CrossRef]
- Malmierca, M.G.; Cardoza, R.E.; Alexander, N.J.; McCormick, S.P.; Hermosa, R.; Monte, E.; Gutiérrez, S. Involvement of Trichoderma trichothecenes in the biocontrol activity and induction of plant defense-related genes. Appl. Environ. Microbiol. 2012, 78, 4856–4868. [Google Scholar] [CrossRef]
- Kron, A.S.; Zengerer, V.; Bieri, M.; Dreyfuss, V.; Sostizzo, T.; Schmid, M.; Lutz, M.; Remus-Emsermann, M.N.P.; Pelludat, C. Pseudomonas orientalis F9 pyoverdine, safracin, and phenazine mutants remain effective antagonists against erwinia amylovora in apple flowers. Appl. Envsiron. Microbiol. 2020, 86. [Google Scholar] [CrossRef]
| Code | Trichoderma Species | Crop | Source | Municipality | Area |
|---|---|---|---|---|---|
| T002 | T. harzianum | Riñón menudo (bean) | seed | Moscas del Páramo | El Páramo |
| T007 | T. harzianum | Pinta (bean) | seed | Sueros de Cepeda | Astorga |
| T008 | T. citrinoviride | Pinta (bean) | seed | Fresno de la Vega | Esla-Campos |
| T015 | T. harzianum | Riñón menudo (bean) | seed | Veguellina de Fondo | El Páramo |
| T021 | T. harzianum | Pinta (bean) | seed | Altobar de la Encomienda | El Páramo |
| T028 | T. velutinum | Riñón (bean) | soil | Otero de Escarpizo | Astorga |
| T044 | T. harzianum | Riñón (bean) | soil | Javares de los Oteros | Esla-Campos |
| T050 | T. harzianum | Canela (bean) | soil | Bercianos del Páramo | El Páramo |
| T055 | T. harzianum | Sugarbeet | soil | La Milla del Páramo | El Páramo |
| T057 | T. gamsii | Sugarbeet | soil | La Milla del Páramo | El Páramo |
| First Round of Data Collection | Second Round of Data Collection | ||||||||
|---|---|---|---|---|---|---|---|---|---|
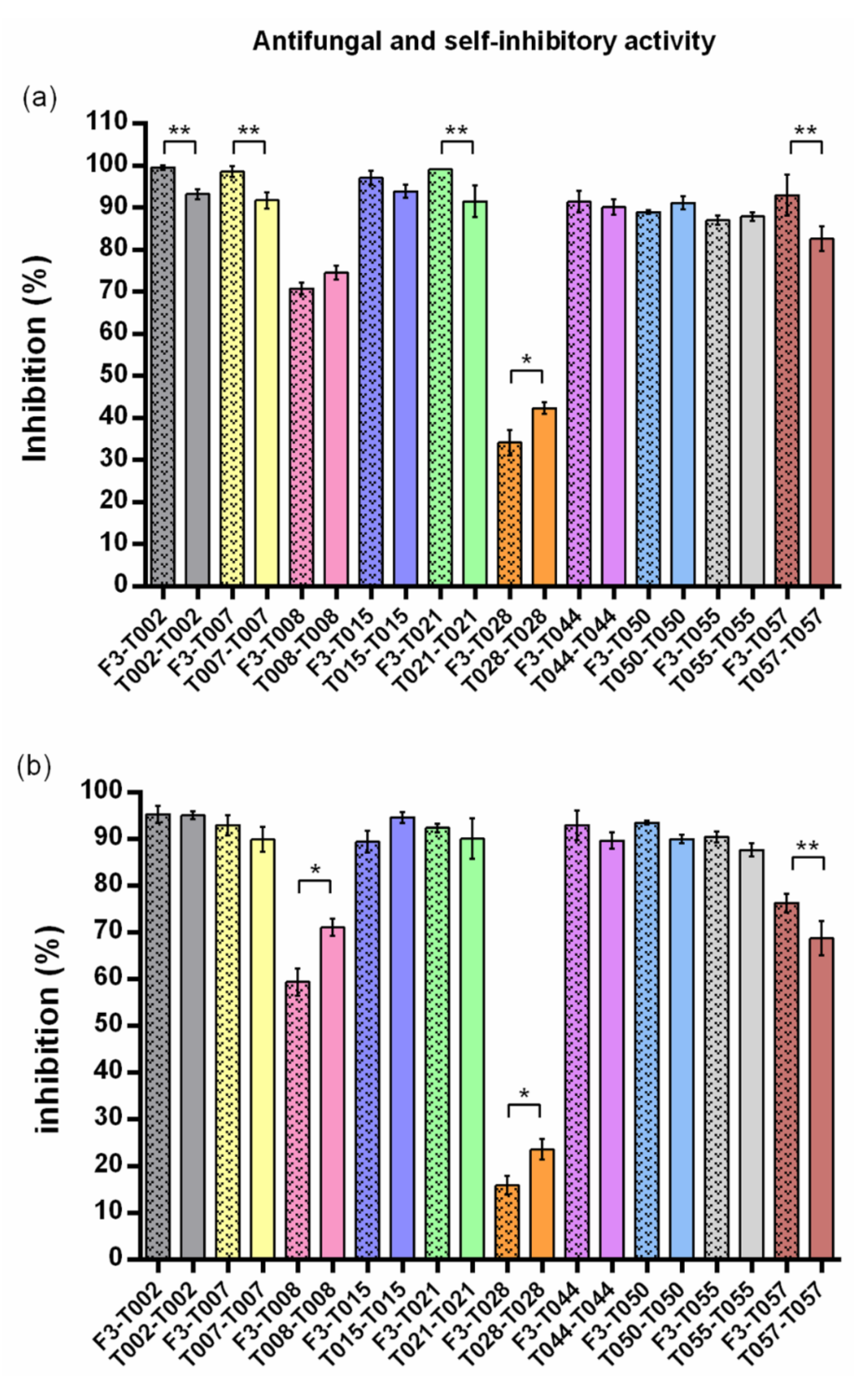
| Code | Trichoderma | Self-Inhibition (% ± SD) | Statistics 1 | F3 Inhibition (% ± SD) | Statistics 2 | Self-Inhibition (% ± SD) | Statistics 1 | F3 Inhibition (% ± SD) | Statistics 2 |
| T002 | T. harzianum | 93.18 ± 1.21 | A | 99.51 ± 0.56 | a | 95.04 ± 0.87 | A | 95.25 ± 1.89 | a |
| T007 | T. harzianum | 91.68 ± 1.89 | A,B | 98.54 ± 1.26 | a | 89.87 ± 2.67 | A,B | 92.93 ± 2.16 | a,b |
| T008 | T. citrinoviride | 74.51 ± 1.66 | D | 70.73 ± 1.38 | e | 71.02 ± 1.85 | C | 59.38 ± 2.93 | d |
| T015 | T. harzianum | 93.86 ± 1.60 | A | 97.07 ± 1.59 | a,b | 94.62 ± 1.21 | A | 89.40 ± 2.28 | b |
| T021 | T. harzianum | 91.43 ± 3.77 | A,B | 99.02 ± 0.00 | a | 90.08 ± 4.25 | A,B | 92.38 ± 0.91 | a,b |
| T028 | T. velutinum | 42.29 ± 1.40 | E | 34.15 ± 3.03 | f | 23.57 ± 2.12 | D | 15.89 ± 1.96 | e |
| T044 | T. harzianum | 90.09 ± 1.79 | A,B | 91.35 ± 2.55 | c,d | 89.66 ± 1.71 | A,B | 92.93 ± 3.22 | a,b |
| T050 | T. harzianum | 91.11 ± 1.48 | A,B | 88.91 ± 0.51 | c,d | 89.98 ± 0.94 | A,B | 93.46 ± 0.42 | a,b |
| T055 | T. harzianum | 87.86 ± 0.97 | B | 86.92 ± 1.12 | d | 87.66 ± 1.35 | B | 90.40 ± 1.11 | a,b |
| T057 | T. gamsii | 82.53 ± 2.91 | C | 92.90 ± 4.86 | b,c | 68.74 ± 3.65 | C | 76.27 ± 2.02 | c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-García, S.; Mayo-Prieto, S.; Gutiérrez, S.; Casquero, P.A. Self-Inhibitory Activity of Trichoderma Soluble Metabolites and Their Antifungal Effects on Fusarium oxysporum. J. Fungi 2020, 6, 176. https://doi.org/10.3390/jof6030176
Álvarez-García S, Mayo-Prieto S, Gutiérrez S, Casquero PA. Self-Inhibitory Activity of Trichoderma Soluble Metabolites and Their Antifungal Effects on Fusarium oxysporum. Journal of Fungi. 2020; 6(3):176. https://doi.org/10.3390/jof6030176
Chicago/Turabian StyleÁlvarez-García, Samuel, Sara Mayo-Prieto, Santiago Gutiérrez, and Pedro Antonio Casquero. 2020. "Self-Inhibitory Activity of Trichoderma Soluble Metabolites and Their Antifungal Effects on Fusarium oxysporum" Journal of Fungi 6, no. 3: 176. https://doi.org/10.3390/jof6030176
APA StyleÁlvarez-García, S., Mayo-Prieto, S., Gutiérrez, S., & Casquero, P. A. (2020). Self-Inhibitory Activity of Trichoderma Soluble Metabolites and Their Antifungal Effects on Fusarium oxysporum. Journal of Fungi, 6(3), 176. https://doi.org/10.3390/jof6030176








