Clinical Usefulness of Susceptibility Breakpoints for Yeasts in the Treatment of Candidemia: A Noninterventional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrollment and Antifungal Susceptibility Data
2.2. Statistical Analyses
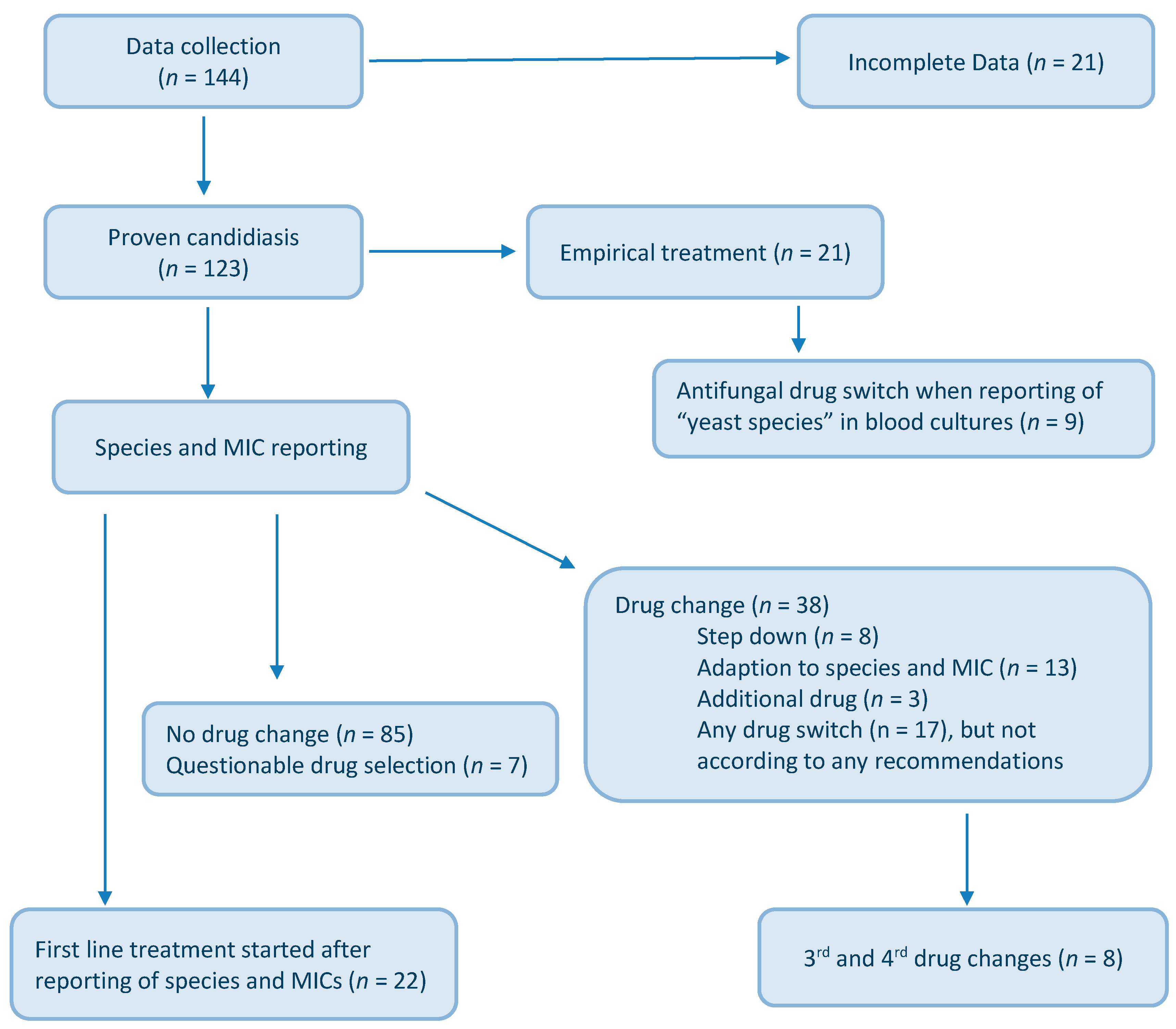
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial Bloodstream Infections in US Hospitals: Analysis of 24,179 Cases from a Prospective Nationwide Surveillance Study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Luzzaro, F.; Ortisi, G.; LaRosa, M.; Drago, M.; Brigante, G.; Gesu, G. Prevalence and epidemiology of microbial pathogens causing bloodstream infections: Results of the OASIS multicenter study. Diagn. Microbiol. Infect. Dis. 2011, 69, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Eggimann, P.; Garbino, J.; Pittet, D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect. Dis. 2003, 3, 685–702. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Andes, D.R.; Diekema, D.; Horn, D.L.; Reboli, A.C.; Rotstein, C.; Franks, B.; Azie, N.E. Epidemiology and Outcomes of Invasive Candidiasis Due to Non-albicans Species of Candida in 2496 Patients: Data from the Prospective Antifungal Therapy (PATH) Registry. PLOS ONE 2014, 9, e101510. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Ebbers, J.; Geurtz, L.; Stefanik, D.; Major, Y.; Edmond, M.B.; Wenzel, R.P.; Seifert, H. Nosocomial bloodstream infections due to Candida spp. in the USA: Species distribution, clinical features and antifungal susceptibilities. Int. J. Antimicrob. Agents 2014, 43, 78–81. [Google Scholar] [CrossRef]
- Posteraro, B.; Sanguinetti, M. The future of fungal susceptibility testing. Futur. Microbiol. 2014, 9, 947–967. [Google Scholar] [CrossRef] [PubMed]
- Weiler, S.; Lass-Flörl, C.; Auberger, J.; Bellmann-Weiler, R.; Stein, M.; Joannidis, M.; Bellmann, R. Triazole-resistant candidaemia following posaconazole exposure. Int. J. Antimicrob. Agents 2009, 33, 494–495. [Google Scholar] [CrossRef] [PubMed]
- Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing. Method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 2008, 14, 398–405. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Epidemiological Cutoff Values for Antifungal Susceptibility Testing, 2nd ed.; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Antifungal Agents. Breakpoint Tables for Interpretation of MICs. Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 16 November 2015).
- Pfaller, M.A.; Diekema, D.; Andes, D.; Arendrup, M.C.; Brown, S.; Lockhart, S.; Motyl, M.; Perlin, D.S. Clinical breakpoints for the echinocandins and Candida revisited: Integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist. Updat. 2011, 14, 164–176. [Google Scholar] [CrossRef]
- Rex, J.H.; Pfaller, M.A.; Walsh, T.J.; Chaturvedi, V.; Espinel-Ingroff, A.; Ghannoum, M.A.; Gosey, L.L.; Odds, F.C.; Rinaldi, M.G.; Sheehan, D.J.; et al. Antifungal Susceptibility Testing: Practical Aspects and Current Challenges. Clin. Microbiol. Rev. 2001, 14, 643–658. [Google Scholar] [CrossRef]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Lausch, K.R.; Søgaard, M.; Rosenvinge, F.; Johansen, H.; Boysen, T.; Røder, B.; Mortensen, K.L.; Nielsen, L.; Lemming, L.; Olesen, B.; et al. High incidence of candidaemia in a nationwide cohort: Underlying diseases, risk factors and mortality. Int. J. Infect. Dis. 2018, 76, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Rammaert, B.; Puyade, M.; Cornely, O.A.; Seidel, D.; Grossi, P.; Husain, S.; Picard, C.; Lass-Flörl, C.; Manuel, O.; Le Pavec, J.; et al. Perspectives on Scedosporium species and Lomentospora prolificans in lung transplantation: Results of an international practice survey from ESCMID fungal infection study group and study group for infections in compromised hosts, and European Confederation of Medical Mycology. Transpl. Infect. Dis. 2019, 21, e13141. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; A Marr, K.; Ostrosky-Zeichner, L.; Reboli, A.; Schuster, M.G.; A Vazquez, J.; Walsh, T.J.; et al. Executive Summary: Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Epelbaum, O.; Chasan, R. Candidemia in the Intensive Care Unit. Clin. Chest Med. 2017, 38, 493–509. [Google Scholar] [CrossRef] [PubMed]
- A Eschenauer, G.; Carver, P.L.; Patel, T.S.; Lin, S.-W.; Klinker, K.P.; Pai, M.P.; Lam, S.W. Survival in Patients with Candida glabrata Bloodstream Infection Is Associated with Fluconazole Dose. Antimicrob. Agents Chemother. 2018, 62, e02566-17. [Google Scholar] [CrossRef]
- Ghanem-Zoubi, N.; Qasum, M.; Khoury, J.; Zorbavel, D.; Arnon, M.; Geffen, Y.; Paul, M. The association between fluconazole dose and MIC with mortality and persistence in candidemia. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1773–1780. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Bassetti, M.; Nucci, M.; Capparella, M.R.; Yan, J.L.; Aram, J.; Hogan, P.A. Anidulafungin for the treatment of candidaemia caused by Candida parapsilosis: Analysis of pooled data from six prospective clinical studies. Mycoses 2017, 60, 663–667. [Google Scholar] [CrossRef]
- Wu, Y.-M.; Huang, C.-T.; Lu, J.-J.; Shie, S.-S.; Ye, J.-J.; Wu, T.-S.; Huang, C.-T. Risk factors and outcomes of candidemia caused by Candida parapsilosis complex in a medical center in northern Taiwan. Diagn. Microbiol. Infect. Dis. 2018, 90, 44–49. [Google Scholar] [CrossRef]
- Choi, H.; Kim, J.H.; Seong, H.; Lee, W.; Jeong, W.; Ahn, J.Y.; Jeong, S.J.; Ku, N.S.; Yeom, J.S.; Kim, Y.K.; et al. Changes in the utilization patterns of antifungal agents, medical cost and clinical outcomes of candidemia from the health-care benefit expansion to include newer antifungal agents. Int. J. Infect. Dis. 2019, 83, 49–55. [Google Scholar] [CrossRef]
- Su, H.-C.; Hua, Y.-M.; Feng, I.-J.; Wu, H.-C. Comparative effectiveness of antifungal agents in patients with hematopoietic stem cell transplantation: A systematic review and network meta-analysis. Infect. Drug Resist. 2019, 12, 1311–1324. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, M.; Lora-Pablos, D.; Puig-Asensio, M.; Padilla, B.; Munoz, P.; Guinea, J.; Pardo, J.R.P.; García-Rodríguez, J.; Cerrada, C.G.; Fortun, J.; et al. Initial Use of Echinocandins Does Not Negatively Influence Outcome in Candida parapsilosis Bloodstream Infection: A Propensity Score Analysis. Clin. Infect. Dis. 2014, 58, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Ghrenassia, E.; Mokart, D.D.; Mayaux, J.; Demoule, A.; Rezine, I.; Kerhuel, L.; Calvet, L.; De Jong, A.; Azoulay, E.; Darmon, M. Candidemia in critically ill immunocompromised patients: Report of a retrospective multicenter cohort study. Ann. Intensiv. Care 2019, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.H.; Pfaller, M.A. Has antifungal susceptibility testing come of age? Clin. Infect Dis. 2002, 35, 982–989. [Google Scholar] [CrossRef]
- Hesstvedt, L.; Gaustad, P.; Müller, F.; Andersen, C.T.; Brunborg, C.; Mylvaganam, H.; Leiva, R.A.; Berdal, J.E.; Ranheim, T.E.; Johnsen, B.O.; et al. The impact of age on risk assessment, therapeutic practice and outcome in candidemia. Infect. Dis. 2019, 51, 425–434. [Google Scholar] [CrossRef]
- Lortholary, O.; Renaudat, C.; Sitbon, K.; Desnos-Ollivier, M.; Bretagne, S.; Dromer, F. French Mycoses Study Group The risk and clinical outcome of candidemia depending on underlying malignancy. Intensiv. Care Med. 2017, 43, 652–662. [Google Scholar] [CrossRef]
- Ko, J.-H.; Peck, K.R.; Jung, D.S.; Lee, J.-Y.; Kim, H.A.; Ryu, S.Y.; Jung, S.-I.; Joo, E.-J.; Cheon, S.; Kim, Y.-S.; et al. Impact of high MIC of fluconazole on outcomes of Candida glabrata bloodstream infection: A retrospective multicenter cohort study. Diagn. Microbiol. Infect. Dis. 2018, 92, 127–132. [Google Scholar] [CrossRef]
- Barchiesi, F.; Orsetti, E.; Osimani, P.; Catassi, C.; Santelli, F.; Manso, E. Factors related to outcome of bloodstream infections due to Candida parapsilosis complex. BMC Infect. Dis. 2016, 16, 387. [Google Scholar] [CrossRef]
- Vena, A.; Bouza, E.; Valerio, M.; Padilla, B.; Paño-Pardo, J.R.; Fernández-Ruiz, M.; Martín, A.D.; Salavert, M.; Mularoni, A.; Puig-Asensio, M.; et al. Candidemia in non-ICU surgical wards: Comparison with medical wards. PLOS ONE 2017, 12, e0185339. [Google Scholar] [CrossRef]
- González-Lara, M.F.; Torres-Gonzalez, P.; Cornejo-Juárez, P.; Velázquez-Acosta, C.; Martinez-Gamboa, A.; Rangel-Cordero, A.; Valle, M.B.-D.; Ostrosky-Zeichner, L.; Ponce-De-León, A.; Sifuentes-Osornio, J. Impact of inappropriate antifungal therapy according to current susceptibility breakpoints on Candida bloodstream infection mortality, a retrospective analysis. BMC Infect. Dis. 2017, 17, 753. [Google Scholar] [CrossRef]
| Species | Number | Anidulafungin | Fluconazole | Voriconazole | Posaconazole | Amphotericin B | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S | R | S | R | S | R | S | R | S | R | ||
| C. albicans | 72 | 96 | 4 | 100 | 0 | 100 | 0 | 95 | 5 | 100 | 0 |
| C. glabrata | 24 | 100 | 0 | 0 | 100 | 80 | 20 | 45 | 55 | 100 | 0 |
| C. parapsilosis | 18 | 5 | 95 | 78 | 22 | 100 | 0 | 94 | 6 | 100 | 0 |
| C. tropicalis | 5 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 |
| C. krusei | 4 | 100 | 0 | 0 | 100 | 100 | 0 | 50 | 50 | 100 | 0 |
| Variables | Number of Patients (%) |
|---|---|
| Demographic parameters | |
| Age, range | Median 63 years, range 22–91 |
| Male gender | 70 (75%) |
| Medical ward | 39 (32%) |
| Surgical ward | 62 (50%) |
| Others | 22 (18%) |
| ICU stay (overall) | 61 (49%) |
| Treatment | |
| Empirical treatment | 53 (43%) |
| Antifungal drugs used as first-line treatment | |
| Caspofungin | 38 (31%) |
| Fluconazole | 35 (28%) |
| Anidulafungin | 31 (25%) |
| Micafungin | 12 (10%) |
| Voriconazole | 5 (4%) |
| Posaconazole | 1 (1%) |
| Fluconazole and Flucytosine | 1 (1%) |
| Days until fungal clearance | Median 5.6 days, range 2–12 |
| Outcome | |
| 14-day fungal free blood cultures | 123 (100%) |
| Species (Patients) | Antifungal Drug Resistance | Primary Therapy (Patients) | Antifungal Switch | 14-Day Fungal Free Blood Cultures | Open Questions Based on International Guideline Recommendations and Candida Species and MIC Values Reported |
|---|---|---|---|---|---|
| C. albicans (n = 3) | ANI | CAS | MICA & l-AmB ISA | ✓ | The switch from CAS to MICA is unclear? |
| CAS | ✓ | The application of ISA, which is not licensed for Candida therapy is unclear? | |||
| CAS | ✓ | The application of CAS despite ANI resistance is unclear? | |||
| C. glabrata (n = 14) | FLU ANI | FLU (7) | FLU & ANI CAS | ✓ | The application of FLU in C. glabrata and FLU resistance being present is unclear? |
| FLU | ✓ | Why not stopping FLU when adding ANI? | |||
| ANI | ✓ | Why a change from ANI to CAS in such case? | |||
| ANI (5) | ✓ | The application of ANI despite ANI resistance being present is unclear? | |||
| C. parapsilosis (n = 6) | ANI ANI/FLU FLU | CAS | MICA | ✓ | The switch from CAS to MICA despite ANI resistance being present is unclear? |
| ANI (2) | ✓ | ANI treatment despite ANI resistance being present? | |||
| CAS | ✓ | CAS therapy despite ANI resistance? | |||
| CAS | ✓ | Why CAS therapy despite ANI resistance? | |||
| FLU | ✓ | The application of FLU despite FLU resistance being present is unclear? | |||
| C. krusei (n = 1) | FLU | MICA | CAS | ✓ | Why change from MIC to CAS in case of FLU resistance being present? |
| Variables | Univariate p Value |
|---|---|
| Age | 0.880 |
| Gender | 0.259 |
| Empirical treatment | 0.881 |
| Targeted treatment | 0.881 |
| MIC (antifungal susceptibility data)-correlated treatment | 0.713 |
| MIC-non-correlated treatment | 0.867 |
| Antifungal drug switch | 0.187 |
| Intensive care unit | 0.744 |
| Candida species involved | 0.570 |
| Resistant isolates involved | 0.721 |
| Susceptible isolates involved | 0.657 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lass-Flörl, C.; Krause, R.; Willinger, B.; Starzengruber, P.; Decristoforo, P.; Neururer, S.; Kreidl, P.; Aigner, M. Clinical Usefulness of Susceptibility Breakpoints for Yeasts in the Treatment of Candidemia: A Noninterventional Study. J. Fungi 2020, 6, 76. https://doi.org/10.3390/jof6020076
Lass-Flörl C, Krause R, Willinger B, Starzengruber P, Decristoforo P, Neururer S, Kreidl P, Aigner M. Clinical Usefulness of Susceptibility Breakpoints for Yeasts in the Treatment of Candidemia: A Noninterventional Study. Journal of Fungi. 2020; 6(2):76. https://doi.org/10.3390/jof6020076
Chicago/Turabian StyleLass-Flörl, Cornelia, Robert Krause, Birgit Willinger, Peter Starzengruber, Petra Decristoforo, Sabrina Neururer, Peter Kreidl, and Maria Aigner. 2020. "Clinical Usefulness of Susceptibility Breakpoints for Yeasts in the Treatment of Candidemia: A Noninterventional Study" Journal of Fungi 6, no. 2: 76. https://doi.org/10.3390/jof6020076
APA StyleLass-Flörl, C., Krause, R., Willinger, B., Starzengruber, P., Decristoforo, P., Neururer, S., Kreidl, P., & Aigner, M. (2020). Clinical Usefulness of Susceptibility Breakpoints for Yeasts in the Treatment of Candidemia: A Noninterventional Study. Journal of Fungi, 6(2), 76. https://doi.org/10.3390/jof6020076








