Applying the Host-Microbe Damage Response Framework to Candida Pathogenesis: Current and Prospective Strategies to Reduce Damage
Abstract
1. Introduction
2. The Damage Response Framework
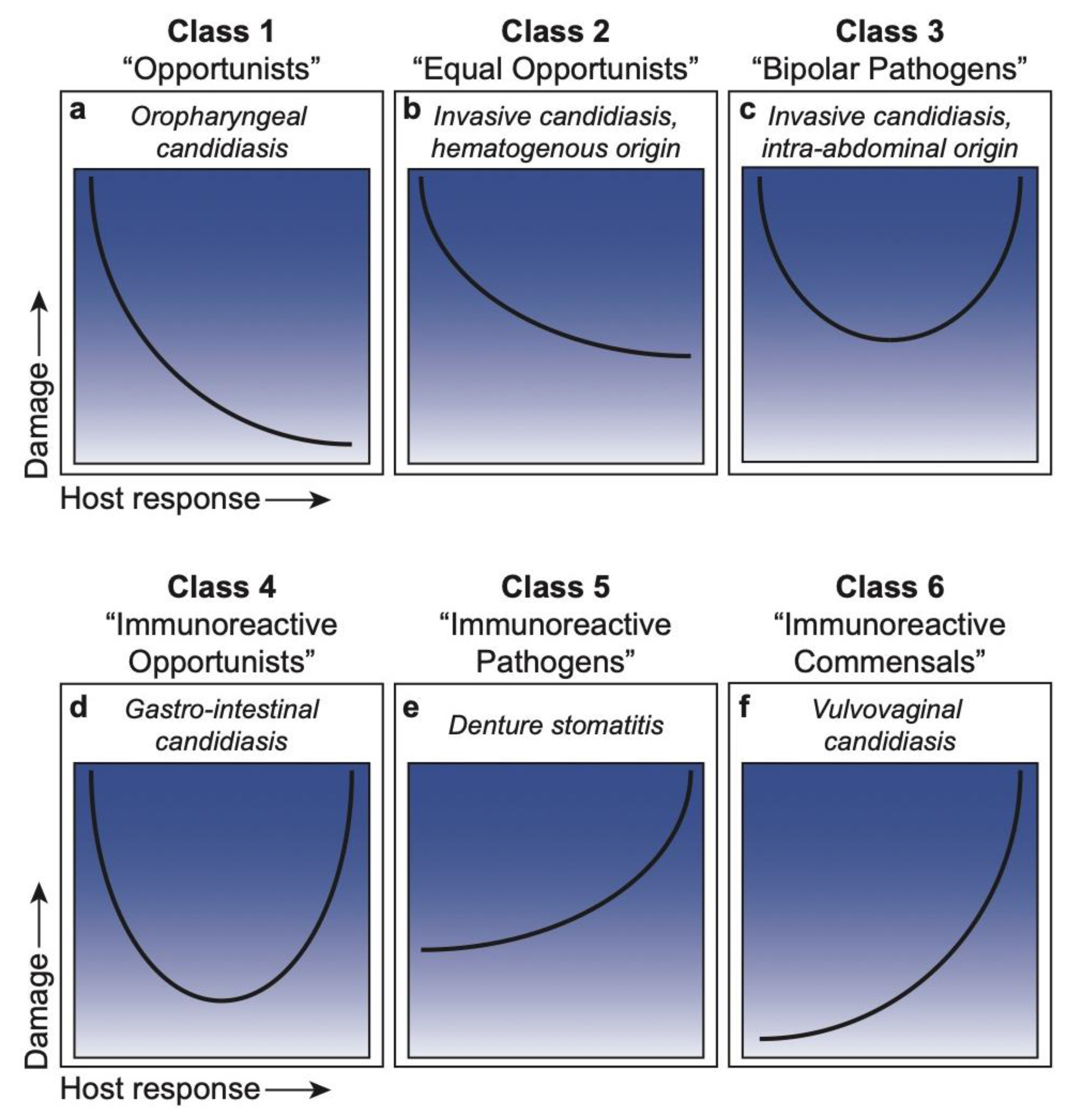
DRF Classification of Microbial Species
3. Candida within the DRF
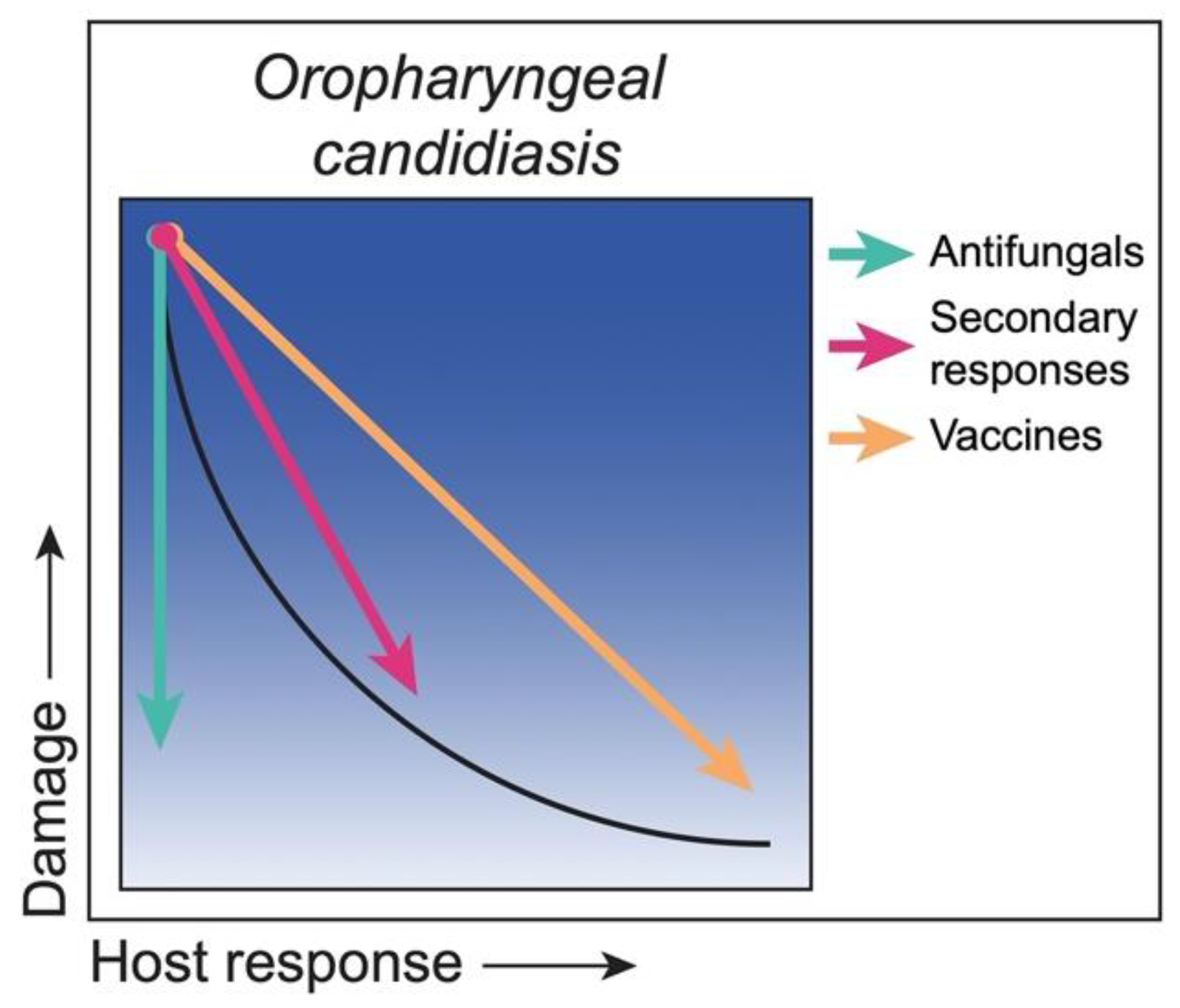
3.1. Class 1. Oropharyngeal Candidiasis: Damage Occurs Only in Situations of a Weakened or Compromised Immune System
Strategies to Reduce Damage
3.2. Class 2. Hematogenously Disseminated Candidiasis (HDC): Damage Occurs in Hosts with Weak or Normal Immune Responses
Strategies to Reduce Damage
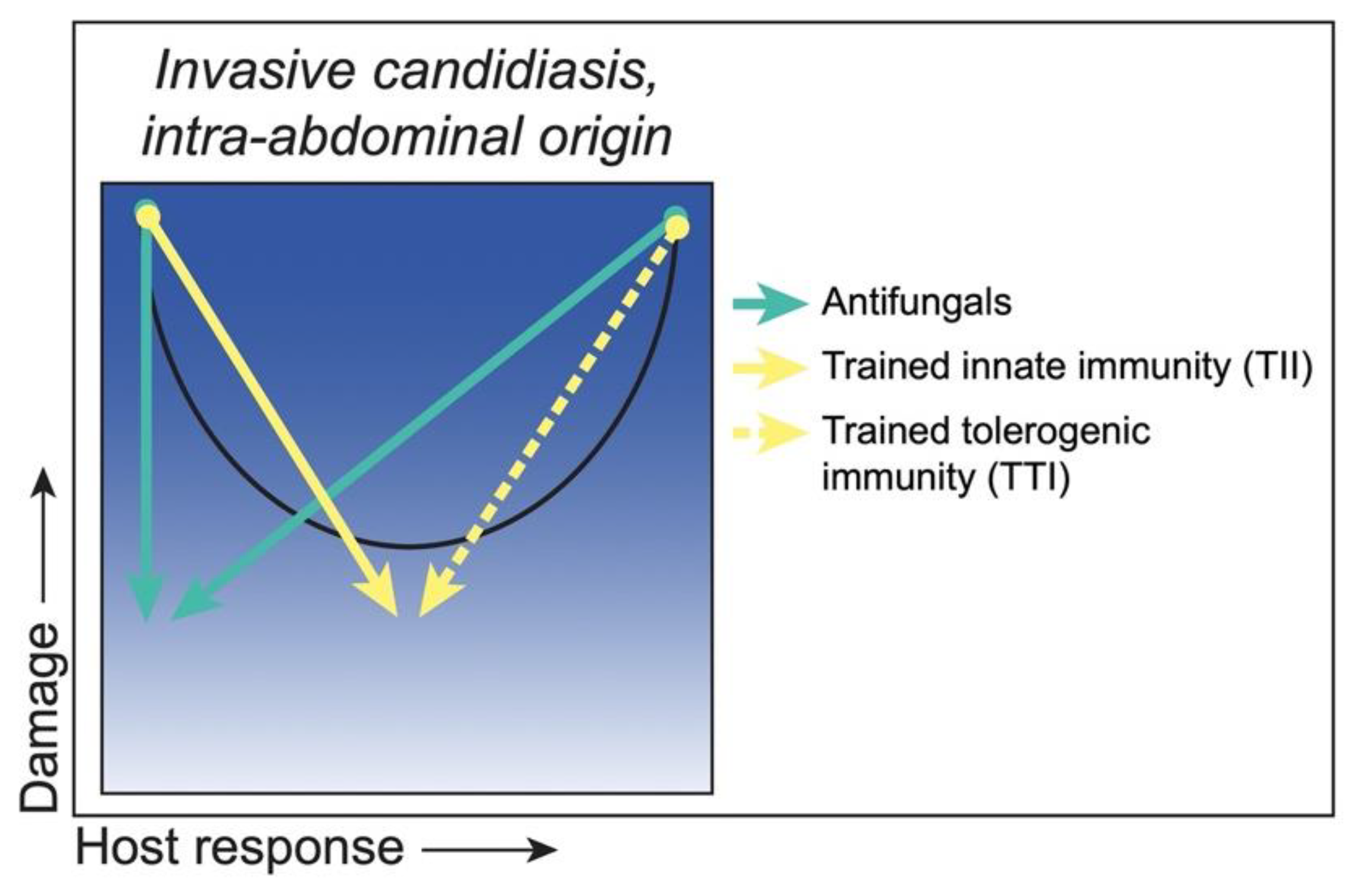
3.3. Class 3. Intra-Abdominal Candidiasis: Damage Occurs throughout the Continuum of Immune Responses but Is Amplified at Extremes of Both Weak and Strong Immune Responses
Strategies to Reduce Damage
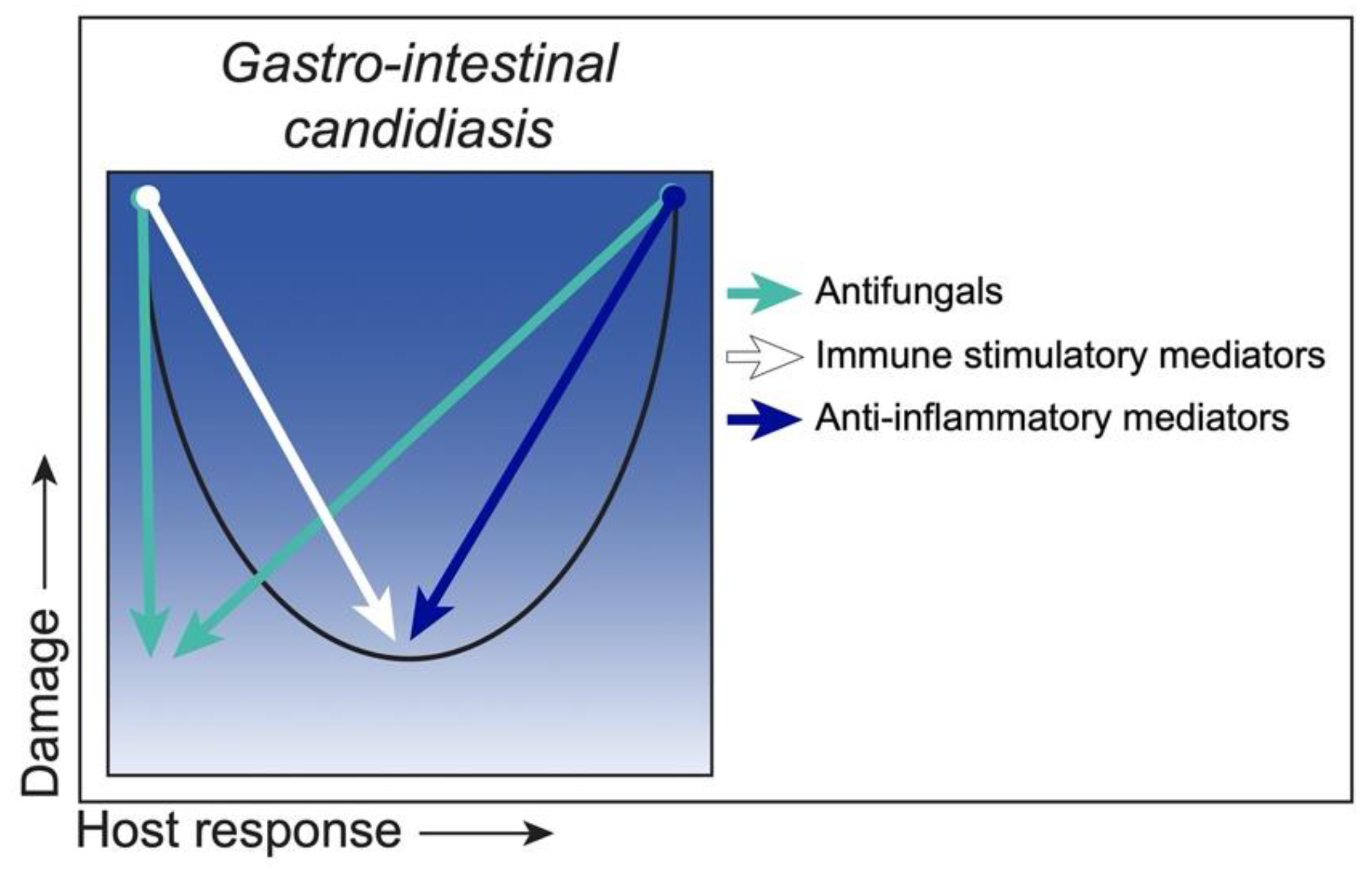
3.4. Class 4. Gastro-Intestinal Candidiasis: Damage Occurs Primarily at the Extremes of Both Weak and Strong Immune Responses
Strategies to Reduce Damage
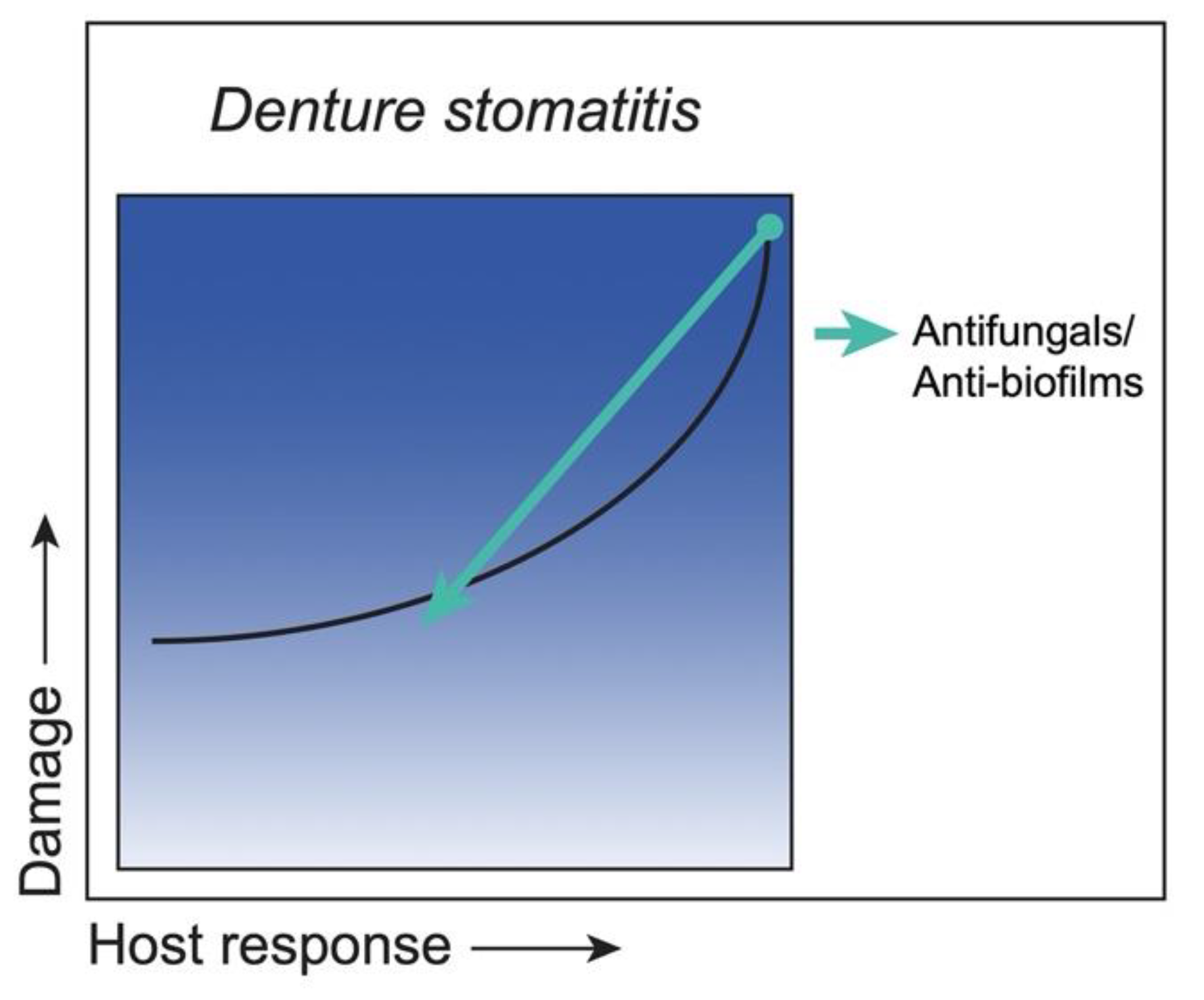
3.5. Class 5. Denture Stomatitis: Damage Occurs across the Spectrum of Immune Responses, but Damage Is Enhanced by Strong Immune Responses
Strategies to Reduce Damage
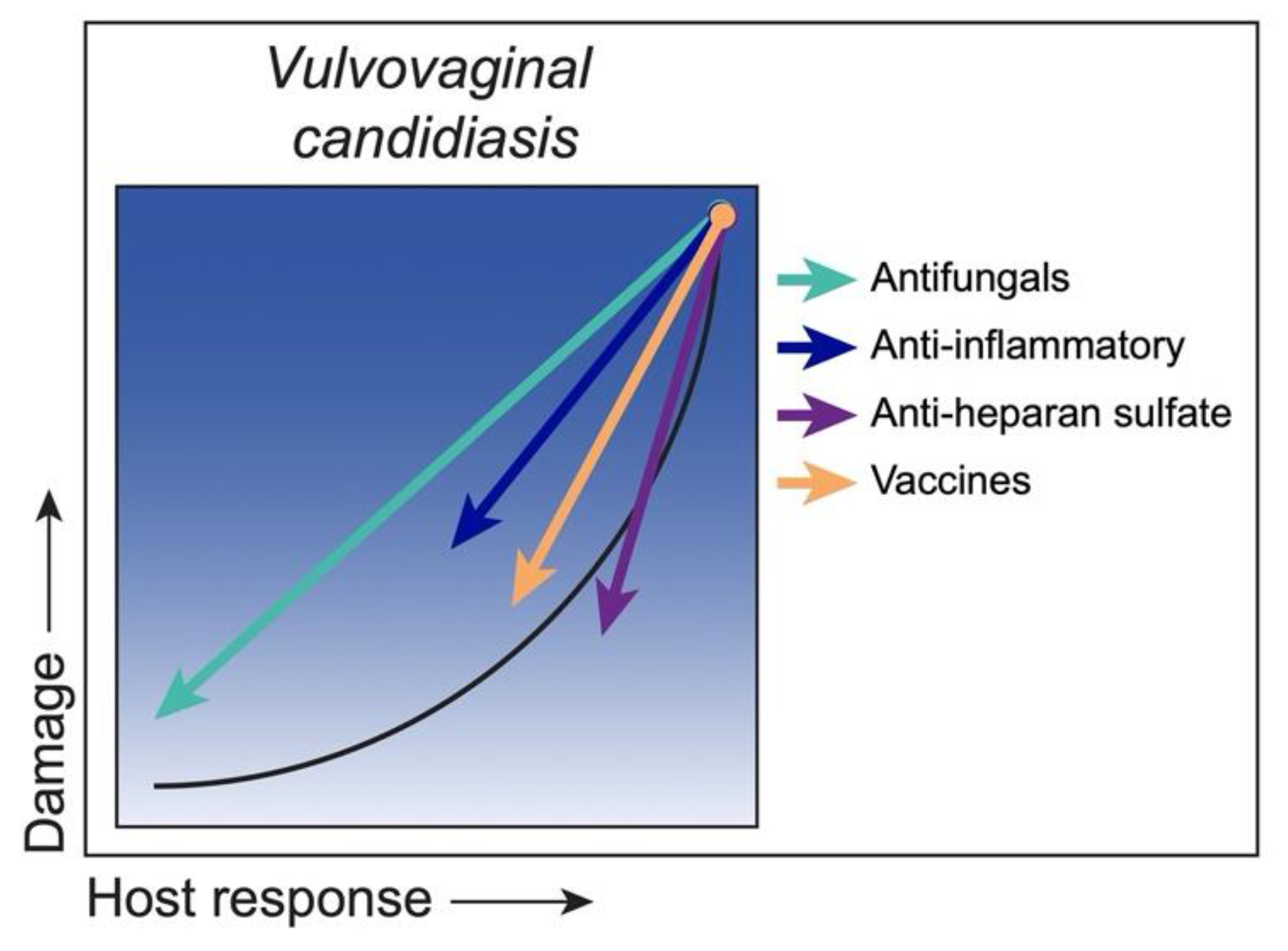
3.6. Class 6. Vulvovaginal Candidiasis: Damage Occurs Only under Conditions of Strong Immune Responses
Strategies to Reduce Damage
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ART | Antiretroviral therapy |
| BCG | Bacillus Calmette–Guérin |
| CAGTA | C. albicans germ tube antibodies |
| DABCO | 1,4-Diazabicyclo[2.2.2]octane |
| DRF | Damage response framework |
| DS | Denture stomatitis |
| DSC | Deep-seated candidiasis |
| GI | Gastrointestinal |
| HDC | Hematogenously disseminated candidiasis |
| HIV | Human immunodeficiency virus |
| HS | Heparan sulfate |
| HSPC | Hematopoietic stem and progenitor cell |
| IAC | Intra-abdominal candidiasis |
| IAI | Intra-abdominal infection |
| ICU | Intensive care unit |
| IFN | Interferon |
| IL | Interleukin |
| MDSC | Myeloid-derived suppressor cell |
| NAC | Non-albicans Candida |
| NSAID | Non-steroidal anti-inflammatory drug |
| nTh17 | Natural T helper type 17 |
| OPC | Oropharyngeal candidiasis |
| PDI | Photodynamic inactivation |
| PMN | Polymorphonuclear neutrophil |
| RVVC | Recurrent vulvovaginal candidiasis |
| SAP | Secretory aspartyl proteinase |
| Th | T helper |
| TII | Trained innate immunity |
| TTI | Trained tolerogenic immunity |
| VVC | Vulvovaginal candidiasis |
References
- Casadevall, A.; Pirofski, L.A. Host-pathogen interactions: Redefining the basic concepts of virulence and pathogenicity. Infect. Immun. 1999, 67, 3703–3713. [Google Scholar] [CrossRef]
- Calderone, R.A. (Ed.) Candida and Candidiasis; ASM Press: Washington, DC, USA, 2012. [Google Scholar]
- Williams, D.; Jordan, R.; Wei, X.-Q.; Alves, C.; Wise, M.; Wilson, M.; Lewis, M. Interactions of Candida albicans with host epithelial surfaces. Oral Microbiol. 2013, 5, 22434. [Google Scholar] [CrossRef]
- Naglik, J.R.; Fidel, P.L.; Odds, F.C. Animal models of mucosal Candida infection. FEMS Microbiol. Lett. 2008, 283, 129–139. [Google Scholar] [CrossRef]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microb. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.-A. What Is a Host? Incorporating the microbiota into the damage-response framework. Infect. Immun. 2015, 83, 2–7. [Google Scholar] [CrossRef]
- Jabra-Rizk, M.A.; Kong, E.F.; Tsui, C.; Nguyen, M.H.; Clancy, C.J.; Fidel, P.L., Jr.; Noverr, M. Candida albicans Pathogenesis: Fitting within the Host-Microbe Damage Response Framework. Infect. Immun. 2016, 84, 2724–2739. [Google Scholar] [CrossRef] [PubMed]
- Fidel, P.L., Jr. Candida-Host Interactions in HIV Disease: Implications for Oropharyngeal Candidiasis. Adv. Dent. Res. 2011, 23, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Fidel, P.L., Jr. Candida-host interactions in HIV disease: Relationships in oropharyngeal candidiasis. Adv. Dent. Res. 2006, 19, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cuesta, C.; Sarrion-Pérez, M.; Bagán, J. Current treatment of oral candidiasis: A literature review. J. Clin. Exp. Dent. 2014, 6, e576–e582. [Google Scholar] [CrossRef] [PubMed]
- Fidel, P., Jr. Immunity to Candida. Oral Dis. 2002, 8, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Redding, S.W.; Zellars, R.C.; Kirkpatrick, W.R.; McAtee, R.K.; Caceres, M.A.; Fothergill, A.G.; Lopez-Ribot, J.L.; Bailey, C.W.; Rinaldi, M.G.; Paterson, T.F. Epidemiology of oropharyngeal Candida colonization and infection in patients receiving radiation for head and neck cancer. J. Clin. Microbiol. 1999, 37, 3896–3900. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Lewis, M. Pathogenesis and treatment of oral candidosis. Oral Microbiol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.R.; Newport, G.; White, T.C.; Fernandes-Naglik, L.L.; Greenspan, J.S.; Greenspan, D.; Sweet, S.P.; Challacombe, S.J.; Agabian, N. In vivo analysis of secreted aspartyl proteinase expression in human oral candidiasis. Infect. Immun. 1999, 67, 2482–2490. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.F.; Tsui, C.; Boyce, H.; Ibrahim, A.; Hoag, S.W.; Karlsson, A.J.; Meiller, T.F.; Jabra-Rizk, M.A. Development and in vivo evaluation of a novel histatin-5 bioadhesive hydrogel formulation against oral candidiasis. Antimicrob. Agents Chemother. 2015. [Google Scholar] [CrossRef]
- Khan, S.; Fidel, P., Jr.; Al Thunayyan, A.; Meiller, T.; Jabra-Rizk, M. Impaired histatin-5 level and salivary antimicrobial activity against C. albicans in HIV-infected individuals. J. AIDS Clin. Res. 2013, 4, 1–6. [Google Scholar] [CrossRef]
- Edgerton, M.; Koshlukova, S.E.; Lo, T.E.; Chrzan, B.G.; Straubinger, R.M.; Raj, P.A. Candidacidal activity of salivary histatins. J. Biol. Chem. 1998, 272, 20438–20447. [Google Scholar] [CrossRef]
- Peters, B.; Shirtliff, M.; Jabra-Rizk, M. Antimicrobial Peptides: Primeval Molecules or Future Drugs? PLoS Pathog. 2010, 6, e1001067. [Google Scholar] [CrossRef]
- Ashman, R.; Farah, C.; Wanasaengsakul, S.; Hu, V.; Pang, G.; Clancy, R. Innate versus adaptive immunity in Candida albicans infection. Immunol. Cell Biol. 2004, 82, 196–204. [Google Scholar] [CrossRef]
- Farah, C.; Elahi, S.; Drysdale, K.; Pang, G.; Gotjamanos, T.; Seymour, G.; Clancy, R.; Ashman, R. Primary role for CD4(+) T lymphocytes in recovery from oropharyngeal candidiasis. Infect. Immun. 2001, 70, 724–731. [Google Scholar] [CrossRef]
- Solis, N.; Filler, S. Mouse model of oropharyngeal candidiasis. Nat. Prot. 2012, 7, 637–642. [Google Scholar] [CrossRef]
- Pirofski, L.-A.; Casadevall, A. Rethinking T cell immunity in oropharyngeal candidiasis. J. Exp. Med. 2009, 206, 269. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.L.; Hernandez-Santos, N.; Peterson, A. IL-17 Signaling in host defense against Candida albicans. Immunol. Res. 2011, 50, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Conti, H.; Shen, F.; Nayyar, N.; Stocum, E.; Sun, J.; Lindemann, M.; Ho, A.; Hai, J.; Yu, J.; Jung, J.; et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009, 206, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Tan, X.; Luxenberg, D.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef]
- Verma, A.H.; Richardson, J.P.; Zhou, C.; Coleman, B.M.; Moyes, D.L.; Ho, J.; Huppler, A.R.; Ramani, K.; McGeachy, M.J.; Mufazalov, I.A.; et al. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef]
- Conti, H.R.; Peterson, A.C.; Brane, L.; Huppler, A.R.; Hernandez-Santos, N.; Whibley, N.; Garg, A.V.; Simpson-Abelson, M.R.; Gibson, G.A.; Mamo, A.J.; et al. Oral-resident natural Th17 cells and gammadelta T cells control opportunistic Candida albicans infections. J. Exp. Med. 2014, 211, 2075–2084. [Google Scholar] [CrossRef]
- Harriott, M.; Lilly, E.; Rodriguez, T.; Fidel, P., Jr.; Noverr, M. Candida albicans forms biofilms on the vaginal mucosa. Microbiol. 2010, 156, 3635–3644. [Google Scholar] [CrossRef]
- Hernandez-Santos, N.; Huppler, A.R.; Peterson, A.C.; Khader, S.A.; McKenna, K.C.; Gaffen, S.L. Th17 cells confer long-term adaptive immunity to oral mucosal Candida albicans infections. Mucosal. Immunol. 2013, 6, 900–910. [Google Scholar] [CrossRef]
- Schmidt, C.S.; White, C.J.; Ibrahim, A.S.; Filler, S.G.; Fu, Y.; Yeaman, M.R.; Edwards, J.E., Jr.; Hennessey, J.P., Jr. NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine 2012, 30, 7594–7600. [Google Scholar] [CrossRef]
- Edwards, J.E., Jr.; Schwartz, M.M.; Schmidt, C.S.; Sobel, J.D.; Nyirjesy, P.; Schodel, F.; Marchus, E.; Lizakowski, M.; DeMontigny, E.A.; Hoeg, J.; et al. A Fungal Immunotherapeutic Vaccine (NDV-3A) for Treatment of Recurrent Vulvovaginal Candidiasis-A Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 2018, 66, 1928–1936. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef] [PubMed]
- Weiner-Lastinger, L.M.; Abner, S.; Edwards, J.R.; Kallen, A.J.; Karlsson, M.; Magill, S.S.; Pollock, D.; See, I.; Soe, M.M.; Walters, M.S.; et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control. Hosp. Epidemiol. 2019, 41, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in U.S. hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health care-associated infections. New Eng. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Parker, J.C., Jr.; McCloskey, J.J.; Knauer, K.A. Pathobiologic features of human candidiasis. A common deep mycosis of the brain, heart and kidney in the altered host. Am. J. Clin. Pathol. 1976, 65, 991–1000. [Google Scholar] [CrossRef]
- Louria, D.; Stiff, D.; Bennett, B. Disseminated moniliasis in the adult. Medicine 1962, 41, 307–338. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. Finding the "missing 50%" of invasive candidiasis: How nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin. Infect. Dis. 2013, 56, 1284–1292. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. Non-Culture Diagnostics for Invasive Candidiasis: Promise and Unintended Consequences. J. Fungi 2018, 4, 27. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. Diagnosing Invasive Candidiasis. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef]
- Morrell, M.; Fraser, V.; Kollef, M. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: A potential risk factor for hospital mortality. Antimicrob. Agents Chemother. 2005, 49, 3640–3645. [Google Scholar] [CrossRef] [PubMed]
- Pasqualotto, A.C.; Nedel, W.L.; Machado, T.S.; Severo, L.C. Risk factors and outcome for nosocomial breakthrough candidaemia. J. Infect. 2006, 52, 216–222. [Google Scholar] [CrossRef]
- Eggimann, P.; Pittet, D. Candida colonization index and subsequent infection in critically ill surgical patients: 20 years later. Intensive Care Med. 2014, 40, 1429–1448. [Google Scholar] [CrossRef] [PubMed]
- Wall, G.; Montelongo-Jauregui, D.; Vidal Bonifacio, B.; Lopez-Ribot, J.L.; Uppuluri, P. Candida albicans biofilm growth and dispersal: Contributions to pathogenesis. Curr. Opin. Microbiol. 2019, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tsui, C.; Kong, E.F.; Jabra-Rizk, M.A. Pathogenesis of Candida albicans biofilm. Pathog. Dis. 2016, 74, ftw018. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Righi, E.; Montravers, P.; Cornely, O.A. What has changed in the treatment of invasive candidiasis? A look at the past 10 years and ahead. J. Antimicrob. Chemother. 2018, 73, i14–i25. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B. Novel insights into disseminated candidiasis: Pathogenesis research and clinical experience converge. PLoS Pathog. 2008. [Google Scholar] [CrossRef]
- Vonk, A.; Netea, M.; van der Meer, J.; Kullberg, B. Host defence against disseminated Candida albicans infection and implications for antifungal immunotherapy. Expert. Opin. Biol. Ther. 2006, 6, 891–903. [Google Scholar] [CrossRef]
- Pappas, P.G. Invasive candidiasis. Infect. Dis. Clin. N. Am. 2006, 20, 485–506. [Google Scholar] [CrossRef]
- Mylonakis, E.; Clancy, C.J.; Ostrosky-Zeichner, L.; Garey, K.W.; Alangaden, G.J.; Vazquez, J.A.; Groeger, J.S.; Judson, M.A.; Vinagre, Y.M.; Heard, S.O.; et al. T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: A clinical trial. Clin. Infect. Dis. 2015, 60, 892–899. [Google Scholar] [CrossRef]
- Clancy, C.J.; Pappas, P.G.; Vazquez, J.; Judson, M.A.; Kontoyiannis, D.P.; Thompson, G.R., 3rd; Garey, K.W.; Reboli, A.; Greenberg, R.N.; Apewokin, S.; et al. Detecting Infections Rapidly and Easily for Candidemia Trial, Part 2 (DIRECT2): A Prospective, Multicenter Study of the T2Candida Panel. Clin. Infect. Dis. 2018, 66, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, M.; Calandra, T.; Sanguinetti, M.; Poulain, D.; Viscoli, C. The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: Recommendations from the Third European Conference on Infections in Leukemia. Crit. Care 2010, 14, R222. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Quintin, J.; Cheng, S.C.; van der Meer, J.W.; Netea, M.G. Innate immune memory: Towards a better understanding of host defense mechanisms. Curr. Opin. Immunol. 2014, 29, 1–7. [Google Scholar] [CrossRef]
- Quintin, J.; Saeed, S.; Martens, J.H.A.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.J.; Wijmenga, C.; et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef]
- Saeed, S.; Quintin, J.; Kerstens, H.H.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345, 1251086. [Google Scholar] [CrossRef]
- Mitroulis, I.; Ruppova, K.; Wang, B.; Chen, L.S.; Grzybek, M.; Grinenko, T.; Eugster, A.; Troullinaki, M.; Palladini, A.; Kourtzelis, I.; et al. Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell 2018, 172, 147–161 e112. [Google Scholar] [CrossRef]
- Lilly, E.A.; Yano, J.; Esher, S.K.; Hardie, E.; Fidel, P.L., Jr.; Noverr, M.C. Spectrum of Trained Innate Immunity Induced by Low-Virulence Candida Species against Lethal Polymicrobial Intra-abdominal Infection. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonca, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.C.; et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 2018, 172, 176–190 e119. [Google Scholar] [CrossRef]
- Rusek, P.; Wala, M.; Druszczynska, M.; Fol, M. Infectious Agents as Stimuli of Trained Innate Immunity. Int. J. Mol. Sci. 2018, 19, 456. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M. New insights into innate immune control of systemic candidiasis. Med. Mycol. 2014, 52, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, R.; Schroder, C.; Monnens, L.; Cornelissen, E.; Warris, A. Fungal periotonitis in children on peritoneal dialysis. Pediatric Nephrol. 2007, 22, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo, M.; Sarria, J. Candida peritonitis intra-abdominal fungal infections. Curr. Opin. Infect. Dis. 2013, 26, 441–446. [Google Scholar] [PubMed]
- Montravers, P.; Dupont, H.; Eggimann, P. Intra-abdominal candidiasis: The guidelines—forgotten non-candidemic invasive candidiasis. Intens Care Med. 2013, 39, 2226–2230. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Clancy, C.; Xu, W.; Schneider, F.; Hao, B.; Mitchell, A.; Nguyen, M.-H. Profiling of Candida albicans gene expression during intra-abdominal candidiasis identifies biologic processes involved in pathogenesis. J. Infect. Dis. 2013, 208, 1529–1537. [Google Scholar] [CrossRef]
- Koh, A.; Köhler, J.; Coggshall, K.; Van Rooijen, N.; Pier, G. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog. 2008, 8, e35. [Google Scholar] [CrossRef]
- Vergidis, P.; Clancy, C.J.; Shields, R.K.; Park, S.Y.; Wildfeuer, B.N.; Simmons, R.L.; Nguyen, M.H. Intra-Abdominal Candidiasis: The Importance of Early Source Control and Antifungal Treatment. PLoS ONE 2016, 11, e0153247. [Google Scholar] [CrossRef]
- Wu, S.Y.; Weng, C.L.; Jheng, M.J.; Kan, H.W.; Hsieh, S.T.; Liu, F.T.; Wu-Hsieh, B.A. Candida albicans triggers NADPH oxidase-independent neutrophil extracellular traps through dectin-2. PLoS Pathog. 2019, 15, e1008096. [Google Scholar] [CrossRef]
- Wojtowicz, A.; Tissot, F.; Lamoth, F.; Orasch, C.; Eggimann, P.; Siegemund, M.; Zimmerli, S.; Flueckiger, U.M.; Bille, J.; Calandra, T.; et al. Polymorphisms in tumor necrosis factor-alpha increase susceptibility to intra-abdominal Candida infection in high-risk surgical ICU patients. Crit. Care Med. 2014, 42, e304–e308. [Google Scholar] [CrossRef]
- Zhao, Y.; Prideaux, B.; Nagasaki, Y.; Lee, M.H.; Chen, P.Y.; Blanc, L.; Ho, H.; Clancy, C.J.; Nguyen, M.H.; Dartois, V.; et al. Unraveling Drug Penetration of Echinocandin Antifungals at the Site of Infection in an Intra-abdominal Abscess Model. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Dupont, H.; Paugam-Burtz, C.; Muller-Serieys, C.; Fierobe, L.; Chosidow, D.; Marmuse, J.P.; Mantz, J.; Desmonts, J.M. Predictive factors of mortality due to polymicrobial peritonitis with Candida isolation in peritoneal fluid in critically ill patients. Arch. Surg. 2002, 137, 1341–1346; discussion 1347. [Google Scholar] [CrossRef] [PubMed]
- Montravers, P.; Gauzit, R.; Muller, C.; Marmuse, J.P.; Fichelle, A.; Desmonts, J.M. Emergence of antibiotic-resistant bacteria in cases of peritonitis after intraabdominal surgery affects the efficacy of empirical antimicrobial therapy. Clin. Infect. Dis. 1996, 23, 486–494. [Google Scholar] [CrossRef]
- Calandra, T.; Bille, J.; Schneider, R.; Mosimann, F.; Francioli, P. Clinical significance of Candida isolated from peritoneum in surgical patients. Lancet 1989, 2, 1437–1440. [Google Scholar] [CrossRef]
- Blot, S.I.; Vandewoude, K.H.; De Waele, J.J. Candida peritonitis. Curr. Opin. Crit. Care 2007, 13, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.; Noverr, M. Candida albicans-Staphylococcus aureus polymicrobial peritonitis modulates host innate immunity. Infect. Immun. 2013 81, 2178–2189. [CrossRef]
- Nash, E.; Peters, B.; Palmer, G.; Fidel, P., Jr.; Noverr, M. Morphogenesis is not required for Candida albicans-Staphylococcus aureus intra-abdominal infection-mediated dissemination and lethal sepsis. Infect. Immun. 2014, 82, 3426–3435. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arons, M.M.; Wheeler, A.P.; Bernard, G.R.; Christman, B.W.; Russell, J.A.; Schein, R.; Summer, W.R.; Steinberg, K.P.; Fulkerson, W.; Wright, P.; et al. Effects of ibuprofen on the physiology and survival of hypothermic sepsis. Ibuprofen in Sepsis Study Group. Crit. Care Med. 1999, 27, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Bernard, G.R.; Wheeler, A.P.; Russell, J.A.; Schein, R.; Summer, W.R.; Steinberg, K.P.; Fulkerson, W.J.; Wright, P.E.; Christman, B.W.; Dupont, W.D.; et al. The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group. N. Engl. J. Med. 1997, 336, 912–918. [Google Scholar] [CrossRef]
- Haupt, M.T.; Jastremski, M.S.; Clemmer, T.P.; Metz, C.A.; Goris, G.B. Effect of ibuprofen in patients with severe sepsis: A randomized, double-blind, multicenter study. The Ibuprofen Study Group. Crit. Care Med. 1991, 19, 1339–1347. [Google Scholar] [CrossRef]
- Memis, D.; Karamanlioglu, B.; Turan, A.; Koyuncu, O.; Pamukcu, Z. Effects of lornoxicam on the physiology of severe sepsis. Crit. Care 2004, 8, R474–R482. [Google Scholar] [CrossRef] [PubMed]
- Ikeh, M.A.C.; Fidel, P.L., Jr.; Noverr, M.C. Identification of Specific Components of the Eicosanoid Biosynthetic and Signaling Pathway Involved in Pathological Inflammation during Intra-abdominal Infection with Candida albicans and Staphylococcus aureus. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef] [PubMed]
- Lilly, E.A.; Ikeh, M.; Nash, E.E.; Fidel, P.L., Jr.; Noverr, M.C. Immune Protection against Lethal Fungal-Bacterial Intra-Abdominal Infections. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Esher, S.K.; Fidel, P.L., Jr.; Noverr, M.C. Candida/Staphylococcal Polymicrobial Intra-Abdominal Infection: Pathogenesis and Perspectives for a Novel Form of Trained Innate Immunity. J. Fungi 2019, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Koh, A. Gastrointestinal Colonization of Fungi. Curr. Fungal Infect. Rep. 2013, 7, 144–151. [Google Scholar] [CrossRef]
- Rashid, M.U.; Rosenborg, S.; Panagiotidis, G.; Soderberg-Lofdal, K.; Weintraub, A.; Nord, C.E. Ecological Effect of Ceftaroline-Avibactam on the Normal Human Intestinal Microbiota. Antimicrob. Agents Chemother. 2015, 59, 4504–4509. [Google Scholar] [CrossRef] [PubMed]
- Lacour, M.; Zunder, T.; Huber, R.; Sander, A.; Daschner, F.; Frank, U. The pathogenetic significance of intestinal Candida colonization-a systematic review from an interdisciplinary and environmental medical point of view. Int, J. Hyg. Environ. Health 2002, 205, 257–268. [Google Scholar] [CrossRef]
- Wheeler, M.L.; Limon, J.J.; Bar, A.S.; Leal, C.A.; Gargus, M.; Tang, J.; Brown, J.; Funari, V.A.; Wang, H.L.; Crother, T.R.; et al. Immunological Consequences of Intestinal Fungal Dysbiosis. Cell Host Microbe 2016, 19, 865–873. [Google Scholar] [CrossRef]
- Erdogan, A.; Rao, S. Small intestinal fungal overgrowth. Curr. Gastroenterol Rep. 2015, 17, 16. [Google Scholar] [CrossRef]
- Schulze, J.; Sonnenborn, U. Yeasts in the gut: From commensals to infectious agents. Dtsch. Arztebl. Int. 2009, 106, 837–842. [Google Scholar]
- Cantorna, M.; Balish, E. Acquired immunity to systemic candidiasis in immunodeficient mice. J. Infect. Dis. 1991, 164, 936–943. [Google Scholar] [CrossRef]
- Cantorna, M.; Balish, E. Mucosal and systemic candidiasis in congenitally immunodeficient mice. Infect. Immun. 1990, 58, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Balish, E.; Jensen, J.; Warner, T.; Brekke, J.; Leonard, B. Mucosal and disseminated candidiasis in gnotobiotic SCID mice. J. Med. Vet. Mycol. 1993, 31, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Balish, E.; Balish, M.; Salkowski, C.; Lee, K.; Bartizal, K. Colonization of congenitally athymic, gnotobiotic mice by Candida albicans. Appl. Environ. Microbiol. 1984, 47, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Cenci, E.; Mencacci, A.; Spaccapelo, R.; Tonnetti, L.; Mosci, P.; Enssle, K.; Puccetti, P.; Romani, L.; Bistoni, F. T helper cell type 1 (Th1)- and Th2-like responses are present in mice with gastric candidiasis but protective immunity is associated with Th1 development. J. Infect. Dis. 1995, 171, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Jones-Carson, J.; Vazquez-Torres, A.; Warner, T.; Balish, E. Disparate requirement for T cells in resistance to mucosal and acute systemic candidiasis. Infect. Immun. 2000, 68, 2363–2365. [Google Scholar] [CrossRef] [PubMed]
- Koh, A. Murine models of Candida gastrointestinal colonization and dissemination. Eukaryot. Cell. 2013, 12, 1416–1422. [Google Scholar] [CrossRef]
- Fan, D.; Coughlin, L.A.; Neubauer, M.M.; Kim, J.; Kim, M.S.; Zhan, X.; Simms-Waldrip, T.R.; Xie, Y.; Hooper, L.V.; Koh, A.Y. Activation of HIF-1alpha and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat. Med. 2015, 21, 808–814. [Google Scholar] [CrossRef]
- Mishra, A.A.; Koh, A.Y. Adaptation of Candida albicans during gastrointestinal tract colonization. Curr. Clin. Microbiol. Rep. 2018, 5, 165–172. [Google Scholar] [CrossRef]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef]
- Webb, B.C.; Thomas, C.J.; Willcox, M.D.; Harty, D.W.; Knox, K.W. Candida-associated denture stomatitis. Aetiology and management: A review. Part 3. Treatment of oral candidosis. Aust. Dent. J. 1998, 43, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Tomsett, K.; Wickes, B.L.; Lopez-Ribot, J.L.; Redding, S.W. Denture stomatitis: A role for Candida biofilms. Oral Surg Oral Med. Oral Pathol Oral Radiol Endod 2004, 98, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Budtz-Jörgensen, E. The significance of Candida albicans in denture stomatitis. Scand. J. Dent. Res. 1974, 82, 151–190. [Google Scholar] [CrossRef] [PubMed]
- Arendorf, T.M.; Walker, D.M. Denture stomatitis: A review. J. Oral Rehabil. 1987, 14, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Budtz-Jorgensen, E. Oral mucosal lesions associated with the wearing of removable dentures. J. Oral Pathol. 1981, 10, 65–80. [Google Scholar] [CrossRef]
- Shulman, J.D.; Rivera-Hidalgo, F.; Beach, M.M. Risk factors associated with denture stomatitis in the United States. J. Oral Pathol. Med. 2005, 34, 340–346. [Google Scholar] [CrossRef]
- Redding, S.; Bhatt, B.; Rawls, H.R.; Siegel, G.; Scott, K.; Lopez-Ribot, J. Inhibition of Candida albicans biofilm formation on denture material. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 669–672. [Google Scholar] [CrossRef]
- Radford, D.R.; Challacombe, S.J.; Walter, J.D. Denture plaque and adherence of Candida albicans to denture-base materials in vivo and in vitro. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 1999, 10, 99–116. [Google Scholar] [CrossRef]
- Nett, J.E.; Marchillo, K.; Spiegel, C.A.; Andes, D.R. Development and Validation of an In Vivo Candida albicans Biofilm Denture Model. Infect. Immun. 2010, 78, 3650–3659. [Google Scholar] [CrossRef]
- Offenbacher, S.; Barros, S.P.; Bencharit, S.; Yu, N.; Preisser, J.; Moss, K.; Loewy, Z.G. Differential Mucosal Gene Expression Patterns in Candida-Associated, Chronic Oral Denture Stomatitis. J. Prosthodonts 2019, 28, 202–208. [Google Scholar] [CrossRef]
- Cumming, C.G.; Wight, C.; Blackwell, C.L.; Wray, D. Denture stomatitis in the elderly. Oral Microbiol. Immunol. 1990, 5, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Dorocka-Bobkowska, B.; Konopka, K. Susceptibility of candida isolates from denture-related stomatitis to antifungal agents in vitro. Int. J. Prosthodont 2007, 20, 504–506. [Google Scholar] [PubMed]
- Zomorodian, K.; Haghighi, N.N.; Rajaee, N.; Pakshir, K.; Tarazooie, B.; Vojdani, M.; Sedaghat, F.; Vosoghi, M. Assessment of Candida species colonization and denture-related stomatitis in complete denture wearers. Med. Mycol. 2011, 49, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Vanden Abbeele, A.; de Meel, H.; Ahariz, M.; Perraudin, J.P.; Beyer, I.; Courtois, P. Denture contamination by yeasts in the elderly. Gerodontology 2008, 25, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.A.; Toledo, B.C.; Santos, C.T.; Pereira Costa, A.C.; Back-Brito, G.N.; Kaminagakura, E.; Jorge, A.O. Opportunistic microorganisms in individuals with lesions of denture stomatitis. Diagn. Microbiol. Infect. Dis. 2013, 76, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Peric, M.; Zivkovic, R.; Milic Lemic, A.; Radunovic, M.; Milicic, B.; Arsic Arsenijevic, V. The severity of denture stomatitis as related to risk factors and different Candida spp. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 41–47. [Google Scholar] [CrossRef]
- Sanita, P.V.; Pavarina, A.C.; Giampaolo, E.T.; Silva, M.M.; Mima, E.G.; Ribeiro, D.G.; Vergani, C.E. Candida spp. prevalence in well controlled type 2 diabetic patients with denture stomatitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 726–733. [Google Scholar] [CrossRef]
- Coco, B.J.; Bagg, J.; Cross, L.J.; Jose, A.; Cross, J.; Ramage, G. Mixed Candida albicans and Candida glabrata populations associated with the pathogenesis of denture stomatitis. Oral Microbiol. Immunol. 2008, 23, 377–383. [Google Scholar] [CrossRef]
- Lee, H.; Yu, A.; Johnson, C.C.; Lilly, E.A.; Noverr, M.C.; Fidel, P.L., Jr. Fabrication of a multi-applicable removable intraoral denture system for rodent research. J. Oral Rehabil. 2011, 38, 686–690. [Google Scholar] [CrossRef]
- Johnson, C.C.; Yu, A.; Lee, H.; Fidel, P.L., Jr.; Noverr, M.C. Development of a contemporary animal model of Candida albicans-associated denture stomatitis using a novel intraoral denture system. Infect. Immun. 2012, 80, 1736–1743. [Google Scholar] [CrossRef]
- Yano, J.; Yu, A.; Fidel, P.L., Jr.; Noverr, M.C. Transcription Factors Efg1 and Bcr1 Regulate Biofilm Formation and Virulence during Candida albicans-Associated Denture Stomatitis. PLoS ONE 2016, 11, e0159692. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.; Yu, A.; Fidel, P.L., Jr.; Noverr, M.C. Candida glabrata Has No Enhancing Role in the Pathogenesis of Candida-Associated Denture Stomatitis in a Rat Model. mSphere 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Konopka, K.; Dorocka-Bobkowska, B.; Gebremedhin, S.; Duzgunes, N. Susceptibility of Candida biofilms to histatin 5 and fluconazole. Antonie van Leeuwenhoek 2010, 97, 413–417. [Google Scholar] [CrossRef]
- Cross, L.J.; Williams, D.W.; Sweeney, C.P.; Jackson, M.S.; Lewis, M.A.; Bagg, J. Evaluation of the recurrence of denture stomatitis and Candida colonization in a small group of patients who received itraconazole. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Bergendal, T. Status and treatment of denture stomatitis patients: A 1-year follow-up study. Scand. J. Dent. Res. 1982, 90, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Lamb, D.J.; Douglas, C.W. Treatment of denture stomatitis by a sustained drug-delivery device: A preliminary study. J. Dent. 1988, 16, 219–221. [Google Scholar] [CrossRef]
- Herman, J.L.; Wang, Y.; Lilly, E.A.; Lallier, T.E.; Peters, B.M.; Hamdan, S.; Xu, X.; Fidel, P.L., Jr.; Noverr, M.C. Synthesis, Antifungal Activity, and Biocompatibility of Novel 1,4-Diazabicyclo[2.2.2]Octane (DABCO) Compounds and DABCO-Containing Denture Base Resins. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Sultan, A.S.; Rizk, A.M.; Vila, T.; Ji, Y.; Masri, R.; Jabra-Rizk, M.A. Digital Design of a Universal Rat Intraoral Device for Therapeutic Evaluation of a Topical Formulation against Candida-Associated Denture Stomatitis. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef]
- Minagi, S.; Miyake, Y.; Inagaki, K.; Tsuru, H.; Suginaka, H. Hydrophobic interaction in Candida albicans and Candida tropicalis adherence to various denture base resin materials. Infect. Immun. 1985, 47, 11–14. [Google Scholar] [CrossRef]
- Klotz, S.A.; Drutz, D.J.; Zajic, J.E. Factors governing adherence of Candida species to plastic surfaces. Infect. Immun. 1985, 50, 97–101. [Google Scholar] [CrossRef]
- Yoshijima, Y.; Murakami, K.; Kayama, S.; Liu, D.; Hirota, K.; Ichikawa, T.; Miyake, Y. Effect of substrate surface hydrophobicity on the adherence of yeast and hyphal Candida. Mycoses 2010, 53, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Namangkalakul, W.; Benjavongkulchai, S.; Pochana, T.; Promchai, A.; Satitviboon, W.; Howattanapanich, S.; Phuprasong, R.; Ungvijanpunya, N.; Supakanjanakanti, D.; Chaitrakoonthong, T.; et al. Activity of chitosan antifungal denture adhesive against common Candida species and Candida albicans adherence on denture base acrylic resin. J. Prosthet. Dent. 2019, 123, 181.e1–181.e7. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Wang, R.S.; Hsu, Y.C.; Chuang, C.C.; Chan, H.R.; Chiu, H.C.; Wang, Y.B.; Chen, K.Y.; Fu, E. Antifungal effect of tissue conditioners containing poly(acryloyloxyethyltrimethyl ammonium chloride)-grafted chitosan on Candida albicans growth in vitro. J. Dent. Sci. 2018, 13, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Ohshima, T.; Maeda, N.; Ohkubo, C. Inhibitory effect of coated mannan against the adhesion of Candida biofilms to denture base resin. Dent. Mater. J. 2013, 32, 355–360. [Google Scholar] [CrossRef]
- Freire, F.; de Barros, P.P.; Pereira, C.A.; Junqueira, J.C.; Jorge, A.O.C. Photodynamic inactivation in the expression of the Candida albicans genes ALS3, HWP1, BCR1, TEC1, CPH1, and EFG1 in biofilms. Lasers Med. Sci. 2018, 33, 1447–1454. [Google Scholar] [CrossRef]
- Alrabiah, M.; Alsahhaf, A.; Alofi, R.S.; Al-Aali, K.A.; Abduljabbar, T.; Vohra, F. Efficacy of photodynamic therapy versus local nystatin in the treatment of denture stomatitis: A randomized clinical study. Photodiagnosis Photodyn. Ther. 2019, 28, 98–101. [Google Scholar] [CrossRef]
- de Senna, A.M.; Vieira, M.M.F.; Machado-de-Sena, R.M.; Bertolin, A.O.; Nunez, S.C.; Ribeiro, M.S. Photodynamic inactivation of Candida spp. on denture stomatitis. A clinical trial involving palatal mucosa and prosthesis disinfection. Photodiagnosis Photodyn. Ther. 2018, 22, 212–216. [Google Scholar] [CrossRef]
- Sobel, J.D.; Faro, S.; Force, R.W.; Foxman, B.; Ledger, W.J.; Nyirjesy, P.R.; Reed, B.D.; Summers, P.R. Vulvovaginal candidiasis: Epidemiologic, diagnostic, and therapeutic considerations. Am. J. Obstet. Gynecol. 1998, 178, 203–211. [Google Scholar] [CrossRef]
- Sobel, J.D.; Chaim, W. Vaginal microbiology of women with acute recurrent vulvovaginal candidiasis. J. Clin. Microbiol. 1996, 34, 2497–2499. [Google Scholar] [CrossRef]
- McClelland, R.S.; Richardson, B.A.; Hassan, W.M.; Graham, S.M.; Kiarie, J.; Baeten, J.M.; Mandaliya, K.; Jaoko, W.; Ndinya-Achola, J.O.; Holmes, K.K. Prospective study of vaginal bacterial flora and other risk factors for vulvovaginal candidiasis. J. Infect. Dis. 2009, 199, 1883–1890. [Google Scholar] [CrossRef]
- Fidel, P., Jr. History and new insights into host defense against vaginal candidiasis. Trends Microbiol. 2004, 12, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin. Infect. Dis 1992, 14, S148–S153. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.; Sobel, J.D.; Nyirjesy, P.; Sobel, R.; Williams, V.L.; Yu, Q.; Noverr, M.C.; Fidel, P.L., Jr. Current patient perspectives of vulvovaginal candidiasis: Incidence, symptoms, management and post-treatment outcomes. BMC Women’s Health 2019, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Vaginitis. N. Engl. J. Med. 1997, 337, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Cu-Uvin, S.; Hogan, J.W.; Warren, D.; Klein, R.S.; Peipert, J.; Schuman, P.; Holmberg, S.; Anderson, J.; Schoenbaum, E.; Vlahov, D.; et al. Prevalence of lower genital tract infections among human immunodeficiency virus (HIV)-seropositive and high-risk HIV-seronegative women. HIV Epidemiology Research Study Group. Clin. Infect. Dis. 1999, 29, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, D.M.; Barousse, M.M.; Fidel, P.L., Jr. Role for dendritic cells in immunoregulation during experimental vaginal candidiasis. Infect. Immun. 2006, 74, 3213–3221. [Google Scholar] [CrossRef]
- Taylor, B.N.; Saavedra, M.; Fidel, P.L., Jr. Local Th1/Th2 cytokine production during experimental vaginal candidiasis: Potential importance of transforming growth factor-beta. Med. Mycol. 2000, 38, 419–431. [Google Scholar] [CrossRef]
- Wormley, F.L., Jr.; Steele, C.; Wozniak, K.; Fujihashi, K.; McGhee, J.R.; Fidel, P.L., Jr. Resistance of T-cell receptor delta-chain-deficient mice to experimental Candida albicans vaginitis. Infect. Immun. 2001, 69, 7162–7164. [Google Scholar] [CrossRef]
- Leigh, J.E.; Barousse, M.; Swoboda, R.K.; Myers, T.; Hager, S.; Wolf, N.A.; Cutright, J.L.; Thompson, J.; Sobel, J.D.; Fidel, P.L., Jr. Candida-specific systemic cell-mediated immune reactivities in human immunodeficiency virus-positive persons with mucosal candidiasis. J. Infect. Dis. 2001, 183, 277–285. [Google Scholar] [CrossRef]
- Fidel, P.L., Jr.; Cutright, J.L.; Sobel, J.D. Effects of systemic cell-mediated immunity on vaginal candidiasis in mice resistant and susceptible to Candida albicans infections. Infect. Immun. 1995, 63, 4191–4194. [Google Scholar] [CrossRef]
- Fidel, P.L., Jr.; Lynch, M.E.; Sobel, J.D. Circulating CD4 and CD8 T cells have little impact on host defense against experimental vaginal candidiasis. Infect. Immun. 1995, 63, 2403–2408. [Google Scholar] [CrossRef] [PubMed]
- Wormley, F.L., Jr.; Cutright, J.; Fidel, P.L., Jr. Multiple experimental designs to evaluate the role of T-cell-mediated immunity against experimental vaginal Candida albicans infection. Med. Mycol. 2003, 41, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, K.L.; Wormley, F.L., Jr.; Fidel, P.L., Jr. Candida-specific antibodies during experimental vaginal candidiasis in mice. Infect. Immun. 2002, 70, 5790–5799. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.; Fidel, P.L., Jr. Protocols for vaginal inoculation and sample collection in the experimental mouse model of Candida vaginitis. J. Vis. Exp. 2011. [Google Scholar] [CrossRef]
- Yano, J.; Lilly, E.; Barousse, M.; Fidel, P., Jr. Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infect. Immun. 2010, 78, 5126–5137. [Google Scholar] [CrossRef]
- Peters, B.M.; Palmer, G.E.; Nash, A.K.; Lilly, E.A.; Fidel, P.L., Jr.; Noverr, M.C. Fungal morphogenetic pathways are required for the hallmark inflammatory response during Candida albicans vaginitis. Infect. Immun. 2014, 82, 532–543. [Google Scholar] [CrossRef]
- Yano, J.; Kolls, J.K.; Happel, K.I.; Wormley, F.; Wozniak, K.L.; Fidel, P.L., Jr. The acute neutrophil response mediated by S100 alarmins during vaginal Candida infections is independent of the Th17-pathway. PLoS ONE 2012, 7, e46311. [Google Scholar] [CrossRef]
- Peters, B.M.; Coleman, B.M.; Willems, H.M.E.; Barker, K.S.; Aggor, F.E.Y.; Cipolla, E.; Verma, A.H.; Bishu, S.; Huppler, A.H.; Bruno, V.M.; et al. The IL-17R/IL-22R signaling axis is dispensable for vulvovaginal candidiasis regardless of estrogen status. J. Infect. Dis. 2019. [Google Scholar] [CrossRef]
- Yano, J.; Noverr, M.C.; Fidel, P.L., Jr. Vaginal Heparan Sulfate Linked to Neutrophil Dysfunction in the Acute Inflammatory Response Associated with Experimental Vulvovaginal Candidiasis. mBio 2017, 8. [Google Scholar] [CrossRef]
- Richardson, J.P.; Willems, H.M.E.; Moyes, D.L.; Shoaie, S.; Barker, K.S.; Tan, S.L.; Palmer, G.E.; Hube, B.; Naglik, J.R.; Peters, B.M. Candidalysin Drives Epithelial Signaling, Neutrophil Recruitment, and Immunopathology at the Vaginal Mucosa. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef]
- Yano, J.; Lilly, E.A.; Steele, C.; Fortenberry, D.; Fidel, P.L., Jr. Oral and vaginal epithelial cell anti-Candida activity is acid labile and does not require live epithelial cells. Oral Microbiol Immunol. 2005, 20, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Barousse, M.M.; Espinosa, T.; Dunlap, K.; Fidel, P.L., Jr. Vaginal epithelial cell anti-Candida albicans activity is associated with protection against symptomatic vaginal candidiasis. Infect. Immun. 2005, 73, 7765–7767. [Google Scholar] [CrossRef] [PubMed]
- Lilly, E.A.; Yano, J.; Fidel, P.L., Jr. Annexin-A1 identified as the oral epithelial cell anti-Candida effector moiety. Mol. Oral Microbiol. 2010, 25, 293–304. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, G.; Wozniak, K.; Wallig, M.A.; Fidel, P.L., Jr.; Trupin, S.R.; Hoyer, L.L. Comparison between Candida albicans agglutinin-like sequence gene expression patterns in human clinical specimens and models of vaginal candidiasis. Infect. Immun. 2005, 73, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.J.; Ibrahim, A.S.; Avanesian, V.; Fu, Y.; Myers, C.; Phan, Q.T.; Filler, S.G.; Yeaman, M.R.; Edwards, J.E., Jr. Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J. Infect. Dis. 2006, 194, 256–260. [Google Scholar] [CrossRef]
- Yano, J.; Palmer, G.E.; Eberle, K.E.; Peters, B.M.; Vogl, T.; McKenzie, A.N.; Fidel, P.L., Jr. Vaginal epithelial cell-derived S100 alarmins induced by Candida albicans via pattern recognition receptor interactions are sufficient but not necessary for the acute neutrophil response during experimental vaginal candidiasis. Infect. Immun. 2014, 82, 783–792. [Google Scholar] [CrossRef]
- Naglik, J.R.; Moyes, D.; Makwana, J.; Kanzaria, P.; Tsichlaki, E.; Weindl, G.; Tappuni, A.R.; Rodgers, C.A.; Woodman, A.J.; Challacombe, S.J.; et al. Quantitative expression of the Candida albicans secreted aspartyl proteinase gene family in human oral and vaginal candidiasis. Microbiology 2008, 154, 3266–3280. [Google Scholar] [CrossRef]
- Pericolini, E.; Gabrielli, E.; Amacker, M.; Kasper, L.; Roselletti, E.; Luciano, E.; Sabbatini, S.; Kaeser, M.; Moser, C.; Hube, B.; et al. Secretory Aspartyl Proteinases Cause Vaginitis and Can Mediate Vaginitis Caused by Candida albicans in Mice. mBio 2015, 6, e00724. [Google Scholar] [CrossRef]
- Swidsinski, A.; Guschin, A.; Tang, Q.; Dorffel, Y.; Verstraelen, H.; Tertychnyy, A.; Khayrullina, G.; Luo, X.; Sobel, J.D.; Jiang, X. Vulvovaginal candidiasis: Histologic lesions are primarily polymicrobial and invasive and do not contain biofilms. Am. J. Obstet. Gynecol. 2019, 220, 91.e91–91.e98. [Google Scholar] [CrossRef]
- Fidel, P.L., Jr.; Vazquez, J.A.; Sobel, J.D. Candida glabrata: Review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 1999, 12, 80–96. [Google Scholar] [CrossRef]
- Kennedy, M.A.; Sobel, J.D. Vulvovaginal Candidiasis Caused by Non-albicans Candida Species: New Insights. Curr. Infect. Dis. Rep. 2010, 12, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Ventolini, G.; Baggish, M.S.; Walsh, P.M. Vulvovaginal candidiasis from non-albicans species: Retrospective study of recurrence rate after fluconazole therapy. J. Reprod. Med. 2006, 51, 475–478. [Google Scholar] [PubMed]
- Tati, S.; Davidow, P.; McCall, A.; Hwang-Wong, E.; Rojas, I.G.; Cormack, B.; Edgerton, M. Candida glabrata Binding to Candida albicans Hyphae Enables Its Development in Oropharyngeal Candidiasis. PLoS Pathog. 2016, 12, e1005522. [Google Scholar] [CrossRef] [PubMed]
- Rossoni, R.D.; Barbosa, J.O.; Vilela, S.F.; dos Santos, J.D.; de Barros, P.P.; Prata, M.C.; Anbinder, A.L.; Fuchs, B.B.; Jorge, A.O.; Mylonakis, E.; et al. Competitive Interactions between C. albicans, C. glabrata and C. krusei during Biofilm Formation and Development of Experimental Candidiasis. PLoS ONE 2015, 10, e0131700. [Google Scholar] [CrossRef]
- Nash, E.E.; Peters, B.M.; Lilly, E.A.; Noverr, M.C.; Fidel, P.L., Jr. A Murine Model of Candida glabrata Vaginitis Shows No Evidence of an Inflammatory Immunopathogenic Response. PLoS ONE 2016, 11, e0147969. [Google Scholar] [CrossRef]
- Willems, H.M.E.; Lowes, D.J.; Barker, K.S.; Palmer, G.E.; Peters, B.M. Comparative Analysis of the Capacity of the Candida Species To Elicit Vaginal Immunopathology. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef]
- Yano, J.; Peters, B.M.; Noverr, M.C.; Fidel, P.L., Jr. Novel Mechanism behind the Immunopathogenesis of Vulvovaginal Candidiasis: "Neutrophil Anergy". Infect. Immun. 2018, 86. [Google Scholar] [CrossRef]
- Cassone, A. Vulvovaginal Candida albicans infections: Pathogenesis, immunity and vaccine prospects. BJOG 2015, 122, 785–794. [Google Scholar] [CrossRef]
- De Bernardis, F.; Amacker, M.; Arancia, S.; Sandini, S.; Gremion, C.; Zurbriggen, R.; Moser, C.; Cassone, A. A virosomal vaccine against candidal vaginitis: Immunogenicity, efficacy and safety profile in animal models. Vaccine 2012, 30, 4490–4498. [Google Scholar] [CrossRef]
- Pietrella, D.; Rachini, A.; Torosantucci, A.; Chiani, P.; Brown, A.J.; Bistoni, F.; Costantino, P.; Mosci, P.; d’Enfert, C.; Rappuoli, R.; et al. A beta-glucan-conjugate vaccine and anti-beta-glucan antibodies are effective against murine vaginal candidiasis as assessed by a novel in vivo imaging technique. Vaccine 2010, 28, 1717–1725. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.A. A Therapeutic Vaccine for Recurrent Vulvovaginal Candidiasis. Clin. Infect. Dis. 2018, 66, 1937–1939. [Google Scholar] [CrossRef] [PubMed]
- White, D.J.; Vanthuyne, A.; Wood, P.M.; Ayres, J.G. Zafirlukast for severe recurrent vulvovaginal candidiasis: An open label pilot study. Sex. Transm. Infect. 2004, 80, 219–222. [Google Scholar] [CrossRef][Green Version]
- Beck, B.R.; Park, G.S.; Lee, Y.H.; Im, S.; Jeong, D.Y.; Kang, J. Whole Genome Analysis of Lactobacillus plantarum Strains Isolated From Kimchi and Determination of Probiotic Properties to Treat Mucosal Infections by Candida albicans and Gardnerella vaginalis. Front. Microbiol. 2019, 10, 433. [Google Scholar] [CrossRef]
- Santos, C.M.A.; Pires, M.C.V.; Leao, T.L.; Silva, A.K.S.; Miranda, L.S.; Martins, F.S.; Silva, A.M.; Nicoli, J.R. Anti-inflammatory effect of two Lactobacillus strains during infection with Gardnerella vaginalis and Candida albicans in a HeLa cell culture model. Microbiology 2018, 164, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, V.H.; Ishikawa, K.H.; Ando-Suguimoto, E.S.; Bueno-Silva, B.; Nakamae, A.E.M.; Mayer, M.P.A. Probiotic Bacteria Alter Pattern-Recognition Receptor Expression and Cytokine Profile in a Human Macrophage Model Challenged with Candida albicans and Lipopolysaccharide. Front. Microbiol. 2017, 8, 2280. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.M.; Kim, K.A.; Myoung, K.S.; Ahn, Y.T.; Lee, J.H.; Huh, C.S.; Han, M.J.; Kim, D.H. Lactobacillus helveticus HY7801 ameliorates vulvovaginal candidiasis in mice by inhibiting fungal growth and NF-kappaB activation. Int. Immunopharmacol. 2012, 14, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, E.; Pericolini, E.; Ballet, N.; Roselletti, E.; Sabbatini, S.; Mosci, P.; Decherf, A.C.; Pelerin, F.; Perito, S.; Justen, P.; et al. Saccharomyces cerevisiae-based probiotic as novel anti-fungal and anti-inflammatory agent for therapy of vaginal candidiasis. Benef. Microbes 2018, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Rodero, C.F.; Fioramonti Calixto, G.M.; Cristina Dos Santos, K.; Sato, M.R.; Aparecido Dos Santos Ramos, M.; Miro, M.S.; Rodriguez, E.; Vigezzi, C.; Bauab, T.M.; Sotomayor, C.E.; et al. Curcumin-Loaded Liquid Crystalline Systems for Controlled Drug Release and Improved Treatment of Vulvovaginal Candidiasis. Mol. Pharm. 2018, 15, 4491–4504. [Google Scholar] [CrossRef]
- Uppuluri, P.; Singh, S.; Alqarihi, A.; Schmidt, C.S.; Hennessey, J.P., Jr.; Yeaman, M.R.; Filler, S.G.; Edwards, J.E.; Ibrahim, A.S. Human Anti-Als3p Antibodies Are Surrogate Markers of NDV-3A Vaccine Efficacy Against Recurrent Vulvovaginal Candidiasis. Front. Immunol. 2018, 9, 1349. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Luo, G.; Gebremariam, T.; Lee, H.; Schmidt, C.S.; Hennessey, J.P., Jr.; French, S.W.; Yeaman, M.R.; Filler, S.G.; Edwards, J.E., Jr. NDV-3 protects mice from vulvovaginal candidiasis through T- and B-cell immune response. Vaccine 2013, 31, 5549–5556. [Google Scholar] [CrossRef]
- Park, S.J.; Mehrad, B. Innate immunity to Aspergillus species. Clin. Microbiol. Rev. 2009, 22, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Pirofski, L.A.; Casadevall, A. Immune-Mediated Damage Completes the Parabola: Cryptococcus neoformans Pathogenesis Can Reflect the Outcome of a Weak or Strong Immune Response. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fidel, P.L., Jr.; Yano, J.; Esher, S.K.; Noverr, M.C. Applying the Host-Microbe Damage Response Framework to Candida Pathogenesis: Current and Prospective Strategies to Reduce Damage. J. Fungi 2020, 6, 35. https://doi.org/10.3390/jof6010035
Fidel PL Jr., Yano J, Esher SK, Noverr MC. Applying the Host-Microbe Damage Response Framework to Candida Pathogenesis: Current and Prospective Strategies to Reduce Damage. Journal of Fungi. 2020; 6(1):35. https://doi.org/10.3390/jof6010035
Chicago/Turabian StyleFidel, Paul L., Jr., Junko Yano, Shannon K. Esher, and Mairi C. Noverr. 2020. "Applying the Host-Microbe Damage Response Framework to Candida Pathogenesis: Current and Prospective Strategies to Reduce Damage" Journal of Fungi 6, no. 1: 35. https://doi.org/10.3390/jof6010035
APA StyleFidel, P. L., Jr., Yano, J., Esher, S. K., & Noverr, M. C. (2020). Applying the Host-Microbe Damage Response Framework to Candida Pathogenesis: Current and Prospective Strategies to Reduce Damage. Journal of Fungi, 6(1), 35. https://doi.org/10.3390/jof6010035




