Abstract
Antifungal susceptibility testing is an important tool for managing patients with invasive fungal infections, as well as for epidemiological surveillance of emerging resistance. For routine testing in clinical microbiology laboratories, ready-to-use commercial methods are more practical than homemade reference techniques. Among commercially available methods, the concentration gradient Etest strip technique is widely used. It combines an agar-based diffusion method with a dilution method that determinates a minimal inhibitory concentration (MIC) in µg/mL. Many studies have evaluated the agreement between the gradient strip method and the reference methods for both yeasts and filamentous fungi. This agreement has been variable depending on the antifungal, the species, and the incubation time. It has also been shown that the gradient strip method could be a valuable alternative for detection of emerging resistance (non-wild-type isolates) as Etest epidemiological cutoff values have been recently defined for several drug-species combinations. Furthermore, the Etest could be useful for direct antifungal susceptibility testing on blood samples and basic research studies (e.g., the evaluation of the in vitro activity of antifungal combinations). This review summarizes the available data on the performance and potential use of the gradient strip method.
1. Introduction
Antifungal susceptibility testing (AFST) is now widely used and recommended for management of patients with invasive fungal infections such as candidiasis and aspergillosis [1,2,3]. AFST has also become an important tool for better epidemiological knowledge in rare fungal diseases [4,5,6,7]. There are currently broth microdilution reference techniques for both yeasts [8,9] and molds [10,11] that have been developed and standardized by the Clinical and Laboratory Standards Institute (CLSI) and by the European Committee for Antimicrobial Susceptibility Testing (EUCAST). Nevertheless, these reference techniques are time-consuming and more adapted for reference laboratories and large epidemiological surveillance studies. For routine testing in clinical microbiology laboratories, commercially available and ready-to-use methods may be a better alternative, as far as they are able to produce similar results with those obtained with the reference techniques. These methods may be based on different principles including microdilution broth (e.g., YeastOne) or agar diffusion (e.g., NeoSensitabs).
2. Principle of the Concentration Gradient Strip (Etest)
The concentration gradient strip technique is a combination of an agar-based diffusion method with a dilution method that determinates a minimal inhibitory concentration (MIC). A predefined exponential gradient of antifungal drug is immobilized on a plastic (Etest, bioMérieux, France) or impregnated on a paper (MTS, Liophilchem, Italy) strip. After homogenous inoculation of an agar plate, the strip is applied onto the agar surface and the drug is immediately released from the carrier to produce a continuous drug gradient in the agar medium. After incubation, an ellipse of growth inhibition is obtained, and the MIC is determined at the intersection of the ellipse with the scale on the upper side of the strip. The recommended medium used for testing is RPMI 1640 MOPS supplemented with 2% glucose and the incubation time is variable depending on the tested species [12].
3. Etest as a Routine AFST Method
3.1. Inhibition Patterns and Reading Problems
Inhibition patterns and reading endpoints (Figure 1 and Figure 2) are dependent on the drug and organism tested. Amphotericin B, in general, gives a sharp ellipse of inhibition that allows an easy MIC determination, since any colony inside the ellipse is important. The reading endpoint is a 100% inhibition for both yeasts and filamentous fungi [13,14]. Similar to most AFST methods, including the reference EUCAST and CLSI techniques, a trailing phenomenon [15] can be observed with the gradient strip method when azoles are tested against yeasts. With Etest, the trailing is visible as the presence of a lawn of microcolonies in the inhibition ellipse (Figure 1). The trailing is particularly important when testing specific species such as C. albicans, C. glabrata, and C. tropicalis and may lead to difficulties regarding the MIC endpoint. The reading endpoint for yeasts is an 80% inhibition. In contrast, a sharp and clear ellipse is generally observed when testing azoles against the filamentous fungi such as Aspergillus spp. (Figure 2), and therefore a 100% inhibition (trailing free ellipse) is the MIC endpoint [14]. For the echinocandins, the inhibition patterns are also different for both yeasts and filamentous fungi. When testing yeasts, a clear ellipse is generally seen which allows an easy MIC determination. The reading endpoint is an 80% inhibition (trailing within the ellipse). Nevertheless, two specific phenomena can be observed. The first one is the paradoxical effect corresponding to enhanced growth at high supra-MIC concentrations [16,17]. By the Etest method, it appears as the presence of microcolonies around the strip at the highest concentrations, within the inhibition ellipse [18]. The paradoxical effect is also observed using both CLSI and EUCAST reference microdilution broth techniques [19,20], but is not indicative of in vitro resistance or associated with therapeutic failure [21]. The second phenomenon is the dip effect, corresponding to a narrow inhibition zone at sub-MIC values, which can complicate the MIC determination [22]. When testing echinocandins against filamentous fungi, residual or trailing growth within the inhibition ellipse is generally observed and should be ignored for MIC determination [23]. The same phenomenon of partial inhibition is seen with broth microdilution techniques. Flucytosine is only tested against yeasts and, generally, gives large and clear ellipses. A 90% inhibition endpoint is used. It has been shown that testing flucytosine (on RPMI medium) against Cryptococcus neoformans can lead to erroneously high MICs [24,25,26].
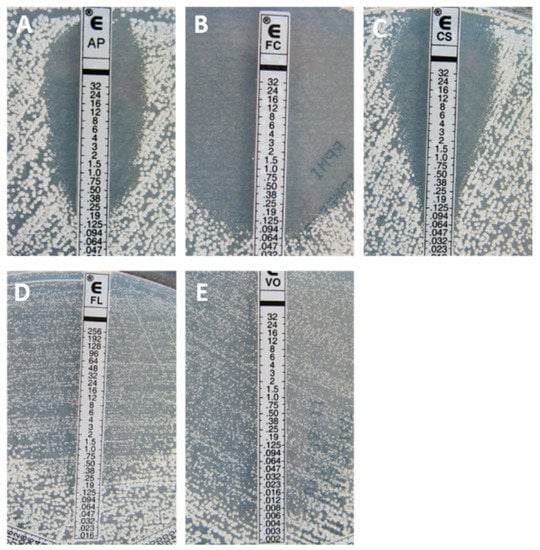
Figure 1.
Typical inhibition pattern of amphotericin B (A), flucytosine (B), caspofungin (C), fluconazole (D), and voriconazole (E) against a wild-type (WT) isolate of Candida albicans.
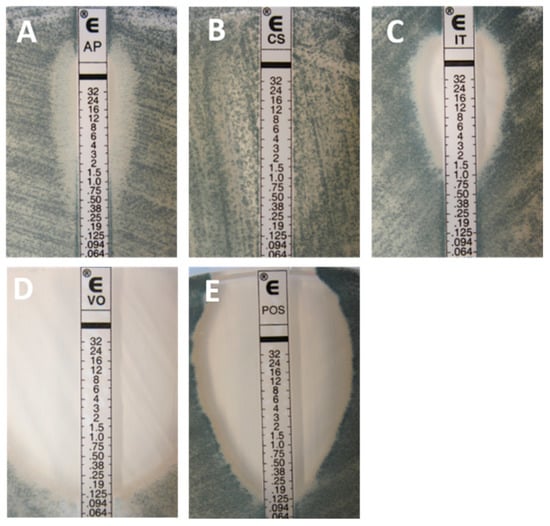
Figure 2.
Typical inhibition pattern of amphotericin B (A), caspofungin (B), itraconazole (C), voriconazole (D), and posaconazole (E) tested against a wild-type (WT) isolate of Aspergillus fumigatus.
3.2. Interlaboratory Reproducibility
Several studies have tested the interlaboratory reproducibility of Etest [26,27,28,29]. In one study, in which 83 strains of Candida spp. were tested in two laboratories [27], the overall reproducibility on RPMI, at +/−1 log2 dilution, was good for fluconazole (96%), ketoconazole (90%), flucytosine (84%), much lower for itraconazole (63%), and very poor for amphotericin B (4%). The same drugs have been evaluated in another study that tested two quality control strains in four laboratories [28]. The interlaboratory reproducibility at +/−1 log2 dilution was 99% to 100% for all five antifungals. In a large multicenter evaluation of reproducibility, 20 isolates (18 Candida spp. and 2 C. neoformans) were tested in 10 laboratories against amphotericin B, flucytosine, fluconazole, and itraconazole [26]. Overall, it was concluded that Etest is suitable to test amphotericin B and flucytosine against Candida spp. and less reliable for the azoles. In a more recent study, 198 isolates of Candida spp. were tested in four laboratories against amphotericin B and caspofungin [29]. The interlaboratory reproducibility, at +/−2 log2 dilutions, was very good at 97.5% and 97.1% for amphotericin B and caspofungin, respectively.
3.3. Correlation with Reference Techniques
In most correlation studies, results of gradient strips are compared to results obtained by the CLSI or EUCAST microdilution broth reference techniques. The two main parameters used for comparison are the essential agreement (EA) and the categorical agreement (CA). The EA is the percentage of isolates for which MIC values by the gradient strip method are within +/−1 or +/−2 log2 dilutions of the values obtained by the reference method. In most of the studies, a threshold at +/−2 log2 dilutions is used. The CA is the percentage of isolates for which the same categorization (susceptible/intermediate/resistant) is obtained by the two methods. The availability of reference clinical breakpoints (CBPs) is a prerequisite for the calculation of CA percentages. Many problems are evident with most of these early categorizations and comparisons. Both triazole and echinocandin CPBs were adjusted to lower cutoffs as the molecular mechanisms of resistance were understood and more clinical data were available and also, different “resistant” amphotericin B cutoffs were used for these comparisons. Epidemiological cutoff values determined by the EUCAST (ECOFFs) or CLSI (ECVs) define the wild-type (WT) population and could be used for comparing results obtained by Etest and reference techniques. As the ECOFF and the ECV represent the same parameter, for simplification, the term ECV is used throughout this review.
3.3.1. Yeasts
Many studies have evaluated the agreement between gradient concentration strip methods and EUCAST/CLSI methods. Overall, a good level of agreement was generally found.
Amphotericin B
Since the reference RPMI broth appeared to lack the ability to detect amphotericin B-resistant Candida isolates, other media such as the antibiotic medium 3 (AM3) were either compared to the CLSI data [30,31] or used instead of the RPMI agar in one of the comparisons with the EUCAST MICs (Table 1) [32]. However, in some instances the EA was not as good as that with the RPMI agar (Table 1) and lot-to-lot variation was also a problem when using the AM3 medium [33]. In one of the studies that evaluated the EA between the Etest and the CLSI method by using both AM3 and RPMI agars, results with the AM3 were lower than those with the RPMI agar for C. parapsilosis and C. tropicalis (overall EA 90% versus 97% with RPMI agar) [31]. Nevertheless, the overall EA between the CLSI and Etest RPMI agar was mostly >96% within the acceptable ±2 dilution range (1994 to 2011) for the most prevalent Candida species (C. albicans, C. glabrata, C. parapsilosis, and C. tropicalis) [30,31,34,35,36] and C. neoformans [37] (Table 1). The exceptions were the lower percentages of EA in one study where the Etest data were evaluated at both 24 and 48 h versus the CLSI method [38], for various species, including C. lusitaniae and C. neoformans [30,39]. The Etest was also evaluated for testing C. krusei with amphotericin B with an acceptable EA [30,34].

Table 1.
Percent essential agreement (EA) (+/− two dilutions) of Etest and reference amphotericin B minimal inhibitory concentrations (MICs).
However, the comparison of Etest and EUCAST MICs yielded consistently and unusually low EA percentages, despite the fact that the Etest MICs were determined on both RPMI [32] and AM3 [25] agars (Table 1).
Regarding rare Candida spp., one study provided Etest amphotericin B data for the emerging C. auris, as well as for the rare C. haemulonii and C. pseudohaemulonii on Mueller-Hinton (MH) agar supplemented with glucose and methylene blue as the “reference” method [36]. The comparison was against the Etest data on RPMI agar, other commercial assays and both reference methods (Table 1). All isolates were identified by sequencing the internal transcribed spacer (ITS) and D1/D2 regions of the 26S ribosomal DNA. The aim was to identify the best method for the detection of amphotericin B resistance. It was found that the Etest with MH yielded the widest amphotericin B MIC range and better “discriminated the susceptibility” of amphotericin B to these three species, 0.125–0.5 µg/ml for C. auris, and 4–32 µg/mL for the other two species. Blood isolates from three amphotericin B therapeutic failure patients were included in the study (MICs, 32 µg/mL) [36].
In summary, although good interlaboratory agreement has been observed for testing Candida spp. using Etest amphotericin B strips, data or isolates recovered after failure or during treatment are very scarce.
Flucytosine
Flucytosine Etest data for yeasts were compared to those by the CLSI method in three studies [34,35,40] and with the EUCAST in two studies [25,32] (Table 2). The EA was excellent for all the Candida species tested, except in one study for C. albicans (EA 89%) [35] and in another one for C. neoformans (EA 70%) [40]. When both incubation times for the Etest results were evaluated [40], the 48 h Etest MICs consistently produced the higher EA percentages versus the CLSI macrodilution method for all the species evaluated, excluding C. glabrata (EA 100% with the 24 h Etest versus 83% with the 48 h results). It is noteworthy, as discussed below for the triazoles, that when testing fluconazole the first Etest reading yielded the highest EA for some species (Table 3).

Table 2.
Essential agreement (EA) (+/− two dilutions) of flucytosine Etest and reference MICs.

Table 3.
Percentage essential agreement (EA) (+/− two dilutions) of azoles (fluconazole, itraconazole, posaconazole, and voriconazole) Etest and reference MICs.
When Etest and EUCAST data were compared, contradictory EA results were reported again for all the Candida species tested (EA 62% to 82% versus 92% to 100%) [25,32]; the EA percentage for C. neoformans and flucytosine was unusually low (EA 35%) [25] (Table 2).
The Triazoles
- Fluconazole
The published EA percentages between both CLSI and Etest fluconazole MICs for six species of Candida and C. neoformans [31,34,35,38,39,40,41,42,43] and the EUCAST [25,32] are presented in Table 3. Although the EA for fluconazole was >90% versus the Etest data for Candida spp. and C. neoformans in most studies, unacceptable EA percentages of <90% were also reported for each of the Candida spp. evaluated (Table 3) [34,35,38,40,41]. There are important caveats regarding these equivalences which are described next. The comparisons were mostly versus the CLSI macrodilution format. More important, some of these studies demonstrated the influence of the incubation time for the Etest data. For example, the overall percentages of EA were mostly higher at the Etest 24 h incubation times for C. albicans (82% to 97% versus 75% to 96%), C. glabrata (37% to 100% versus 34% to 83%) and C. tropicalis (56% to 100% versus 67% to 93%) [38,40,41]. However, for C. parapsilosis, the EA was best with the Etest 48 h MICs (89% to 100% versus 98% to 100%) [38,40]. These results could reflect the different growth rates, as well as the presence of higher amounts of trailing growth at the second incubation time. The fluconazole Etest was also evaluated using two other agar compositions (casitone an MH) against the CLSI; while the casitone agar yielded acceptable EA percentages, on the MH agar some EA values were below 90% [42].
The evaluation of the Etest against the EUCAST methodology once more provided disappointingly low percentages of agreement (Table 3) [25,32].
- Itraconazole, Posaconazole, and Voriconazole
The search of the literature provided less comparative data between the Etest and the CLSI or EUCAST methods for itraconazole [25,32,34,35,38], posaconazole [34,44,45], and voriconazole [25,32,34,37,38,39,43,46] (Table 3). We also found less information regarding the influence of the incubation time (24 versus 48 h) among the comparisons of the Etest with the CLSI method for these three triazoles [38,44]. Overall, the percentages of EA agreement were similar to those for fluconazole with both acceptable (>90%) and unacceptable (<90%) EA percentages. It is noteworthy that among the four most common Candida spp., the EA between the CLSI and Etest methods for C. glabrata was mostly below 90% for testing fluconazole and itraconazole [34,38,41,43]. The lowest EA values were among the evaluations for itraconazole, with similar and same percentages of EA at 24 and 48 h for the four most common Candida species, including C. parapsilosis [38] (Table 3). On the other hand, the EA indicated that posaconazole, and to a certain extent voriconazole, Etest MICs should be read at 24 h (higher or similar EA percentages at both incubation times) for C. albicans, C. dubliniensis, and C. glabrata. However, as for fluconazole, the Etest for C. parapsilosis should be read at 48 h (Table 3) [38,44]. Nevertheless, conclusions about the best incubation time are based on single studies and sometimes with a small number of isolates and species. It is noteworthy that the manufacturers advocate the first time reading, but that the confirmatory result should be at 48 h [12].
EA percentages between Etest and the CLSI microdilution method were acceptable at 48 h for C. neoformans versus fluconazole [39,40,42], posaconazole [44], and voriconazole [37,39]; some of those evaluations included large amounts of isolates and in some instances the first Etest incubation time reading provided the highest EA values (Table 3).
Fewer studies evaluated the Etest for less prevalent species such as C. dubliniensis with posaconazole [44]; C. guilliermondii with fluconazole [43], posaconazole [44,45], and voriconazole [43,46]; C. krusei with fluconazole [34,40,42,43], itraconazole [34], posaconazole, and voriconazole [34,43,46]; and C. lusitaniae with fluconazole [34,40,42,43], itraconazole and posaconazole [34,44], and voriconazole [34,43] (Table 3). Overall, the EA agreement was >90% with a few exceptions for one or two triazoles. In addition, one study evaluated Etest data for 11 C. kefyr, 10 C. rugosa, eight C. lipolytica and eight C. pelliculosa resulting in EA agreement >90% with fluconazole and voriconazole (Table 3) [43].
Only two publications had EA data between the Etest and EUCAST methods for the triazoles [25,32]; the EA percentages were contradictory and disappointingly low with the following exceptions: fluconazole versus C. albicans and itraconazole and voriconazole versus C. parapsilosis (Table 3) [25,32].
The Echinocandins
Since the echinocandins were among the latest licensed agents to be evaluated for clinical use, with the exception of isavuconazole, fewer publications were found reporting EA percentages between the Etest and either the CLSI [34,47,48,49,50] or the EUCAST [25,51,52] methods (Table 4). Although all these studies were conducted with the CLSI microdilution format, the length of incubation was either 24 h (anidulafungin and micafungin) or 48 h (caspofungin). The EA for caspofungin Etest MICs against the CLSI method only produced consistently acceptable percentages in the three studies for C. glabrata and C. krusei [34,49,50]. The EA between the Etest and CLSI methods for anidulafungin was variable among the species, <90% values for all the species evaluated in either one of the two studies, with the exception of the EA for C. tropicalis (100%) (Table 4) [48,49]. However, the EA was >90% for all CLSI and Etest micafungin MIC pairs, except for the data for C. parapsilosis (87%) and C. guilliermondii (79%) (Table 4) [47,49]. Although the relationship between incubation time and EA percentages is not consistent, the impact of the incubation length is evident in several studies.

Table 4.
Essential agreement (EA) (+/− two dilutions) of echinocandins (caspofungin, micafungin, and anidulafungin) Etest and reference MICs.
As for the other agents, only three evaluations were found in the literature on the EA between Etest and the EUCAST for the three echinocandins [25,51,52]. In the caspofungin study, the RPMI broth was replaced by the AM3 medium and the EA values were unacceptable for the species tested (<90%) [25]. In one of the two micafungin studies both 24 h and 48 h incubation times were evaluated; the pattern was similar to that discussed above for the triazoles, higher EA percentages for C. parapsilosis at 48 h and at 24 h for C. albicans, and C. tropicalis [52]. Therefore, it appears that as of now, the best Etest data for the echinocandins are those obtained for micafungin.
Evaluation of EA According to MIC Ranges
As mentioned before, the EA was also reported as reference and Etest MIC ranges instead of the individual EA percentages for amphotericin B [29,53], flucytosine [53], fluconazole, voriconazole [53,54,55], and caspofungin [29,53,54]. The CLSI and Etest MIC ranges (microdilution and Etest MICs at 48 h in three of the four studies) were within the accepted <2 dilution difference for fluconazole [53,55], voriconazole, and flucytosine with the exception of C. krusei [53] and C. tropicalis [55]. However, for caspofungin and amphotericin B, CLSI and Etest MIC ranges appeared to be >2 dilutions for most of the species tested [29,53,54]. These discrepancies reflected the reported overall agreement for caspofungin from 77% to 97% [29,53,54] and for amphotericin B >89% [29,53]. When both incubation times were evaluated in a single study [54], the highest EA percentages were obtained for the first MIC readings by both methods (overall EA 98% for voriconazole and 100% for caspofungin). Other important observations also were reported in one of four studies [55], i.e., the presence of double growth zones within the whole Etest ellipse when testing some isolates of C. albicans and C. glabrata with fluconazole, as well as a substantial trailing among C. tropicalis and C. glabrata isolates. This heavy trailing precluded the definition of Etest fluconazole MICs for a substantial number of isolates of these two species [55].
Although results were similar for the comparison of the Etest and EUCAST methods for voriconazole, the incubation time did not influence the comparison for caspofungin MICs (94% and 95%) [54].
3.3.2. Filamentous Fungi
Correlation between gradient strip and reference microdilution methods for filamentous fungi has been mostly evaluated for Aspergillus spp. [23,44,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78], Fusarium spp. [60,63,65,66,68,73,76,79,80,81,82], Scedosporium spp. [60,63,66,73,76,83], and Mucorales species [44,63,65,66,73,76,81,84,85,86].
Aspergillus spp.
For Aspergillus spp, more than 25 comparative studies have been performed (Table 5), in which more than 3000 isolates were tested against different drugs.

Table 5.
Agreement between Etest and reference techniques (European Committee for Antimicrobial Susceptibility Testing (EUCAST) and Clinical and Laboratory Standards Institute (CLSI)) for Aspergillus spp.
For amphotericin B, the overall EA between Etest and reference techniques was mostly between 80% and 100% (Table 5 and Figure 3). Most of the studies showed a better EA when the Etest was read at 24 hours [44,60,61,67,69,71]. This may be due to the higher amphotericin B MICs by the Etest when the incubation time is extended to 48 hours, while similar MICs were noted among CLSI MICs at both incubation times. Almost all studies showed that overall, higher amphotericin B MICs were obtained by Etest than those obtained by the CLSI method [44,60,61,66,67,69,71,76]. This was more pronounced for A. flavus and A. terreus and could explain the lowest EA percentages for these two species [60,61,66,69,76]. Indeed, major discrepancies have been reported in some studies for A. terreus and A. flavus with EA of 16% and 40%, respectively [66,69] and more recently of 79.7% [78].
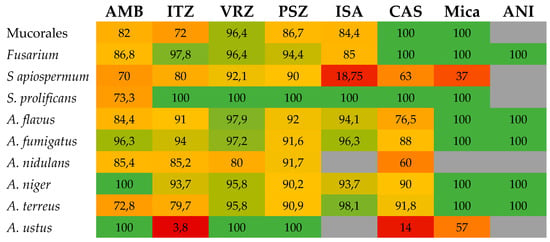
Figure 3.
Heatmap showing the level of essential agreement (EA) between Etest and microdilution broth reference techniques for the different drug-bug combinations for filamentous fungi. When several studies were available, a mean EA was calculated. Numbers represent the percentage of EA and boxes are colored from red (low EA) to green (high EA). AMB, amphotericin B; CAS, caspofungin; ISA, isavuconazole; ITZ, itraconazole; Mica, micafungin; PSZ, posaconazole; VRZ, voriconazole; ANI, anidulafungin.
The correlation between azole Etest and mostly CLSI MICs for Aspergillus spp. has been reported for itraconazole, voriconazole, posaconazole, and isavuconazole. Most EA have been >90% (Table 5 and Figure 3), but lower percentages (64% to 88%) have also been noted [65,66,67,69,71,72]; the lowest EA (64%) was for the combination of posaconazole and A. terreus [66]. As for amphotericin B MICs, Etest itraconazole MICs were usually higher than CLSI values [60,65,67,69,76,78,87]. In contrast, Etest voriconazole, posaconazole, and isavuconazole MICs were generally lower than CLSI endpoints [57,58,61,63,66,72,74,87].
Echinocandin MICs by the Etest have been evaluated in several studies, mainly for caspofungin [23,62,66,68,74,78] and to a lesser extent for micafungin [66,68,78] and anidulafungin [68,74,78]. EA was generally good (up to 100%), but lower values also have been reported in some of the studies (Table 5). It must be noted that for some specific species such as A. ustus, performance of Etest was much lower with EAs of 57% and 14% for micafungin and caspofungin, respectively [66].
Overall, Etest is an alternative to reference methods for testing Aspergillus spp., particularly for A. fumigatus versus the triazoles, since Etest results are usually above the ECV for mutant strains (non-WT). Although a good correlation between results obtained by Etest and by reference techniques are reported, the recent ESCMID-ECMM guidelines on diagnosis and management of Aspergillus diseases only marginally support the use of Etest for antifungal susceptibility testing of Aspergillus clinical isolates [3].
Mucorales
Various studies have compared the performance of the Etest with CLSI or EUCAST methods for the antifungal susceptibility testing of the Mucorales (Table 6), mostly for amphotericin B, itraconazole, and posaconazole. Moderate to good percentages of EA were generally observed. Although the EA was >90% in several studies for amphotericin B [44,66,76], the range was between 70% to 80% in other reports [81,84,86]. For posaconazole, the EA ranged from 77% to 100% [44,65,66,81,84,85,86]. A CA of 67% and 87% (by using CBPs of ≥1 µg/ml) were reported for amphotericin B and posaconazole, respectively [85]. For itraconazole, the EA was 50% to 83% [65,73,76,86], while an acceptable EA of 84% was observed during the only study that evaluated the Etest for testing isavuconazole against 45 isolates of Mucorales (62). Some studies have evaluated voriconazole, micafungin, and caspofungin with, not surprisingly, good agreement of 90% to 100% for these agents devoid of activity against Mucorales [65,66]. Therefore, the only acceptable EA was for testing amphotericin B by the Etest and the Mucorales.

Table 6.
Agreement between Etest and reference techniques (EUCAST and CLSI) for Mucorales.
Fusarium spp.
Antifungal susceptibility testing of Fusarium spp. is now recommended in patient care [88]. The comparisons of Etest results for Fusarium spp. to those obtained by the CLSI or EUCAST are depicted in Table 7. Three studies evaluated amphotericin B Etest strips for Fusarium isolates, the EA was either >90% [66,79,81,82] or lower [60,76,80]. In another study, the Etest was compared with both reference methods and the EA was either 95% and 90% against EUCAST and CLSI MICs, respectively [79]. In the latter study, the CA (based on a 4 µg/ml ECV for Fusarium spp.) was 100% with the EUCAST, but 85% with the CLSI MICs [79]. Among the azoles, the EA have ranged from 80% to 100% in most studies for itraconazole, voriconazole, and posaconazole [60,65,73,76,79,80,82]. A high level of CA between Etest voriconazole (90%, based on a 4 µg/mL ECV) and posaconazole (95%, based on a 2 µg/mL ECV) and both CLSI and EUCAST MICs have also been reported [79]. The only study that evaluated Etest isavuconazole strips for Fusarium spp. (20 isolates) reported an 85% EA with CLSI MICs [63]. When testing the echinocandins, which exhibited no in vitro activity against Fusarium spp., a 100% agreement was found between Etest caspofungin, micafungin, and anidulafungin and CLSI MICs [66,68,74]. Overall, the Etest could to be a good alternative method for testing of Fusarium spp., but unusually high MICs should be confirmed by the CLSI method since ECVs for some of these species only are available by this method.

Table 7.
Agreement between Etest and reference techniques (EUCAST and CLSI) for Fusarium spp.
Scedosporium
Some studies also have evaluated the agreement between Etest and CLSI MICs for Scedosporium isolates (Table 8) [60,63,66,73,76,83]. Overall, the agreement was dependent on the antifungal and the species tested (i.e., S. apiospermum vs. S. prolificans). For amphotericin B, an EA of 80% to 100% has been reported in two studies [60,66] with an unacceptable lower agreement (20%) elsewhere due to higher Etest MICs [76]. Methodological differences may account for these troublesome discrepancies, especially the incubation time. Among the azoles, the EA was >90% between Etest itraconazole, voriconazole, and posaconazole and CLSI MICs in four studies [60,66,73,83], but 50% when itraconazole MICs for S. apiospermum were evaluated [76]. Isavuconazole strips only have been evaluated in one study where the EA was 100% for S. prolificans, but unacceptably lower (18.7%) for S. apiospermum [63]. For the echinocandins, the EA between Etest and CLSI MICs was better for S. prolificans (100% for both caspofungin and micafungin) than for S. apiospermum (63% for caspofungin and 37% for micafungin) [66]. Although the Etest could be a valuable alternative, there is a need to further evaluate the optimal incubation times for isolates of S. prolificans and S. apiospermum and the various agents.

Table 8.
Agreement between Etest and reference techniques (EUCAST and CLSI) for Scedosporium spp.
3.4. Ability to Detect Acquired Resistance
An important point is to know if the gradient strip Etest method can correctly detect isolates with decreased susceptibility to antifungal agents. CBPs are not available for any commercial method but ECVs have been defined for the Etest and SYO methods for some Candida and Aspergillus species [89]. Therefore, we cannot talk about resistance by any of the commercial methods. However, the ability to identify isolates, as either mutants (non-WT) or WT, can be evaluated using method specific ECVs. This ability to detect non-WT isolates has been thoroughly reviewed recently for testing some yeast species against amphotericin B and the echinocandins for prevalent Candida spp(Espinel-A and Dannaoui E. Under preparation) .
Briefly, for amphotericin B, it has been shown in several studies that Etest was able to better detect the decreased susceptibility of some yeasts than reference methods [30,31,36,90,91,92,93]. Etest was better than the CLSI in recognizing non-WT isolates among C. neoformans [92] and Candida lusitaniae [90,93]. For the filamentous fungi, one study compared Etest and CLSI methods for some A. flavus isolates [94] and the Etest results better correlated with the data from an experimental model of systemic aspergillosis.
For echinocandins, studies have compared the Etest to either the CLSI or EUCAST methods for detecting non-WT Candida isolates [49,89,95,96,97,98,99]. Overall, Etest reliably detected Candida fks mutants and in some studies better discriminated between WT and mutant isolates than the reference techniques [95,96]. In addition, in the largest study that comprised data from 140 molecularly defined echinocandin mutants of Candida spp., anidulafungin Etest classified 92% of the Candida fks mutants as non-WT, while the detection was lower for caspofungin (75%) and micafungin (84%) [89].
For the triazoles, itraconazole, voriconazole, posaconazole, and isavuconazole, Etest MICs have been evaluated for the detection of A. fumigatus mutants (CYP51A substitutions associated with azole resistance) [57,100,101,102,103]. Globally, it have shown that while the Etest itraconazole ECV for this species was a good detector [101], it was not as efficient as the CLSI method for testing posaconazole [103]. In summary, in some reports, the Etest was better in detecting the potentially itraconazole resistant A. fumigatus isolates as well better discriminated between amphotericin B MICs for some Candida spp. and C. neoformans.
3.5. Etest for Direct Antifungal Susceptibility Testing on Blood Samples
When a blood culture is positive, it is necessary to subculture the strain on agar medium for performing antifungal susceptibility testing. This step implies a 24 h to 48 h further delay for reporting the susceptibility results. For this reason, the performance of antifungal susceptibility testing directly from blood culture bottles without that extra step was evaluated.
The Etest method for direct MIC determination from positive blood culture bottles was evaluated and described below [104,105,106,107,108]. The first evaluation was conducted on 138 positive blood cultures containing yeasts (mainly Candida spp.). The agreement was acceptable (81.8% to 89.4%) against the CLSI method for testing amphotericin B, flucytosine, fluconazole, and ketoconazole, but not for itraconazole (69.7% [105]). In another study, direct Etest was again compared to the CLSI microdilution method on 328 blood culture samples (195 collected and 133 laboratory prepared); the direct test performance was good with a low rate of false susceptibility detection (i.e., very major errors) for fluconazole, voriconazole, isavuconazole, and caspofungin [107]. Lower agreement was found for amphotericin B and posaconazole. In a prospective study that compared Etest to the CLSI disk diffusion method, a CA of 100% for fluconazole, voriconazole, amphotericin B and 86.2% for caspofungin was reported between the two techniques [108]. Two other studies focused on the ability of direct Etest to detect resistance to fluconazole and echinocandins in blood cultures [104,106]. In the first study, the Etest was evaluated on blood cultures for the detection of fluconazole resistance in C. albicans. The results showed 100% CA with reference broth microdilution techniques (both CLSI and EUCAST) when trailing was ruled out [106]. In the second study, direct susceptibility by Etest was performed for micafungin and anidulafungin-susceptible and -resistant strains. Overall, both the EA and CA between direct Etest and conventional EUCAST were >97% [104].
In summary, the direct Etest could be a reliable method for antifungal susceptibility testing of blood yeasts isolates for the faster detection of both fluconazole and echinocandin resistance in some Candida isolates.
3.6. Etest Specific ECVs
Because ECVs are method dependent, several studies have gathered and analyzed results from multiple and independent laboratories to define Etest-specific ECVs [89,101,109]. Etest-specific ECVs are currently available for several species of Candida and Aspergillus (Table 9 and Table 10).

Table 9.
Specific Etest ECVs for amphotericin B and azoles as compared to CLSI and EUCAST for Candida spp. and Aspergillus spp.

Table 10.
Specific Etest ECVs for echinocandins as compared to CLSI and EUCAST for Candida spp. and A. fumigatus.
4. Etest as an AFST Research Tool
As the gradient concentration strip method is easy-to-use and mostly reproducible, it has been widely used in the research area for different purposes. In some instances, it has been used for isolation of flucytosine-resistance progeny in C. tropicalis [110] or for testing fluconazole susceptibility of laboratory C. albicans mutants [111,112]. Nevertheless, one of the most important research applications of Etest has been the in vitro evaluation of antifungal combinations against a wide range of fungal species.
Combination Studies
The activity of antifungal combinations is most commonly evaluated by the checkerboard method, performed following the guidelines of the broth microdilution CLSI or EUCAST techniques. Time-kill curves are also used to evaluate the combined fungicidal activity of antifungal agents. Nevertheless, these techniques are time-consuming. In a comparison of several such techniques (Etest, checkerboard, and time-kill), the Etest was simple to use, time-efficient, reproducible, and was proposed as an alternative method [113]. Moreover, because the Etest is an agar diffusion assay, it can also be used to support or confirm results of antifungal interactions detected by checkerboard or time-kill methods [114,115,116,117,118,119,120,121]. Different protocols can be used for testing antifungal combination of a drug A with a drug B by the Etest. The first method is mainly used when one of the partner drugs is not an antifungal agent or when there is no available Etest for this drug. The MIC of drug A is determined by Etest either alone or after drug B has been included in the agar at a fixed concentration [114,115,118,122,123,124,125,126,127]. The second method is used when Etest are available for both drug A and drug B. In this case, after the determination of the MICs for both drugs alone, the combination can be evaluated by the following three main protocols: (i) The fixed ratio protocol where the strip of drug A is applied on the agar for 1 hour, the strip is removed, and the strip of drug B is applied exactly on the same position [113,128]; (ii) the MIC/MIC ratio where the strip of drug A is applied on the agar for 1 hour, removed, and the strip of drug B is applied after vertical transposition such as MICA falls on MICB; and (iii) the cross protocol where the strips of A and B are crossed at 90° angle at the position of their respective MIC [129].
Etest based strategies have mainly been used to evaluate antifungal combinations against Candida spp. [114,116,117,118,119,123,124,127,130], Aspergillus spp. [120,121,122,123,124,125,126,131], and Mucorales [115].
In C. glabrata, the combination of caspofungin with amphotericin B, showed synergy in 40% of the cases and a good concordance of 92% between Etest and time-kill studies was found [116]. For the combination of caspofungin with azoles (fluconazole, itraconazole, and voriconazole), mainly indifference was found with a concordance of 66% to 86% with the time-kill method [117]. In another study [114], the combination of terbinafine with fluconazole or voriconazole against Candida spp. showed a good concordance of the Etest results with those of the checkerboard method and it was concluded that Etest is a suitable method to determine drug interactions. Etest has also been used to test combination of antifungals with non-antifungal drugs against Candida spp [118,119,130]. A synergistic interaction was found between polymyxin B and fluconazole against C. glabrata by time-kill and Etest with a concordance of 60% [119]. In another study that evaluated the combination of polymyxin B with caspofungin, more synergistic interactions were found with Etest than with time-kill [130]. In one study, the interaction of amphotericin B with flucytosine against Cryptococcus neoformans was tested by checkerboard, time-kill, and Etest. Although some synergy was found by checkerboard and time-kill, indifferent interaction was found for all strains by Etest [132]. This lack of concordance between Etest and the other techniques was probably due to the known problems of testing flucytosine against C. neoformans by Etest [24].
In Aspergillus spp., several studies have reported the used of Etest for testing combination of azoles with echinocandins [120,121,131]. In the first two studies, the combination of voriconazole with anidulafungin and the combination of isavuconazole with the three echinocandins against azole-susceptible and -resistant Aspergillus species showed mainly indifferent interactions by Etest. Similar results were obtained by checkerboard, demonstrating a good concordance between the two techniques. In another study, Etest and checkerboard were compared to test combination of azoles with echinocandins against itraconazole-resistant isolates of A. fumigatus [131]. Overall, the results showed variable concordance depending on the combination. Etest was also used against A. fumigatus to visualize antagonism of voriconazole with flucytosine that was initially observed by checkerboard [125].
This Etest strategy has also been evaluated against individual strains to visualize the mode of interaction of antifungal with non-antifungal drugs (incorporated in the agar) against both Candida and Aspergillus [123,124,127]. In that way, it has been shown that radicicol, an inhibitor of hsp90, and cyclosporin A could synergize the activity of caspofungin against A. terreus, and the activity of azoles against C. albicans [123]. The same synergistic interaction between geldanamycin and fluconazole against C. albicans, and between geldanamycin and caspofungin against A. fumigatus, was also demonstrated by a similar approach [124].
In Mucorales, one study evaluated the interaction of cyclosporin A with either amphotericin B or posaconazole and showed synergy both by Etest and by checkerboard [115].
5. Conclusions and Perspectives
The Etest gradient strip method is a valuable alternative to the reference techniques for routine antifungal susceptibility testing in clinical laboratories with some caveats. The optimal incubation time needs to be clarified as this review has provided some insights that this testing condition could be antifungal, agent, and species dependent. For its application for drug-bug combination studies, both the incubation and the appropriate reading endpoints should be further explored. The usefulness of Etest ECVs for the detection of emerging resistance (non-WT isolates) has only been evaluated for some antifungal agent and species combinations, i.e., amphotericin B (for certain Candida spp.), echinocandins (for prevalent Candida spp.), and triazoles (mostly for A. fumigatus). The interlaboratory modal reproducibility of other agent and species combinations need additional evaluation, e.g., the triazoles versus Candida spp. Therefore, further studies are warranted for improving its routine use as a detector of non-WT or emerging resistance, the most important role of any susceptibility method. It is also necessary to determine the degree of correlation with reference techniques or the reproducibility of Etest MICs for less prevalent Candida and filamentous fungal species.
Author Contributions
E.D. and A.E.-I. have performed the bibliographic search, analyzed the results and write the paper.
Funding
This research received no external funding
Conflicts of Interest
During the past 5 years, Eric Dannaoui has received research grants from MSD and Gilead; travel grants from Gilead, MSD, Pfizer, and Astellas, and speaker’s fee from Gilead, MSD, and Astellas.
References
- Arendrup, M.C.; Bille, J.; Dannaoui, E.; Ruhnke, M.; Heussel, C.P.; Kibbler, C. ECIL-3 classical diagnostic procedures for the diagnosis of invasive fungal diseases in patients with leukaemia. Bone Marrow Transpl. 2012, 47, 1030–1045. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Estrella, M.; Verweij, P.E.; Arendrup, M.C.; Arikan-Akdagli, S.; Bille, J.; Donnelly, J.P.; Jensen, H.E.; Lass-Florl, C.; Richardson, M.D.; Akova, M.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Diagnostic procedures. Clin. Microbiol. Infect. 2012, 18 (Suppl. 7), 9–18. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Florl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. 1), e1–e38. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Boekhout, T.; Akova, M.; Meis, J.F.; Cornely, O.A.; Lortholary, O.; European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group; European Confederation of Medical Mycology. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin. Microbiol. Infect. 2014, 20 (Suppl. 3), 76–98. [Google Scholar] [CrossRef]
- Chowdhary, A.; Meis, J.F.; Guarro, J.; de Hoog, G.S.; Kathuria, S.; Arendrup, M.C.; Arikan-Akdagli, S.; Akova, M.; Boekhout, T.; Caira, M.; et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: Diseases caused by black fungi. Clin. Microbiol. Infect. 2014, 20 (Suppl. 3), 47–75. [Google Scholar] [CrossRef]
- Cornely, O.A.; Arikan-Akdagli, S.; Dannaoui, E.; Groll, A.H.; Lagrou, K.; Chakrabarti, A.; Lanternier, F.; Pagano, L.; Skiada, A.; Akova, M.; et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin. Microbiol. Infect. 2014, 20 (Suppl. 3), 5–26. [Google Scholar] [CrossRef]
- Tortorano, A.M.; Richardson, M.; Roilides, E.; van Diepeningen, A.; Caira, M.; Munoz, P.; Johnson, E.; Meletiadis, J.; Pana, Z.D.; Lackner, M.; et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin. Microbiol. Infect. 2014, 20 (Suppl. 3), 27–46. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Hamal, P.; Guinea, J.; Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts. EUCAST Definitive Document E.Def 7.3.1. 2017. Available online: http://www.eucast.org/astoffungi/methodsinantifungalsusceptibilitytesting/susceptibility_testing_of_yeasts/ (accessed on 15 September 2019).
- Clinical and laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard, 3rd ed.; CLSI document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Arendrup, M.C.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Hamal, P.; Guinea, J.; Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds. EUCAST Definitive Document E.Def 9.3.1. 2017. Available online: http://www.eucast.org/astoffungi/methodsinantifungalsusceptibilitytesting/susceptibility_testing_of_moulds/ (accessed on 15 September 2019).
- Clinical and laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 2nd ed.; Approved standard. Document M-38A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- bioMérieux SA. Etest Antifungal Susceptibility Testing Package Insert; bioMérieux SA: Marcy-l’Etoile, France, 2013. [Google Scholar]
- AB Biodisk. Etest for MIC Determination of Antifungal Agents: Reading Guide for Yeast; AB Biodisk: Solna, Sweden, 2006. [Google Scholar]
- bioMérieux SA. Etest Technical Guide No.10. Antifungal Susceptibility Testing of Moulds; bioMérieux SA: Marcy-l'Étoile, France, 2011. [Google Scholar]
- Arthington-Skaggs, B.A.; Lee-Yang, W.; Ciblak, M.A.; Frade, J.P.; Brandt, M.E.; Hajjeh, R.A.; Harrison, L.H.; Sofair, A.N.; Warnock, D.W. Comparison of visual and spectrophotometric methods of broth microdilution MIC end point determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Candida isolates. Antimicrob. Agents Chemother. 2002, 46, 2477–2481. [Google Scholar] [PubMed]
- Stevens, D.A.; Espiritu, M.; Parmar, R. Paradoxical effect of caspofungin: Reduced activity against Candida albicans at high drug concentrations. Antimicrob. Agents Chemother. 2004, 48, 3407–3411. [Google Scholar] [CrossRef]
- Wagener, J.; Loiko, V. Recent insights into the paradoxical effect of echinocandins. J. Fungi 2017, 4. [Google Scholar] [CrossRef]
- Khlif, M.; Bogreau, H.; Michel-Nguyen, A.; Ayadi, A.; Ranque, S. Trailing or paradoxical growth of Candida albicans when exposed to caspofungin is not associated with microsatellite genotypes. Antimicrob. Agents Chemother. 2010, 54, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Lewis, R.E.; Albert, N.; Kontoyiannis, D.P. Paradoxical effect of echinocandins across Candida species in vitro: Evidence for echinocandin-specific and Candida species-related differences. Antimicrob. Agents Chemother. 2007, 51, 2257–2259. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Zambrano, L.J.; Escribano, P.; Sanchez-Carrillo, C.; Bouza, E.; Guinea, J. Frequency of the paradoxical effect measured using the EUCAST procedure with micafungin, anidulafungin, and caspofungin against Candida species isolates causing candidemia. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Rueda, C.; Puig-Asensio, M.; Guinea, J.; Almirante, B.; Cuenca-Estrella, M.; Zaragoza, O.; CANDIPOP Project from GEIH-GEMICOMED (SEIMC) and REIPI. Evaluation of the possible influence of trailing and paradoxical effects on the clinical outcome of patients with candidemia. Clin. Microbiol. Infect. 2017, 23, 49e1–49e8. [Google Scholar] [CrossRef] [PubMed]
- Siopi, M.; Tsala, M.; Siafakas, N.; Zerva, L.; Meletiadis, J. Evaluation of the "dip effect" phenomenon in antifungal susceptibility testing of Candida spp. against echinocandins by use of gradient concentration strips. J. Clin. Microbiol. 2015, 53, 3654–3659. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A. Evaluation of broth microdilution testing parameters and agar diffusion Etest procedure for testing susceptibilities of Aspergillus spp. to caspofungin acetate (MK-0991). J. Clin. Microbiol. 2003, 41, 403–409. [Google Scholar] [CrossRef]
- Dannaoui, E.; Abdul, M.; Arpin, M.; Michel-Nguyen, A.; Piens, M.A.; Favel, A.; Lortholary, O.; Dromer, F. Results obtained with various antifungal susceptibility testing methods do not predict early clinical outcome in patients with cryptococcosis. Antimicrob. Agents Chemother. 2006, 50, 2464–2470. [Google Scholar] [CrossRef]
- Dannaoui, E.; Paugam, A.; Develoux, M.; Chochillon, C.; Matheron, J.; Datry, A.; Bouges-Michel, C.; Bonnal, C.; Dromer, F.; Bretagne, S. Comparison of antifungal MICs for yeasts obtained using the EUCAST method in a reference laboratory and the Etest in nine different hospital laboratories. Clin. Microbiol. Infect. 2010, 16, 863–869. [Google Scholar] [CrossRef]
- Warnock, D.W.; Johnson, E.M.; Rogers, T.R. Multi-centre evaluation of the Etest method for antifungal drug susceptibility testing of Candida spp. and Cryptococcus neoformans. BSAC Working Party on Antifungal Chemotherapy. J. Antimicrob. Chemother. 1998, 42, 321–331. [Google Scholar] [CrossRef][Green Version]
- Espinel-Ingroff, A.; Pfaller, M.; Erwin, M.E.; Jones, R.N. Interlaboratory evaluation of Etest method for testing antifungal susceptibilities of pathogenic yeasts to five antifungal agents by using Casitone agar and solidified RPMI 1640 medium with 2% glucose. J. Clin. Microbiol. 1996, 34, 848–852. [Google Scholar]
- Pfaller, M.A.; Messer, S.A.; Bolmstrom, A.; Odds, F.C.; Rex, J.H. Multisite reproducibility of the Etest MIC method for antifungal susceptibility testing of yeast isolates. J. Clin. Microbiol. 1996, 34, 1691–1693. [Google Scholar] [PubMed]
- Ranque, S.; Lachaud, L.; Gari-Toussaint, M.; Michel-Nguyen, A.; Mallie, M.; Gaudart, J.; Bertout, S. Interlaboratory reproducibility of Etest amphotericin B and caspofungin yeast susceptibility testing and comparison with the CLSI method. J. Clin. Microbiol. 2012, 50, 2305–2309. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pfaller, M.A.; Messer, S.A.; Bolmstrom, A. Evaluation of Etest for determining in vitro susceptibility of yeast isolates to amphotericin B. Diagn. Microbiol. Infect. Dis. 1998, 32, 223–227. [Google Scholar] [CrossRef]
- Wanger, A.; Mills, K.; Nelson, P.W.; Rex, J.H. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for antifungal susceptibility testing: Enhanced ability to detect amphotericin B-resistant Candida isolates. Antimicrob. Agents Chemother. 1995, 39, 2520–2522. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, F.E.; Dias, A.L.; Melhem, M.S.; Szeszs, M.W.; Auler, M.E.; Ruiz, L.S.; Goncalves da Silva, E.; Gandra, R.F.; Paula, C.R. Antifungal susceptibility of bloodstream yeasts isolated at a public children's hospital in Brazil: Comparison of the Etest and the AFST-EUCAST microdilution method. Can. J. Microbiol. 2007, 53, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Chiu, M.; Nelson, P.W.; Lancaster, M.; Pfaller, M.A.; Rex, J.H. Lot-to-lot variability of antibiotic medium 3 used for testing susceptibility of Candida isolates to amphotericin B. J. Clin. Microbiol. 1997, 35, 270–272. [Google Scholar] [PubMed]
- Alexander, B.D.; Byrne, T.C.; Smith, K.L.; Hanson, K.E.; Anstrom, K.J.; Perfect, J.R.; Reller, L.B. Comparative evaluation of Etest and sensititre yeastone panels against the Clinical and Laboratory Standards Institute M27-A2 reference broth microdilution method for testing Candida susceptibility to seven antifungal agents. J. Clin. Microbiol. 2007, 45, 698–706. [Google Scholar] [CrossRef]
- Chryssanthou, E. Trends in antifungal susceptibility among Swedish Candida species bloodstream isolates from 1994 to 1998: Comparison of the E-test and the Sensititre YeastOne Colorimetric Antifungal Panel with the NCCLS M27-A reference method. J. Clin. Microbiol. 2001, 39, 4181–4183. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, M.N.; Jang, S.J.; Ju, M.Y.; Kim, S.H.; Shin, M.G.; Suh, S.P.; Ryang, D.W. Detection of amphotericin B resistance in Candida haemulonii and closely related species by use of the Etest, Vitek-2 Yeast susceptibility system, and CLSI and EUCAST broth microdilution methods. J. Clin. Microbiol. 2012, 50, 1852–1855. [Google Scholar] [CrossRef]
- Maxwell, M.J.; Messer, S.A.; Hollis, R.J.; Diekema, D.J.; Pfaller, M.A. Evaluation of Etest method for determining voriconazole and amphotericin B MICs for 162 clinical isolates of Cryptococcus neoformans. J. Clin. Microbiol. 2003, 41, 97–99. [Google Scholar] [CrossRef]
- Metin, D.Y.; Hilmioglu-Polat, S.; Samlioglu, P.; Doganay-Oflazoglu, B.; Inci, R.; Tumbay, E. Evaluation of antifungal susceptibility testing with microdilution and Etest methods of Candida blood isolates. Mycopathologia 2011, 172, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Mahabeer, Y.; Chang, C.C.; Naidu, D.; Dorasamy, A.; Lewin, S.; Ndung'u, T.; Moosa, M.Y.; French, M.; Mlisana, K.; Coovadia, Y. Comparison of Etests and Vitek 2 (R) to broth microdilution for the susceptibility testing of Cryptococcus neoformans. Diagn. Microbiol. Infect. Dis. 2014, 80, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A. Etest for antifungal susceptibility testing of yeasts. Diagn. Microbiol. Infect. Dis. 1994, 19, 217–220. [Google Scholar] [CrossRef]
- Sewell, D.L.; Pfaller, M.A.; Barry, A.L. Comparison of broth macrodilution, broth microdilution, and E test antifungal susceptibility tests for fluconazole. J. Clin. Microbiol. 1994, 32, 2099–2102. [Google Scholar] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Karlsson, A.; Bolmstrom, A. Evaluation of the Etest method for determining fluconazole susceptibilities of 402 clinical yeast isolates by using three different agar media. J. Clin. Microbiol. 1998, 36, 2586–2589. [Google Scholar]
- Maxwell, M.J.; Messer, S.A.; Hollis, R.J.; Boyken, L.; Tendolkar, S.; Diekema, D.J.; Pfaller, M.A. Evaluation of Etest method for determining fluconazole and voriconazole MICs for 279 clinical isolates of Candida species infrequently isolated from blood. J. Clin. Microbiol. 2003, 41, 1087–1090. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A. Comparison of three commercial assays and a modified disk diffusion assay with two broth microdilution reference assays for testing zygomycetes, Aspergillus spp., Candida spp., and Cryptococcus neoformans with posaconazole and amphotericin B. J. Clin. Microbiol. 2006, 44, 3616–3622. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Messer, S.A.; Mills, K.; Bolmstrom, A.; Jones, R.N. Evaluation of Etest method for determining posaconazole MICs for 314 clinical isolates of Candida species. J. Clin. Microbiol. 2001, 39, 3952–3954. [Google Scholar] [CrossRef][Green Version]
- Pfaller, M.A.; Messer, S.A.; Houston, A.; Mills, K.; Bolmstrom, A.; Jones, R.N. Evaluation of the Etest method for determining voriconazole susceptibilities of 312 clinical isolates of Candida species by using three different agar media. J. Clin. Microbiol. 2000, 38, 3715–3717. [Google Scholar]
- Espinel-Ingroff, A.; Canton, E.; Pelaez, T.; Peman, J. Comparison of micafungin MICs as determined by the Clinical and Laboratory Standards Institute broth microdilution method (M27-A3 document) and Etest for Candida spp. isolates. Diagn. Microbiol. Infect. Dis. 2011, 70, 54–59. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Canton, E.; Peman, J.; Martin-Mazuelo, E. Comparison of anidulafungin MICs determined by the clinical and laboratory standards institute broth microdilution method (M27-A3 document) and Etest for Candida species isolates. Antimicrob. Agents Chemother. 2010, 54, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Castanheira, M.; Diekema, D.J.; Messer, S.A.; Moet, G.J.; Jones, R.N. Comparison of European Committee on Antimicrobial Susceptibility Testing (EUCAST) and Etest methods with the CLSI broth microdilution method for echinocandin susceptibility testing of Candida species. J. Clin. Microbiol. 2010, 48, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Mills, K.; Bolmstrom, A.; Jones, R.N. Evaluation of Etest method for determining caspofungin (MK-0991) susceptibilities of 726 clinical isolates of Candida species. J. Clin. Microbiol. 2001, 39, 4387–4389. [Google Scholar] [CrossRef]
- Bougnoux, M.E.; Dannaoui, E.; Accoceberry, I.; Angoulvant, A.; Bailly, E.; Botterel, F.; Chevrier, S.; Chouaki, T.; Cornet, M.; Dalle, F.; et al. Multicenter comparison of the Etest and EUCAST methods for antifungal susceptibility testing of Candida isolates to micafungin. Antimicrob. Agents Chemother. 2016, 60, 5088–5091. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Zambrano, L.J.; Escribano, P.; Rueda, C.; Zaragoza, O.; Bouza, E.; Guinea, J. Comparison between the EUCAST procedure and the Etest for determination of the susceptibility of Candida species isolates to micafungin. Antimicrob. Agents Chemother. 2013, 57, 5767–5770. [Google Scholar] [CrossRef] [PubMed]
- Fleck, R.; Dietz, A.; Hof, H. In Vitro susceptibility of Candida species to five antifungal agents in a German university hospital assessed by the reference broth microdilution method and Etest. J. Antimicrob. Chemother. 2007, 59, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Chryssanthou, E.; Cuenca-Estrella, M. Comparison of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antibiotic Susceptibility Testing proposed standard and the E-test with the NCCLS broth microdilution method for voriconazole and caspofungin susceptibility testing of yeast species. J. Clin. Microbiol. 2002, 40, 3841–3844. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vandenbossche, I.; Vaneechoutte, M.; Vandevenne, M.; De Baere, T.; Verschraegen, G. Susceptibility testing of fluconazole by the NCCLS broth macrodilution method, E-test, and disk diffusion for application in the routine laboratory. J. Clin. Microbiol. 2002, 40, 918–921. [Google Scholar] [CrossRef]
- Araujo, R.; Espinel-Ingroff, A. Comparison of assessment of oxygen consumption, Etest, and CLSI M38-A2 broth microdilution methods for evaluation of the susceptibility of Aspergillus fumigatus to posaconazole. Antimicrob. Agents Chemother. 2009, 53, 4921–4923. [Google Scholar] [CrossRef][Green Version]
- Arendrup, M.C.; Verweij, P.; Nielsen, H.V. Evaluation of MIC strip isavuconazole test for susceptibility testing of wild-type and non-wild-type Aspergillus fumigatus isolates. Antimicrob. Agents Chemother. 2017, 61, e01659-01616. [Google Scholar] [CrossRef]
- Arikan, S.; Sancak, B.; Alp, S.; Hascelik, G.; McNicholas, P. Comparative in vitro activities of posaconazole, voriconazole, itraconazole, and amphotericin B against Aspergillus and Rhizopus, and synergy testing for Rhizopus. Med. Mycol. 2008, 46, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Escribano, P.; Pelaez, T.; Recio, S.; Bouza, E.; Guinea, J. Characterization of clinical strains of Aspergillus terreus complex: Molecular identification and antifungal susceptibility to azoles and amphotericin B. Clin. Microbiol. Infect. 2012, 18, E24–E26. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A. Comparison of the E-test with the NCCLS M38-P method for antifungal susceptibility testing of common and emerging pathogenic filamentous fungi. J. Clin. Microbiol. 2001, 39, 1360–1367. [Google Scholar] [PubMed]
- Espinel-Ingroff, A.; Rezusta, A. E-Test method for testing susceptibilities of Aspergillus spp. to the new triazoles voriconazole and posaconazole and to established antifungal agents: Comparison with NCCLS broth microdilution method. J. Clin. Microbiol. 2002, 40, 2101–2107. [Google Scholar] [PubMed]
- Fuller, J.; Schofield, A.; Jiwa, S.; Sand, C.; Jansen, B.; Rennie, R. Caspofungin Etest endpoint for Aspergillus isolates shows poor agreement with the reference minimum effective concentration. J. Clin. Microbiol. 2010, 48, 479–482. [Google Scholar] [PubMed]
- Guinea, J.; Pelaez, T.; Recio, S.; Torres-Narbona, M.; Bouza, E. In Vitro antifungal activities of isavuconazole (BAL4815), voriconazole, and fluconazole against 1007 isolates of zygomycete, Candida, Aspergillus, Fusarium, and Scedosporium species. Antimicrob. Agents Chemother. 2008, 52, 1396–1400. [Google Scholar] [CrossRef]
- Howard, S.J.; Harrison, E.; Bowyer, P.; Varga, J.; Denning, D.W. Cryptic species and azole resistance in the Aspergillus niger complex. Antimicrob. Agents Chemother. 2011, 55, 4802–4809. [Google Scholar] [CrossRef]
- Kondori, N.; Svensson, E.; Mattsby-Baltzer, I. In Vitro susceptibility of filamentous fungi to itraconazole, voriconazole and posaconazole by Clinical and Laboratory Standards Institute reference method and E-test. Mycoses 2011, 54, e318–e322. [Google Scholar] [CrossRef]
- Lamoth, F.; Alexander, B.D. Comparing Etest and broth microdilution for antifungal susceptibility testing of the most-relevant pathogenic molds. J. Clin. Microbiol. 2015, 53, 3176–3181. [Google Scholar] [CrossRef]
- Martin-Mazuelos, E.; Peman, J.; Valverde, A.; Chaves, M.; Serrano, M.C.; Canton, E. Comparison of the Sensititre YeastOne colorimetric antifungal panel and Etest with the NCCLS M38-A method to determine the activity of amphotericin B and itraconazole against clinical isolates of Aspergillus spp. J. Antimicrob. Chemother. 2003, 52, 365–370. [Google Scholar] [CrossRef]
- Martos, A.I.; Romero, A.; Gonzalez, M.T.; Gonzalez, A.; Serrano, C.; Castro, C.; Peman, J.; Canton, E.; Martin-Mazuelos, E. Evaluation of the Etest method for susceptibility testing of Aspergillus spp. and Fusarium spp. to three echinocandins. Med. Mycol. 2010, 48, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Meletiadis, J.; Mouton, J.W.; Meis, J.F.; Bouman, B.A.; Verweij, P.E. Comparison of the Etest and the sensititre colorimetric methods with the NCCLS proposed standard for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 2002, 40, 2876–2885. [Google Scholar] [CrossRef] [PubMed]
- Messer, S.A.; Diekema, D.J.; Hollis, R.J.; Boyken, L.B.; Tendolkar, S.; Kroeger, J.; Pfaller, M.A. Evaluation of disk diffusion and Etest compared to broth microdilution for antifungal susceptibility testing of posaconazole against clinical isolates of filamentous fungi. J. Clin. Microbiol. 2007, 45, 1322–1324. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ozkutuk, A.; Ergon, C.; Metin, D.Y.; Yucesoy, M.; Polat, S.H. Comparison of disk diffusion, E-test and broth microdilution test in determination of susceptibility of Aspergillus species to amphotericin B, itraconazole and voriconazole. J. Chemother. 2008, 20, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Boyken, L.; Hollis, R.J.; Diekema, D.J. In Vitro susceptibility testing of filamentous fungi: Comparison of Etest and reference M38-A microdilution methods for determining posaconazole MICs. Diagn. Microbiol. Infect. Dis. 2003, 45, 241–244. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Messer, S.A.; Mills, K.; Bolmstrom, A. In Vitro susceptibility testing of filamentous fungi: Comparison of Etest and reference microdilution methods for determining itraconazole MICs. J. Clin. Microbiol. 2000, 38, 3359–3361. [Google Scholar] [CrossRef]
- Pinto, E.; Lago, M.; Branco, L.; Vale-Silva, L.A.; Pinheiro, M.D. Evaluation of Etest performed in Mueller-Hinton agar supplemented with glucose for antifungal susceptibility testing of clinical isolates of filamentous fungi. Mycopathologia 2014, 177, 157–166. [Google Scholar] [CrossRef]
- Serrano, M.C.; Morilla, D.; Valverde, A.; Chavez, M.; Espinel-Ingroff, A.; Claro, R.; Ramirez, M.; Mazuelos, E.M. Comparison of Etest with modified broth microdilution method for testing susceptibility of Aspergillus spp. to voriconazole. J. Clin. Microbiol. 2003, 41, 5270–5272. [Google Scholar] [CrossRef][Green Version]
- Szekely, A.; Johnson, E.M.; Warnock, D.W. Comparison of E-test and broth microdilution methods for antifungal drug susceptibility testing of molds. J. Clin. Microbiol. 1999, 37, 1480–1483. [Google Scholar]
- Verweij, P.E.; Te Dorsthorst, D.T.; Rijs, A.J.; De Vries-Hospers, H.G.; Meis, J.F. Nationwide survey of in vitro activities of itraconazole and voriconazole against clinical Aspergillus fumigatus isolates cultured between 1945 and 1998. J. Clin. Microbiol. 2002, 40, 2648–2650. [Google Scholar] [CrossRef]
- Imbert, S.; Normand, A.C.; Ranque, S.; Costa, J.M.; Guitard, J.; Accoceberry, I.; Bonnal, C.; Fekkar, A.; Bourgeois, N.; Houze, S.; et al. Species identification and in vitro antifungal susceptibility of Aspergillus terreus species complex clinical isolates from a french multicenter study. Antimicrob. Agents Chemother. 2018, 62, e02315-17. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatmi, A.M.; Normand, A.C.; Ranque, S.; Piarroux, R.; de Hoog, G.S.; Meletiadis, J.; Meis, J.F. Comparative evaluation of Etest, EUCAST, and CLSI methods for amphotericin B, voriconazole, and posaconazole against clinically relevant Fusarium species. Antimicrob. Agents Chemother. 2017, 61, e01671-16. [Google Scholar] [CrossRef] [PubMed]
- Debourgogne, A.; de Hoog, S.; Lozniewski, A.; Machouart, M. Amphotericin B and voriconazole susceptibility profiles for the Fusarium solani species complex: Comparison between the E-test and CLSI M38-A2 microdilution methodology. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 615–618. [Google Scholar] [CrossRef]
- Drogari-Apiranthitou, M.; Mantopoulou, F.D.; Skiada, A.; Kanioura, L.; Grammatikou, M.; Vrioni, G.; Mitroussia-Ziouva, A.; Tsakris, A.; Petrikkos, G. In Vitro antifungal susceptibility of filamentous fungi causing rare infections: Synergy testing of amphotericin B, posaconazole and anidulafungin in pairs. J. Antimicrob. Chemother. 2012, 67, 1937–1940. [Google Scholar] [CrossRef]
- Tortorano, A.M.; Prigitano, A.; Esposto, M.C.; Arsic Arsenijevic, V.; Kolarovic, J.; Ivanovic, D.; Paripovic, L.; Klingspor, L.; Nordoy, I.; Hamal, P.; et al. European Confederation of Medical Mycology (ECMM) epidemiological survey on invasive infections due to Fusarium species in Europe. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Martin-Vicente, A.; Guarro, J.; Gonzalez, G.M.; Lass-Florl, C.; Lackner, M.; Capilla, J. Voriconazole MICs are predictive for the outcome of experimental disseminated scedosporiosis. J. Antimicrob. Chemother. 2017, 72, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Caramalho, R.; Maurer, E.; Binder, U.; Araujo, R.; Dolatabadi, S.; Lass-Florl, C.; Lackner, M. Etest cannot be recommended for in vitro susceptibility testing of mucorales. Antimicrob. Agents Chemother. 2015, 59, 3663–3665. [Google Scholar] [CrossRef]
- Chowdhary, A.; Kathuria, S.; Singh, P.K.; Sharma, B.; Dolatabadi, S.; Hagen, F.; Meis, J.F. Molecular characterization and in vitro antifungal susceptibility of 80 clinical isolates of mucormycetes in Delhi, India. Mycoses 2014, 57 (Suppl. 3), 97–107. [Google Scholar] [CrossRef]
- Torres-Narbona, M.; Guinea, J.; Martinez-Alarcon, J.; Pelaez, T.; Bouza, E. In Vitro activities of amphotericin B, caspofungin, itraconazole, posaconazole, and voriconazole against 45 clinical isolates of zygomycetes: Comparison of CLSI M38-A, Sensititre YeastOne, and the Etest. Antimicrob. Agents Chemother. 2007, 51, 1126–1129. [Google Scholar] [CrossRef][Green Version]
- Pfaller, J.B.; Messer, S.A.; Hollis, R.J.; Diekema, D.J.; Pfaller, M.A. In Vitro susceptibility testing of Aspergillus spp.: Comparison of Etest and reference microdilution methods for determining voriconazole and itraconazole MICs. J. Clin. Microbiol. 2003, 41, 1126–1129. [Google Scholar] [CrossRef]
- Al-Hatmi, A.M.S.; Curfs-Breuker, I.; de Hoog, G.S.; Meis, J.F.; Verweij, P.E. Antifungal Susceptibility Testing of Fusarium: A Practical Approach. J. Fungi 2017, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A.; Arendrup, M.; Canton, E.; Cordoba, S.; Dannaoui, E.; Garcia-Rodriguez, J.; Gonzalez, G.M.; Govender, N.P.; Martin-Mazuelos, E.; Lackner, M.; et al. Multicenter study of method-dependent epidemiological cutoff values for detection of resistance in Candida spp. and Aspergillus spp. to amphotericin B and echinocandins for the Etest agar diffusion method. Antimicrob. Agents Chemother. 2017, 61, e01792-16. [Google Scholar] [CrossRef] [PubMed]
- Favel, A.; Peyron, F.; De Meo, M.; Michel-Nguyen, A.; Carriere, J.; Chastin, C.; Regli, P. Amphotericin B susceptibility testing of Candida lusitaniae isolates by flow cytofluorometry: Comparison with the Etest and the NCCLS broth macrodilution method. J. Antimicrob. Chemother. 1999, 43, 227–232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Law, D.; Moore, C.B.; Denning, D.W. Amphotericin B resistance testing of Candida spp.: A comparison of methods. J. Antimicrob. Chemother. 1997, 40, 109–112. [Google Scholar] [CrossRef]
- Lozano-Chiu, M.; Paetznick, V.L.; Ghannoum, M.A.; Rex, J.H. Detection of resistance to amphotericin B among Cryptococcus neoformans clinical isolates: Performances of three different media assessed by using E-test and National Committee for Clinical Laboratory Standards M27-A methodologies. J. Clin. Microbiol. 1998, 36, 2817–2822. [Google Scholar]
- Peyron, F.; Favel, A.; Michel-Nguyen, A.; Gilly, M.; Regli, P.; Bolmstrom, A. Improved detection of amphotericin B-resistant isolates of Candida lusitaniae by Etest. J. Clin. Microbiol. 2001, 39, 339–342. [Google Scholar] [CrossRef]
- Barchiesi, F.; Spreghini, E.; Sanguinetti, M.; Giannini, D.; Manso, E.; Castelli, P.; Girmenia, C. Effects of amphotericin B on Aspergillus flavus clinical isolates with variable susceptibilities to the polyene in an experimental model of systemic aspergillosis. J. Antimicrob. Chemother. 2013, 68, 2587–2591. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Garcia-Effron, G.; Buzina, W.; Mortensen, K.L.; Reiter, N.; Lundin, C.; Jensen, H.E.; Lass-Florl, C.; Perlin, D.S.; Bruun, B. Breakthrough Aspergillus fumigatus and Candida albicans double infection during caspofungin treatment: Laboratory characteristics and implication for susceptibility testing. Antimicrob. Agents Chemother. 2009, 53, 1185–1193. [Google Scholar] [CrossRef]
- Baixench, M.T.; Aoun, N.; Desnos-Ollivier, M.; Garcia-Hermoso, D.; Bretagne, S.; Ramires, S.; Piketty, C.; Dannaoui, E. Acquired resistance to echinocandins in Candida albicans: Case report and review. J. Antimicrob. Chemother. 2007, 59, 1076–1083. [Google Scholar] [CrossRef]
- Bourgeois, N.; Laurens, C.; Bertout, S.; Balard, Y.; Krasteva, D.; Rispail, P.; Lachaud, L. Assessment of caspofungin susceptibility of Candida glabrata by the Etest(R), CLSI, and EUCAST methods, and detection of FKS1 and FKS2 mutations. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1247–1252. [Google Scholar] [CrossRef]
- Desnos-Ollivier, M.; Dromer, F.; Dannaoui, E. Detection of caspofungin resistance in Candida spp. by Etest. J. Clin. Microbiol. 2008, 46, 2389–2392. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prigent, G.; Ait-Ammar, N.; Levesque, E.; Fekkar, A.; Costa, J.M.; El Anbassi, S.; Foulet, F.; Duvoux, C.; Merle, J.C.; Dannaoui, E.; et al. Echinocandin resistance in Candida species isolates from liver transplant recipients. Antimicrob. Agents Chemother. 2017, 61, e01229-16. [Google Scholar] [CrossRef] [PubMed]
- Burgel, P.R.; Baixench, M.T.; Amsellem, M.; Audureau, E.; Chapron, J.; Kanaan, R.; Honore, I.; Dupouy-Camet, J.; Dusser, D.; Klaassen, C.H.; et al. High prevalence of azole-resistant Aspergillus fumigatus in adults with cystic fibrosis exposed to itraconazole. Antimicrob. Agents Chemother. 2012, 56, 869–874. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Turnidge, J.; Alastruey-Izquierdo, A.; Botterel, F.; Canton, E.; Castro, C.; Chen, Y.C.; Chen, Y.; Chryssanthou, E.; Dannaoui, E.; et al. Method-dependent epidemiological cutoff values for detection of triazole resistance in Candida and Aspergillus species for the sensititre YeastOne colorimetric broth and Etest agar diffusion methods. Antimicrob. Agents Chemother. 2019, 63, e01651-18. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Turnidge, J.; Alastruey-Izquierdo, A.; Dannaoui, E.; Garcia-Effron, G.; Guinea, J.; Kidd, S.; Pelaez, T.; Sanguinetti, M.; Meletiadis, J.; et al. Posaconazole MIC distributions for Aspergillus fumigatus species complex by four methods: Impact of cyp51A mutations on estimation of epidemiological cutoff values. Antimicrob. Agents Chemother. 2018, 62, e01916-17. [Google Scholar] [CrossRef]
- Idelevich, E.A.; Gross, U.; Becker, K.; Bader, O. Comparative evaluation of different gradient diffusion tests for detection of azole resistance in Aspergillus fumigatus. Diagn. Microbiol. Infect. Dis. 2018, 91, 52–54. [Google Scholar] [CrossRef]
- Bordallo-Cardona, M.A.; Marcos-Zambrano, L.J.; Sanchez-Carrillo, C.; Bouza, E.; Munoz, P.; Escribano, P.; Guinea, J. Resistance to echinocandins in Candida can be detected by performing the Etest directly on blood culture samples. Antimicrob. Agents Chemother. 2018, 62, e00162-18. [Google Scholar] [CrossRef]
- Chang, H.C.; Chang, J.J.; Chan, S.H.; Huang, A.H.; Wu, T.L.; Lin, M.C.; Chang, T.C. Evaluation of Etest for direct antifungal susceptibility testing of yeasts in positive blood cultures. J. Clin. Microbiol. 2001, 39, 1328–1333. [Google Scholar] [CrossRef]
- Escribano, P.; Marcos-Zambrano, L.J.; Gomez, A.; Sanchez, C.; Martinez-Jimenez, M.C.; Bouza, E.; Guinea, J. The Etest performed directly on blood culture bottles is a reliable tool for detection of fluconazole-resistant Candida albicans isolates. Antimicrob. Agents Chemother. 2017, 61, e00400-17. [Google Scholar] [CrossRef]
- Guinea, J.; Recio, S.; Escribano, P.; Torres-Narbona, M.; Pelaez, T.; Sanchez-Carrillo, C.; Rodriguez-Creixems, M.; Bouza, E. Rapid antifungal susceptibility determination for yeast isolates by use of Etest performed directly on blood samples from patients with fungemia. J. Clin. Microbiol. 2010, 48, 2205–2212. [Google Scholar] [CrossRef][Green Version]
- Jabeen, K.; Kumar, H.; Farooqi, J.; Mehboob, R.; Brandt, M.E.; Zafar, A. Agreement of direct antifungal susceptibility testing from positive blood culture bottles with the conventional method for Candida species. J. Clin. Microbiol. 2016, 54, 343–348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salse, M.; Gangneux, J.P.; Cassaing, S.; Delhaes, L.; Fekkar, A.; Dupont, D.; Botterel, F.; Costa, D.; Bourgeois, N.; Bouteille, B.; et al. Multicentre study to determine the Etest epidemiological cut-off values of antifungal drugs in Candida spp. and Aspergillus fumigatus species complex. Clin. Microbiol. Infect. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; Lo, H.J.; Wu, C.C.; Ko, H.C.; Chang, T.P.; Yang, Y.L. Loss of heterozygosity of FCY2 leading to the development of flucytosine resistance in Candida tropicalis. Antimicrob. Agents Chemother. 2011, 55, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Vasicek, E.M.; Berkow, E.L.; Bruno, V.M.; Mitchell, A.P.; Wiederhold, N.P.; Barker, K.S.; Rogers, P.D. Disruption of the transcriptional regulator Cas5 results in enhanced killing of Candida albicans by Fluconazole. Antimicrob. Agents Chemother. 2014, 58, 6807–6818. [Google Scholar] [CrossRef]
- Vasicek, E.M.; Berkow, E.L.; Flowers, S.A.; Barker, K.S.; Rogers, P.D. UPC2 is universally essential for azole antifungal resistance in Candida albicans. Eukaryot. Cell 2014, 13, 933–946. [Google Scholar] [CrossRef]
- Lewis, R.E.; Diekema, D.J.; Messer, S.A.; Pfaller, M.A.; Klepser, M.E. Comparison of Etest, chequerboard dilution and time-kill studies for the detection of synergy or antagonism between antifungal agents tested against Candida species. J. Antimicrob. Chemother. 2002, 49, 345–351. [Google Scholar] [CrossRef]
- Canton, E.; Peman, J.; Gobernado, M.; Viudes, A.; Espinel-Ingroff, A. Synergistic activities of fluconazole and voriconazole with terbinafine against four Candida species determined by checkerboard, time-kill, and Etest methods. Antimicrob. Agents Chemother. 2005, 49, 1593–1596. [Google Scholar] [CrossRef]
- Dannaoui, E.; Schwarz, P.; Lortholary, O. In Vitro interactions between antifungals and immunosuppressive drugs against zygomycetes. Antimicrob. Agents Chemother. 2009, 53, 3549–3551. [Google Scholar] [CrossRef]
- Kiraz, N.; Dag, I.; Yamac, M.; Kiremitci, A.; Kasifoglu, N.; Akgun, Y. Antifungal activity of caspofungin in combination with amphotericin B against Candida glabrata: Comparison of disk diffusion, Etest, and time-kill methods. Antimicrob. Agents Chemother. 2009, 53, 788–790. [Google Scholar] [CrossRef]
- Kiraz, N.; Dag, I.; Yamac, M.; Kiremitci, A.; Kasifoglu, N.; Oz, Y. Synergistic activities of three triazoles with caspofungin against Candida glabrata isolates determined by time-kill, Etest, and disk diffusion methods. Antimicrob. Agents Chemother. 2010, 54, 2244–2247. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Wan, Z.; Liu, W.; Li, R. Synergistic activity of chloroquine with fluconazole against fluconazole-resistant isolates of Candida species. Antimicrob. Agents Chemother. 2015, 59, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Pankey, G.; Ashcraft, D.; Kahn, H.; Ismail, A. Time-kill assay and Etest evaluation for synergy with polymyxin B and fluconazole against Candida glabrata. Antimicrob. Agents Chemother. 2014, 58, 5795–5800. [Google Scholar] [CrossRef] [PubMed]
- Planche, V.; Ducroz, S.; Alanio, A.; Bougnoux, M.E.; Lortholary, O.; Dannaoui, E. In Vitro combination of anidulafungin and voriconazole against intrinsically azole-susceptible and -resistant Aspergillus spp. Antimicrob. Agents Chemother. 2012, 56, 4500–4503. [Google Scholar] [CrossRef] [PubMed]
- Raffetin, A.; Courbin, V.; Jullien, V.; Dannaoui, E. In Vitro combination of isavuconazole with echinocandins against azole-susceptible and -resistant Aspergillus spp. Antimicrob. Agents Chemother. 2018, 62, e01382-17. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Lewis, R.E.; Kontoyiannis, D.P. In Vitro interactions among echinocandins against Aspergillus fumigatus: Lack of concordance among methods. Med. Mycol. 2011, 49, 285–288. [Google Scholar] [CrossRef][Green Version]
- Cowen, L.E.; Lindquist, S. Hsp90 potentiates the rapid evolution of new traits: Drug resistance in diverse fungi. Science 2005, 309, 2185–2189. [Google Scholar] [CrossRef]
- Cowen, L.E.; Singh, S.D.; Kohler, J.R.; Collins, C.; Zaas, A.K.; Schell, W.A.; Aziz, H.; Mylonakis, E.; Perfect, J.R.; Whitesell, L.; et al. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc. Natl. Acad. Sci. USA 2009, 106, 2818–2823. [Google Scholar] [CrossRef]
- Dannaoui, E.; Lortholary, O.; Dromer, F. In Vitro evaluation of double and triple combinations of antifungal drugs against Aspergillus fumigatus and Aspergillus terreus. Antimicrob. Agents Chemother. 2004, 48, 970–978. [Google Scholar] [CrossRef][Green Version]
- Kontoyiannis, D.P.; Lewis, R.E.; Sagar, N.; May, G.; Prince, R.A.; Rolston, K.V. Itraconazole-amphotericin B antagonism in Aspergillus fumigatus: An E- test-based strategy. Antimicrob. Agents Chemother. 2000, 44, 2915–2918. [Google Scholar] [CrossRef]
- Lafleur, M.D.; Sun, L.; Lister, I.; Keating, J.; Nantel, A.; Long, L.; Ghannoum, M.; North, J.; Lee, R.E.; Coleman, K.; et al. Potentiation of azole antifungals by 2-adamantanamine. Antimicrob. Agents Chemother. 2013, 57, 3585–3592. [Google Scholar] [CrossRef]
- Vitale, R.G.; Afeltra, J.; Dannaoui, E. Antifungal combinations. Methods Mol. Med. 2005, 118, 143–152. [Google Scholar] [PubMed]
- White, R.L.; Burgess, D.S.; Manduru, M.; Bosso, J.A. Comparison of three different in vitro methods of detecting synergy: Time-kill, checkerboard, and E test. Antimicrob. Agents Chemother. 1996, 40, 1914–1918. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.K.; Ashcraft, D.S.; Pankey, G.A. In Vitro synergistic activity of caspofungin plus polymyxin B against fluconazole-resistant Candida glabrata. Am. J. Med. Sci. 2016, 351, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Denardi, L.B.; Keller, J.T.; de Azevedo, M.I.; Oliveira, V.; Piasentin, F.B.; Severo, C.B.; Santurio, J.M.; Alves, S.H. Comparison between Etest and broth microdilution methods for testing itraconazole-resistant Aspergillus fumigatus susceptibility to antifungal combinations. Mycopathologia 2018, 183, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, P.; Janbon, G.; Dromer, F.; Lortholary, O.; Dannaoui, E. Combination of amphotericin B with flucytosine is active in vitro against flucytosine-resistant isolates of Cryptococcus neoformans. Antimicrob. Agents Chemother. 2007, 51, 383–385. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).