Zinc Binding by Histatin 5 Promotes Fungicidal Membrane Disruption in C. albicans and C. glabrata
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strain, Media, and Reagent Preparation
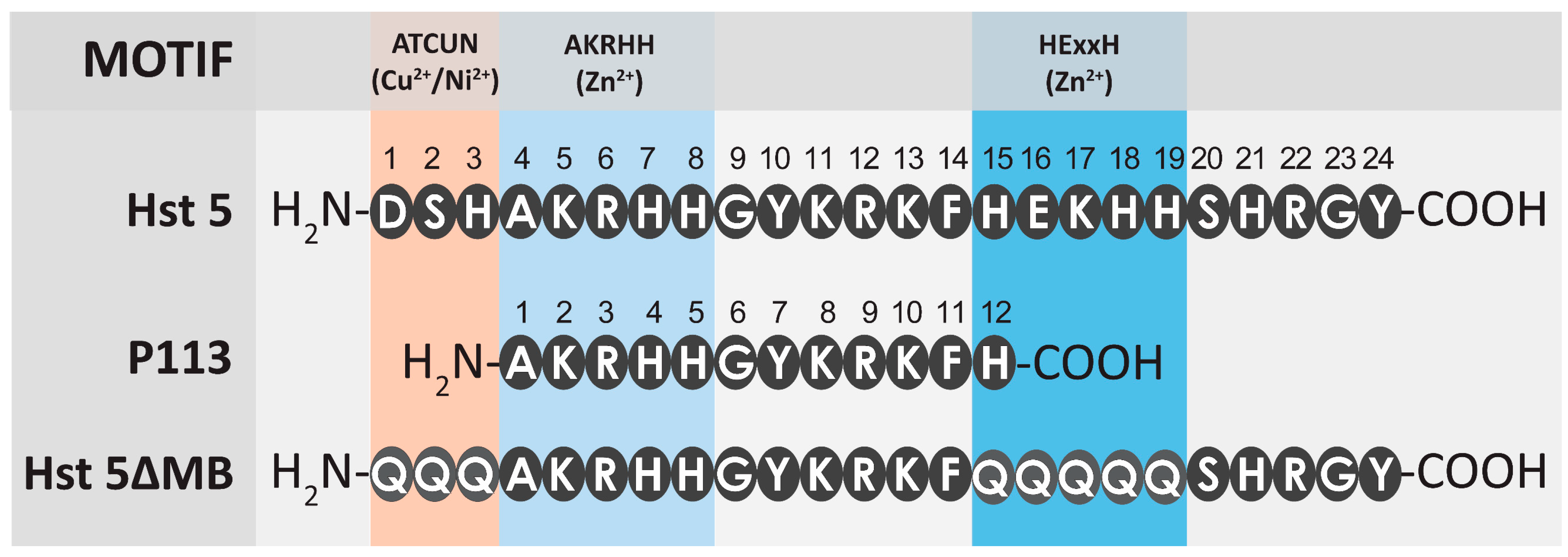
2.2. Peptides
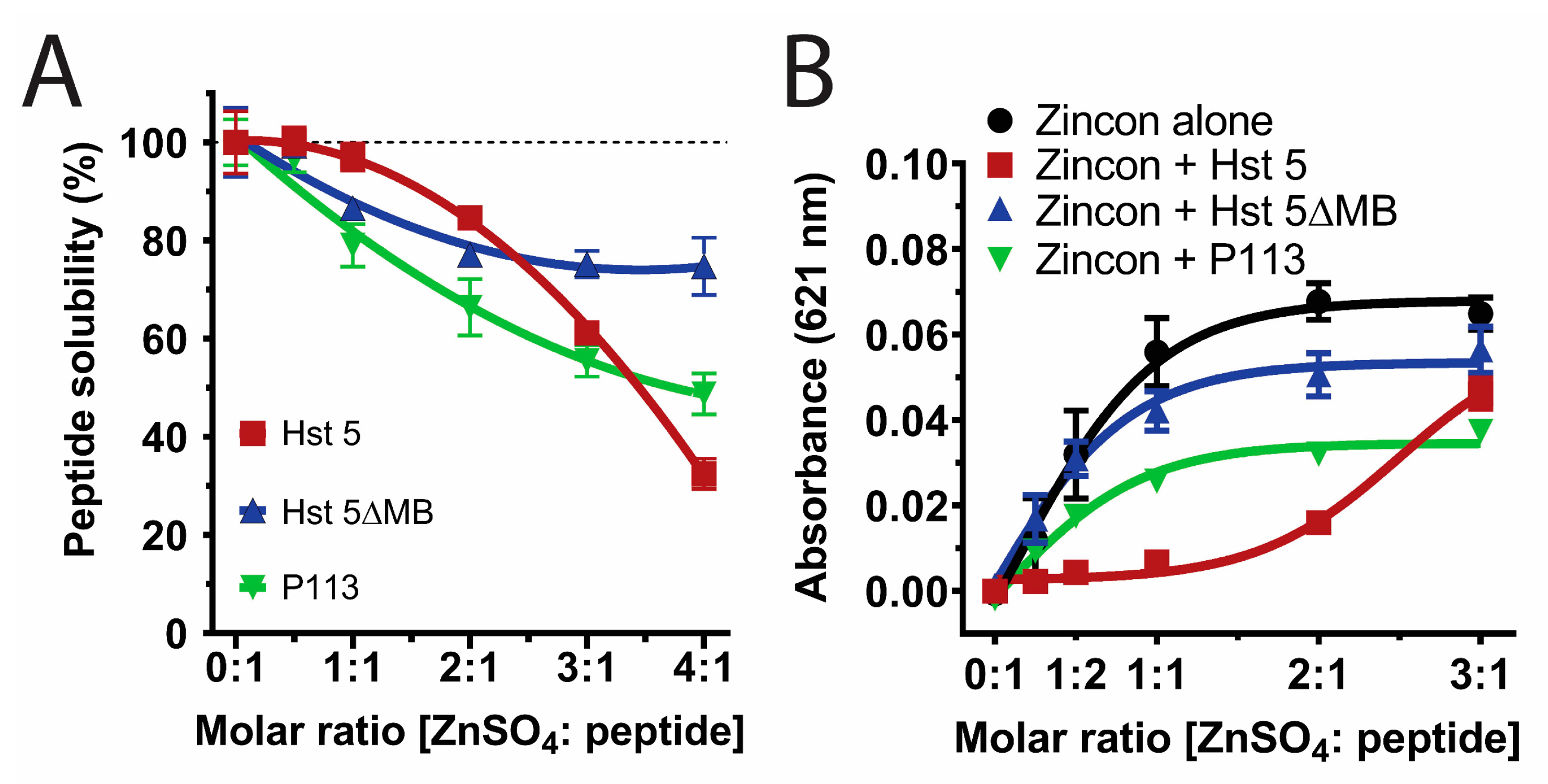
2.3. Peptide Solubility Assay
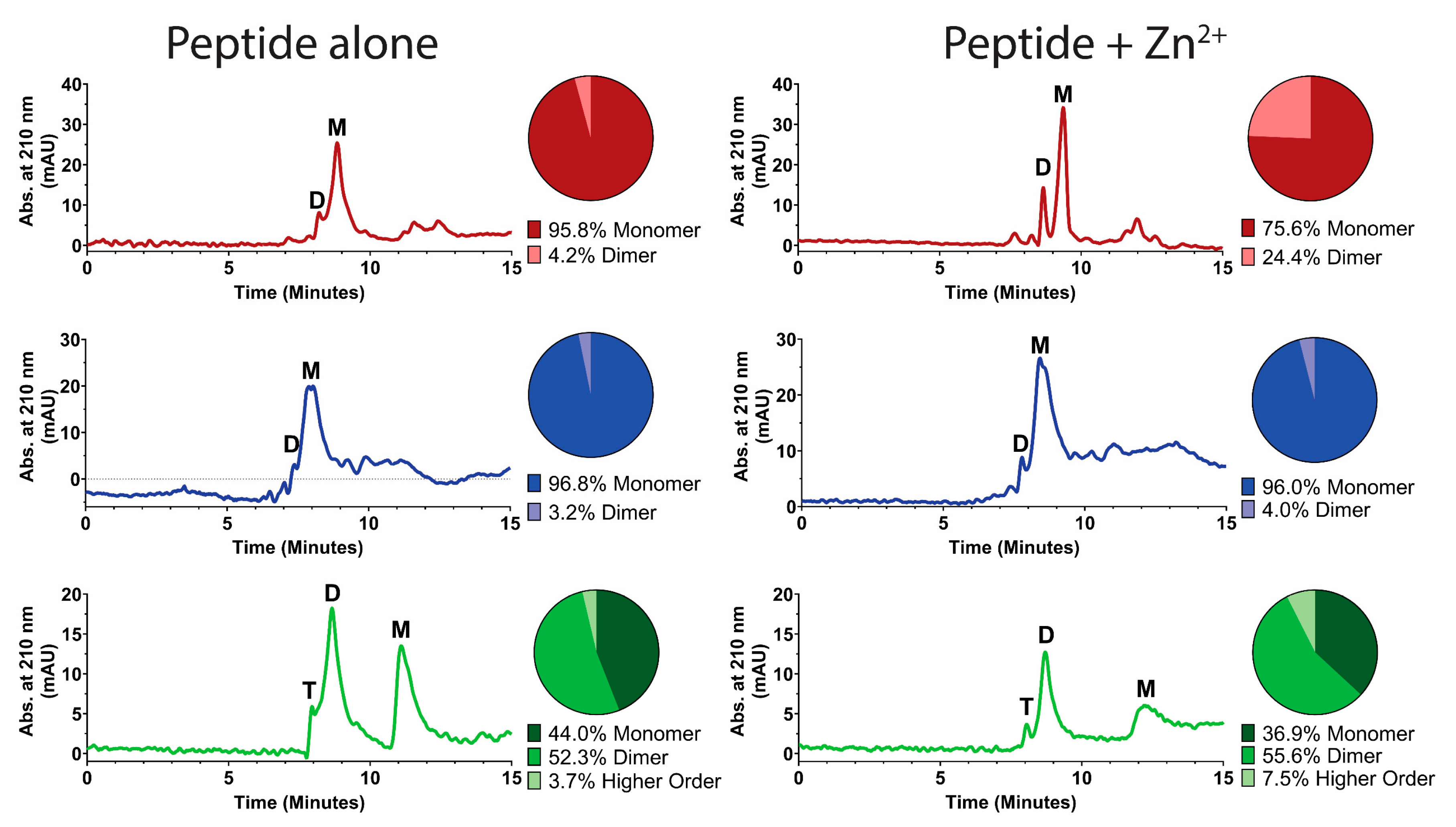
2.4. Zinc-Binding Competition Assay
2.5. High-Performance Liquid Chromatography
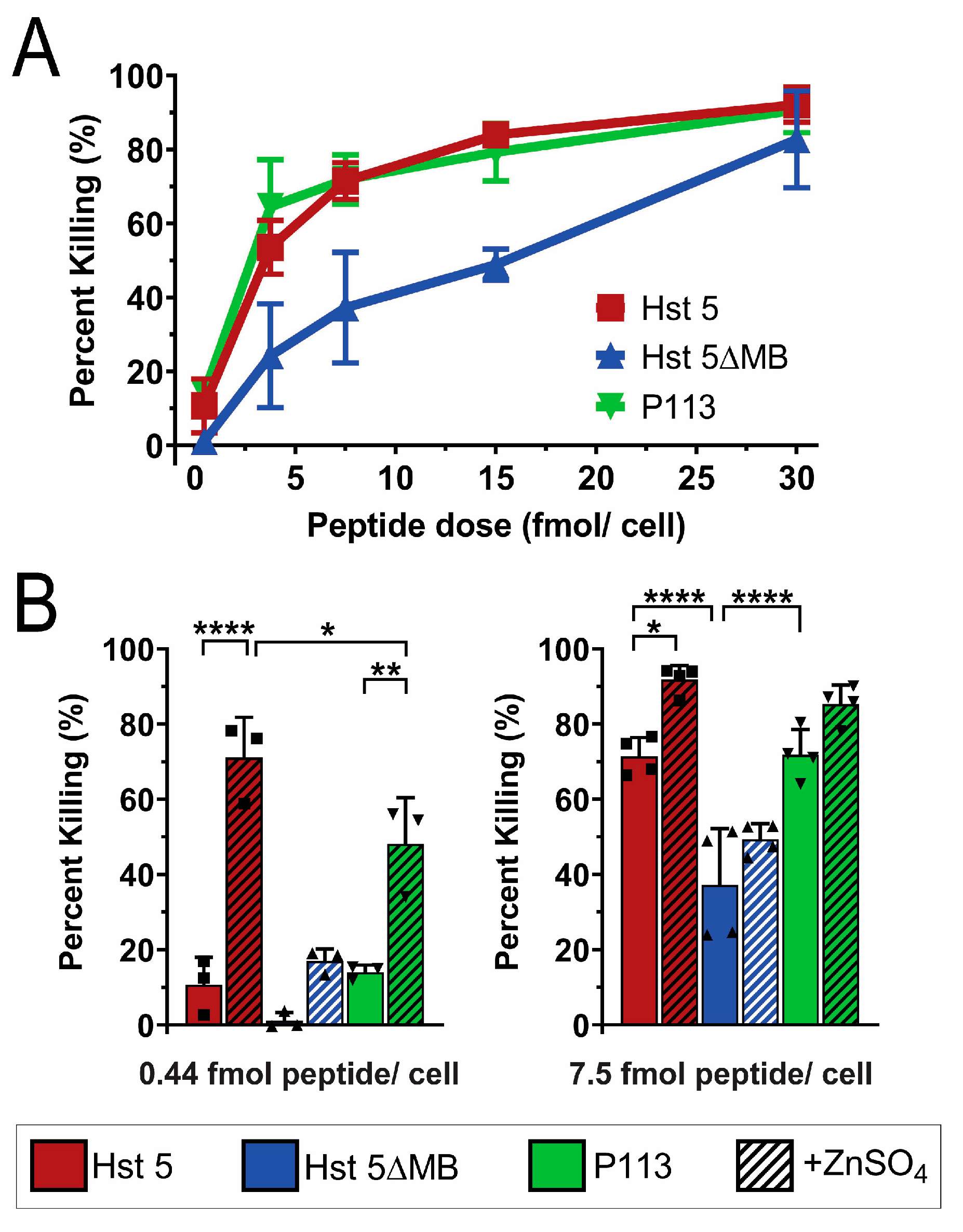
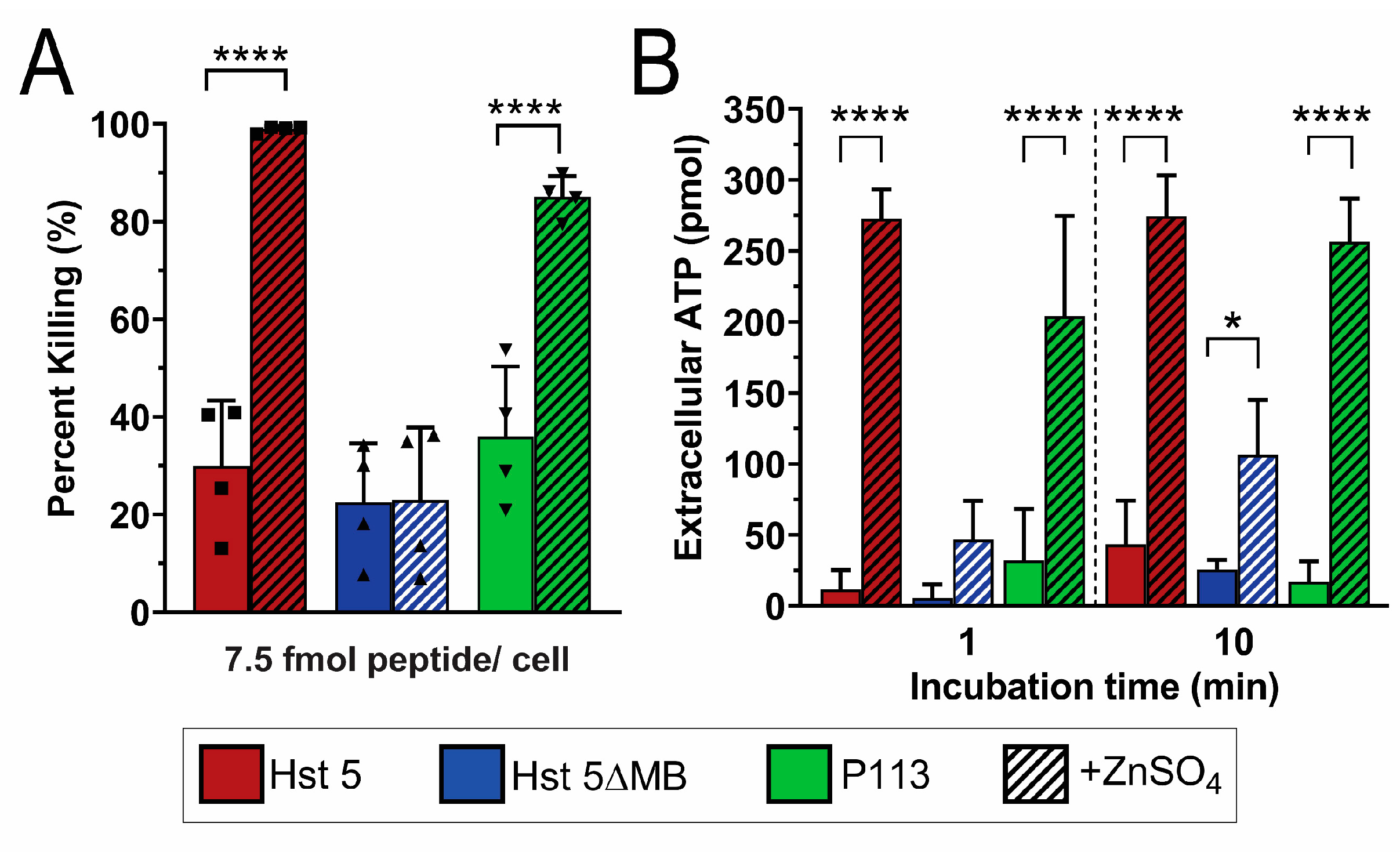
2.6. Candidacidal Assays
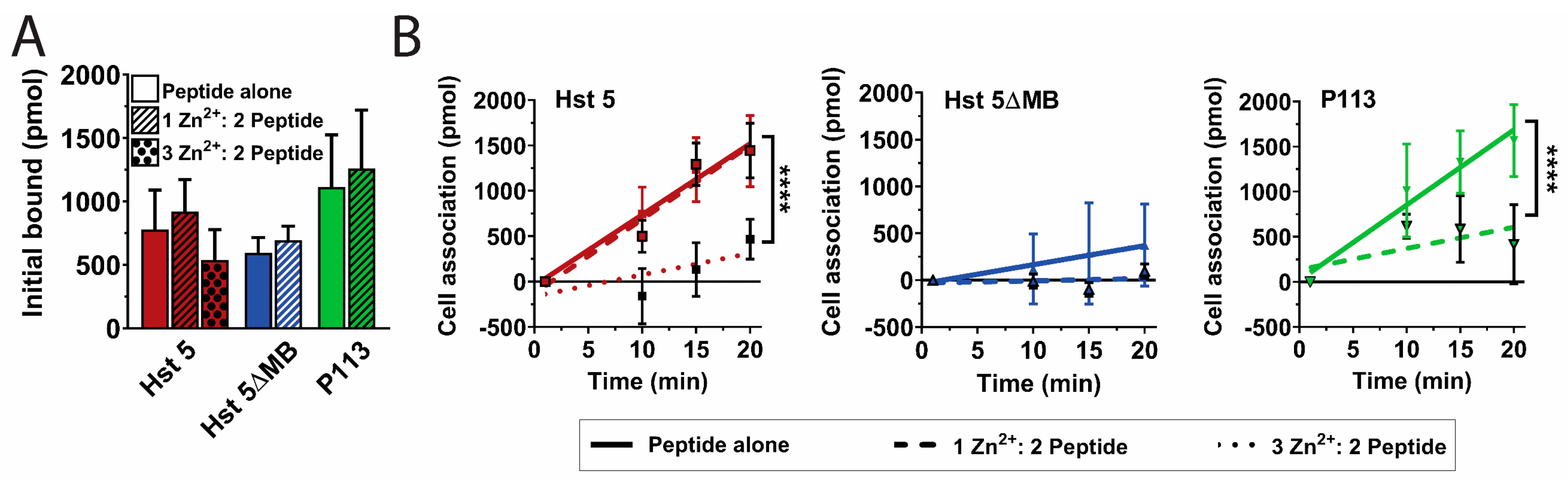
2.7. Peptide Uptake Assay
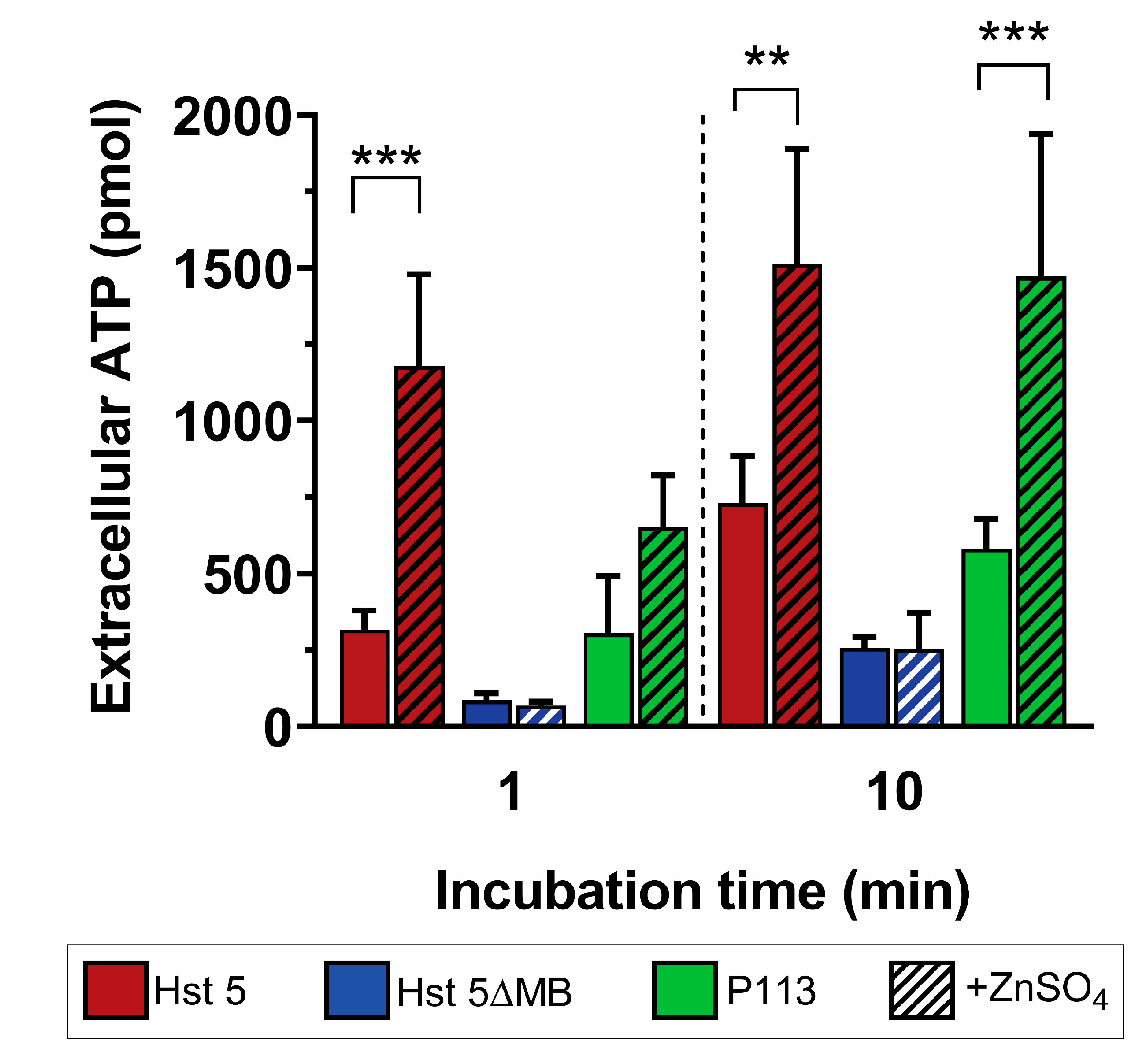
2.8. Extracellular ATP Quantification
2.9. Statistical Analysis
3. Results
3.1. Zinc Binding Characteristics of Hst 5, P113, and Hst 5ΔMB
3.2. Hst 5ΔMB C. albicans Killing is Reduced Accompanying Mutation of one Zn2+ Binding Site While Hst 5 Killing Is Increased in the Presence of Zn2+
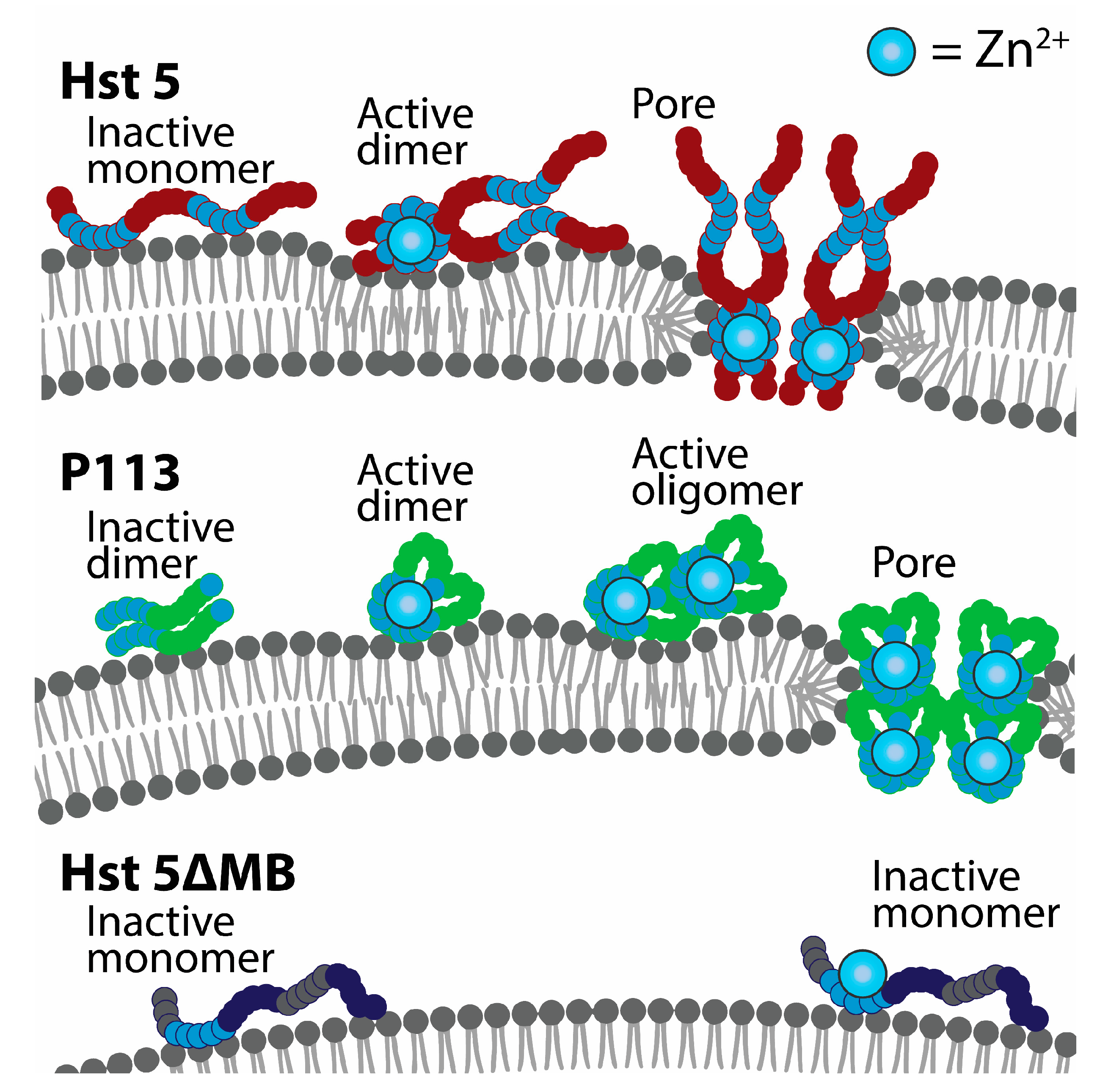
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tsai, H.; Bobek, L.A. Human salivary histatins: Promising anti-fungal therapeutic agents. Crit. Rev. Oral. Biol. Med. 1998, 9, 480–497. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, F.G.; Xu, T.; Mcmillian, F.M.; Levitz, S.M.; Diamond, R.D.; Offner, G.D.; Troxler, R.F. Histatins, A novel family of histidine-rich proteins in human parotid secretion. J. Biol. Chem. 1988, 263, 7472–7477. [Google Scholar] [PubMed]
- Nikawa, H.; Jin, C.; Fukushima, H.; Makihira, S.; Hamada, T. Antifungal activity of Histatin-5 against non-albicans Candida species. Oral. Microbiol. Immunol. 2001, 16, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, R.U.; Friedman, J.; Norris, H.L.; Salvatori, O.; Mccall, A.D.; Kay, J.; Edgerton, M. Fluconazole-resistant Candida auris is susceptible to salivary Histatin 5 killing and to intrinsic host defenses. Antimicrob. Agents Chemother. 2018, 62, 2. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ambudkar, I.; Yamagishi, H.; Swaim, W.; Walsh, T.J.; O’connell, B.C. Histatin 3-mediated killing of Candida albicans: Effect of extracellular salt concentration on binding and internalization. Antimicrob. Agents Chemother. 1999, 43, 2256. [Google Scholar] [CrossRef] [PubMed]
- Koshlukova, S.E.; Lloyd, T.L.; Araujo, M.W.B.; Edgerton, M. Salivary Histatin 5 induces non-lytic release of ATP from Candida albicans leading to cell death. J. Biol. Chem. 1999, 274, 18872–18879. [Google Scholar] [CrossRef]
- Helmerhorst, E.J.; Troxler, R.F.; Oppenheim, F.G. The human salivary peptide Histatin 5 exerts its antifungal activity through the formation of reactive oxygen species. Proc. Natl. Acad. Sci. USA 2001, 98, 14637. [Google Scholar] [CrossRef]
- Baev, D.; Li, X.S.; Dong, J.; Keng, P.; Edgerton, M. Human salivary Histatin 5 causes disordered volume regulation and cell cycle arrest in Candida albicans. Infect. Immun. 2002, 70, 4777–4784. [Google Scholar] [CrossRef]
- Grogan, J.; Mcknight, C.J.; Troxler, R.F.; Oppenheim, F.G. Zinc and copper bind to unique sites of Histatin 5. FEBS Lett. 2001, 491, 76–80. [Google Scholar] [CrossRef]
- Melino, S.; Rufini, S.; Sette, M.; Morero, R.; Grottesi, A.; Paci, M.; Petruzzelli, R. Zn2+ ions selectively induce antimicrobial salivary peptide Histatin-5 to fuse negatively charged vesicles. Identification and characterization of a zinc-binding motif present in the functional domain. Biochemistry 1999, 38, 9626–9633. [Google Scholar] [CrossRef]
- McCaslin, T.G.; Pagba, C.V.; Yohannan, J.; Barry, B.A. Specific metallo-protein interactions and antimicrobial activity in Histatin-5, an intrinsically disordered salivary peptide. Sci. Rep. 2019, 9, 17303. [Google Scholar] [CrossRef] [PubMed]
- Conklin, S.E.; Bridgman, E.C.; Su, Q.; Riggs-Gelasco, P.; Haas, K.L.; Franz, K.J. Specific histidine residues confer histatin peptides with copper-dependent activity against Candida albicans. Biochemistry 2017, 56, 4244–4255. [Google Scholar] [PubMed]
- Rydengard, V.; Andersson Nordahl, E.; Schmidtchen, A. Zinc potentiates the antibacterial effects of histidine-rich peptides against Enterococcus faecalis. FEBS J. 2006, 273, 2399–2406. [Google Scholar] [CrossRef]
- Cragnell, C.; Staby, L.; Lenton, S.; Kragelund, B.B.; Skepö, M. Dynamical oligomerisation of histidine rich intrinsically disordered proteins is regulated through zinc-histidine interactions. Biomolecules 2019, 9, 168. [Google Scholar] [CrossRef]
- Kurut, A.; Henriques, J.; Forsman, J.; Skepo, M.; Lund, M. Role of histidine for charge regulation of unstructured peptides at interfaces and in bulk. Proteins 2014, 82, 657–667. [Google Scholar] [CrossRef][Green Version]
- Puri, S.; Li, R.; Ruszaj, D.; Tati, S.; Edgerton, M. Iron binding modulates candidacidal properties of salivary Histatin 5. J. Dent. Res. 2015, 94, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Chadha, S.; Saraswat, D.; Bajwa, J.S.; Li, R.A.; Conti, H.R.; Edgerton, M. Histatin 5 uptake by Candida albicans utilizes polyamine transporters Dur3 and Dur31 proteins. J. Biol. Chem. 2011, 286, 43748–43758. [Google Scholar] [CrossRef] [PubMed]
- Tati, S.; Jang, W.S.; Li, R.; Kumar, R.; Puri, S.; Edgerton, M. Histatin 5 resistance of Candida glabrata can be reversed by insertion of Candida albicans polyamine transporter-encoding genes DUR3 and DUR31. PLoS ONE 2013, 8, e61480. [Google Scholar] [CrossRef]
- Helmerhorst, E.J.; Van’t Hof, W.; Breeuwer, P.; Veerman, E.C.; Abee, T.; Troxler, R.F.; Amerongen, A.V.; Oppenheim, F.G. Characterization of Histatin 5 with respect to amphipathicity, hydrophobicity, and effects on cell and mitochondrial membrane integrity excludes a candidacidal mechanism of pore formation. J. Biol. Chem. 2001, 276, 5643–5649. [Google Scholar] [CrossRef]
- Den Hertog, A.L.; Wong Fong Sang, H.W.; Kraayenhof, R.; Bolscher, J.G.M.; Van’t Hof, W.; Veerman, E.C.I.; Nieuw Amerongen, A.V. Interactions of Histatin 5 and Histatin 5-derived peptides with liposome membranes: Surface effects, translocation and permeabilization. Biochem. J. 2004, 379, 665–672. [Google Scholar] [CrossRef]
- Den Hertog, A.L.; Van Marle, J.; Van Veen, H.A.; Van’t Hof, W.; Bolscher, J.G.M.; Veerman, E.C.I.; Nieuw Amerongen, A.V. Candidacidal effects of two antimicrobial peptides: Histatin 5 causes small membrane defects, but LL-37 causes massive disruption of the cell membrane. Biochem. J. 2005, 388, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Mochon, A.B.; Liu, H. The antimicrobial peptide Histatin-5 causes a spatially restricted disruption on the Candida albicans surface, allowing rapid entry of the peptide into the cytoplasm. PLoS Pathog. 2008, 4, e1000190. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.S.; Bajwa, J.S.; Sun, J.N.; Edgerton, M. Salivary Histatin 5 internalization by translocation, but not endocytosis, is required for fungicidal activity in Candida albicans. Mol. Microbiol. 2010, 77, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Norris, H.L.; Friedman, J.; Chen, Z.; Puri, S.; Wilding, G.; Edgerton, M. Salivary metals, age, and gender correlate with cultivable oral Candida carriage levels. J. Oral. Microbiol. 2018, 10, 1447216. [Google Scholar] [CrossRef]
- Burnett, G.R.; Stephen, A.S.; Pizzey, R.L.; Bradshaw, D.J. In Vitro effects of novel toothpaste actives on components of oral malodour. Int. Dent. J. 2011, 61, 67–73. [Google Scholar] [CrossRef]
- Shatzman, A.R.; Henkin, R.I. Gustin concentration changes relative to salivary zinc and taste in humans. Proc. Natl. Acad. Sci. USA 1981, 78, 3867–3871. [Google Scholar] [CrossRef]
- Khan, S.A.; Fidel, P.L.; Thunayyan, A.A.J.; Varlotta, S.; Meiller, T.F.; Jabra-Rizk, M.A. Impaired Histatin-5 levels and salivary antimicrobial activity against C albicans in HIV infected individuals. J. AIDS Clin. Res. 2013, 4, 193. [Google Scholar] [CrossRef]
- Łoboda, D.; Kozlowski, H.; Rowinska-Zyrek, M. Antimicrobial peptide-metal ion interactions—A potential way of activity enhancement. New J. Chem. 2018, 42, 7560–7568. [Google Scholar] [CrossRef]
- Earl, C.; Chantry, A.; Mohammad, N.; Glynn, P. Zinc ions stabilize the association of basic protein with brain myelin membranes. J. Neurochem. 1988, 51, 718–724. [Google Scholar] [CrossRef]
- Duay, S.S.; Sharma, G.; Prabhakar, R.; Angeles-Boza, A.M.; May, E.R. Molecular dynamics investigation into the effect of zinc(II) on the structure and membrane interactions of the antimicrobial peptide Clavanin A. J. Phys. Chem. B 2019, 123, 3163–3176. [Google Scholar] [CrossRef]
- Song, C.; Weichbrodt, C.; Salnikov, E.S.; Dynowski, M.; Forsberg, B.O.; Bechinger, B.; Steinem, C.; De Groot, B.L.; Zachariae, U.; Zeth, K. Crystal structure and functional mechanism of a human antimicrobial membrane channel. Proc. Natl. Acad. Sci. USA 2013, 110, 4586–4591. [Google Scholar] [CrossRef] [PubMed]
- Lorenzón, E.N.; Piccoli, J.P.; Santos-Filho, N.A.; Cilli, E.M. Dimerization of antimicrobial peptides: A promising strategy to enhance antimicrobial peptide activity. Protein Pept. Lett. 2019, 26, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Xhindoli, D.; Pacor, S.; Guida, F.; Antcheva, N.; Tossi, A. Native oligomerization determines the mode of action and biological activities of human cathelicidin LL-37. Biochem. J. 2014, 457, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Lorenzón, E.N.; Nobre, T.M.; Caseli, L.; Cilli, E.M.; Da Hora, G.C.A.; Soares, T.A.; Oliveira, O.N. The pre-assembled state of magainin 2 lysine-linked dimer determines its enhanced antimicrobial activity. Colloids Surf. B Biointerfaces 2018, 167, 432–440. [Google Scholar]
- Mani, R.; Tang, M.; Wu, X.; Buffy, J.J.; Waring, A.J.; Sherman, M.A.; Hong, M. Membrane-Bound dimer structure of a β-hairpin antimicrobial peptide from rotational-echo double-resonance solid-state NMR. Biochemistry 2006, 45, 8341–8349. [Google Scholar] [CrossRef]
- Stella, L.; Mazzuca, C.; Venanzi, M.; Palleschi, A.; Didone, M.; Formaggio, F.; Toniolo, C.; Pispisa, B. Aggregation and water-membrane partition as major determinants of the activity of the antibiotic peptide trichogin GA IV. Biophys. J. 2004, 86, 936–945. [Google Scholar] [CrossRef]
- Arnusch, C.J.; Branderhorst, H.; De Kruijff, B.; Liskamp, R.M.J.; Breukink, E.; Pieters, R.J. Enhanced membrane pore formation by multimeric/oligomeric antimicrobial peptides. Biochemistry 2007, 46, 13437. [Google Scholar] [CrossRef]
- Sengupta, D.; Leontiadou, H.; Mark, A.E.; Marrink, S.-J. Toroidal pores formed by antimicrobial peptides show significant disorder. Biochim. Biophys. Acta Biomembr. 2008, 1778, 2308. [Google Scholar] [CrossRef]
- Bechinger, B.; Lohner, K. Detergent-Like actions of linear amphipathic cationic antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1529–1539. [Google Scholar] [CrossRef]
- Fonzi, W.A.; Irwin, M.Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics 1993, 134, 717–728. [Google Scholar]
- Joly, S.; Maze, C.; Mccray, P.B.; Guthmiller, J.M. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J. Clin. Microbiol. 2004, 42, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Tati, S.; Li, R.; Puri, S.; Kumar, R.; Davidow, P.; Edgerton, M. Histatin 5-spermidine conjugates have enhanced fungicidal activity and efficacy as a topical therapeutic for oral candidiasis. Antimicrob. Agents Chemother. 2014, 58, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, D.M.; Spacciapoli, P.; Tran, L.T.; Xu, T.; Roberts, F.D.; Dalla Serra, M.; Buxton, D.K.; Oppenheim, F.G.; Friden, P. Anticandida activity is retained in P-113, a 12-amino-acid fragment of Histatin 5. Antimicrob. Agents Chemother. 2001, 45, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Porciatti, E.; Milenković, M.; Gaggelli, E.; Valensin, G.; Kozlowski, H.; Kamysz, W.; Valensin, D. Structural characterization and antimicrobial activity of the Zn(II) complex with P113 (Demegen), a derivative of Histatin 5. Inorg. Chem. 2010, 49, 8690–8698. [Google Scholar] [CrossRef]
- Kulon, K.; Valensin, D.; Kamysz, W.; Valensin, G.; Nadolski, P.; Porciatti, E.; Gaggelli, E.; Kozłowski, H. The His–His sequence of the antimicrobial peptide Demegen P-113 makes it very attractive ligand for Cu2+. J. Inorg. Biochem. 2008, 102, 960–972. [Google Scholar] [CrossRef]
- Flora, B.; Gusman, H.; Helmerhorst, E.J.; Troxler, R.F.; Oppenheim, F.G. A new method for the isolation of Histatins 1, 3, and 5 from parotid secretion using zinc precipitation. Protein Expr. Purif. 2001, 23, 198–206. [Google Scholar] [CrossRef]
- Gusman, H.; Lendenmann, U.; Grogan, J.; Troxler, R.F.; Oppenheim, F.G. Is salivary Histatin 5 a metallopeptide? Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2001, 1545, 86–95. [Google Scholar] [CrossRef]
- Helmerhorst, E.J.; Venuleo, C.; Beri, A.; Oppenheim, F.G. Candida glabrata is unusual with respect to its resistance to cationic antifungal proteins. Yeast 2005, 22, 705–714. [Google Scholar] [CrossRef]
- Grau-Campistany, A.; Strandberg, E.; Wadhwani, P.; Reichert, J.; Bürck, J.; Rabanal, F.; Ulrich, A.S. Hydrophobic mismatch demonstrated for membranolytic peptides, and their use as molecular rulers to measure bilayer thickness in native cells. Sci. Rep. 2015, 5, 9388. [Google Scholar] [CrossRef]
- Jang, W.S.; Li, X.S.; Sun, J.N.; Edgerton, M. The P-113 fragment of Histatin 5 requires a specific peptide sequence for intracellular translocation in Candida albicans, which is independent of cell wall binding. Antimicrob. Agents Chemother. 2008, 52, 497–504. [Google Scholar] [CrossRef]
- Han, J.; Jyoti, M.A.; Song, H.Y.; Jang, W.S. Antifungal activity and action mechanism of Histatin 5-halocidin hybrid peptides against Candida ssp. PLoS ONE 2016, 11, e0150196. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Edgerton, M. How does it kill?Understanding the candidacidal mechanism of salivary Histatin 5. Eukaryot. Cell 2014, 13, 958–964. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norris, H.L.; Kumar, R.; Ong, C.Y.; Xu, D.; Edgerton, M. Zinc Binding by Histatin 5 Promotes Fungicidal Membrane Disruption in C. albicans and C. glabrata. J. Fungi 2020, 6, 124. https://doi.org/10.3390/jof6030124
Norris HL, Kumar R, Ong CY, Xu D, Edgerton M. Zinc Binding by Histatin 5 Promotes Fungicidal Membrane Disruption in C. albicans and C. glabrata. Journal of Fungi. 2020; 6(3):124. https://doi.org/10.3390/jof6030124
Chicago/Turabian StyleNorris, Hannah L., Rohitashw Kumar, Chih Yean Ong, Ding Xu, and Mira Edgerton. 2020. "Zinc Binding by Histatin 5 Promotes Fungicidal Membrane Disruption in C. albicans and C. glabrata" Journal of Fungi 6, no. 3: 124. https://doi.org/10.3390/jof6030124
APA StyleNorris, H. L., Kumar, R., Ong, C. Y., Xu, D., & Edgerton, M. (2020). Zinc Binding by Histatin 5 Promotes Fungicidal Membrane Disruption in C. albicans and C. glabrata. Journal of Fungi, 6(3), 124. https://doi.org/10.3390/jof6030124





