A Guide to Investigating Suspected Outbreaks of Mucormycosis in Healthcare
Abstract
1. Introduction
1.1. Mucormycosis Background
1.2. Challenges of Investigating Mucormycosis
2. Components of an Outbreak Investigation
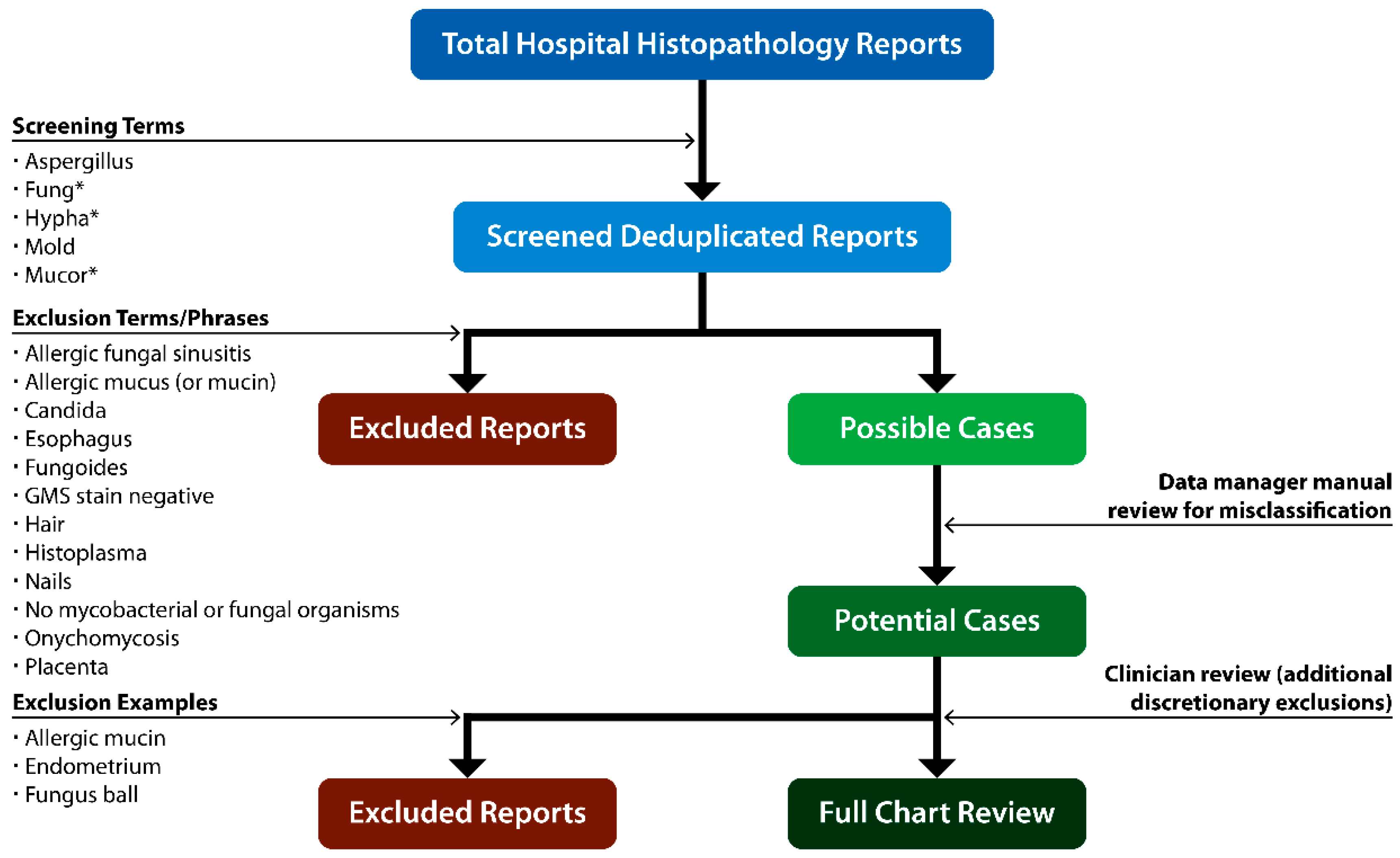
2.1. Verify the Diagnosis and Define a Case
2.1.1. Cutaneous Mucormycosis Case Definition
2.1.2. Laboratory Confirmation
2.2. Evaluate Hospital Trends in Mucormycosis and Other Mold Infections
2.2.1. Assess Trends for All Mold Infections
2.2.2. Using Denominator Data to Calculate Incidence
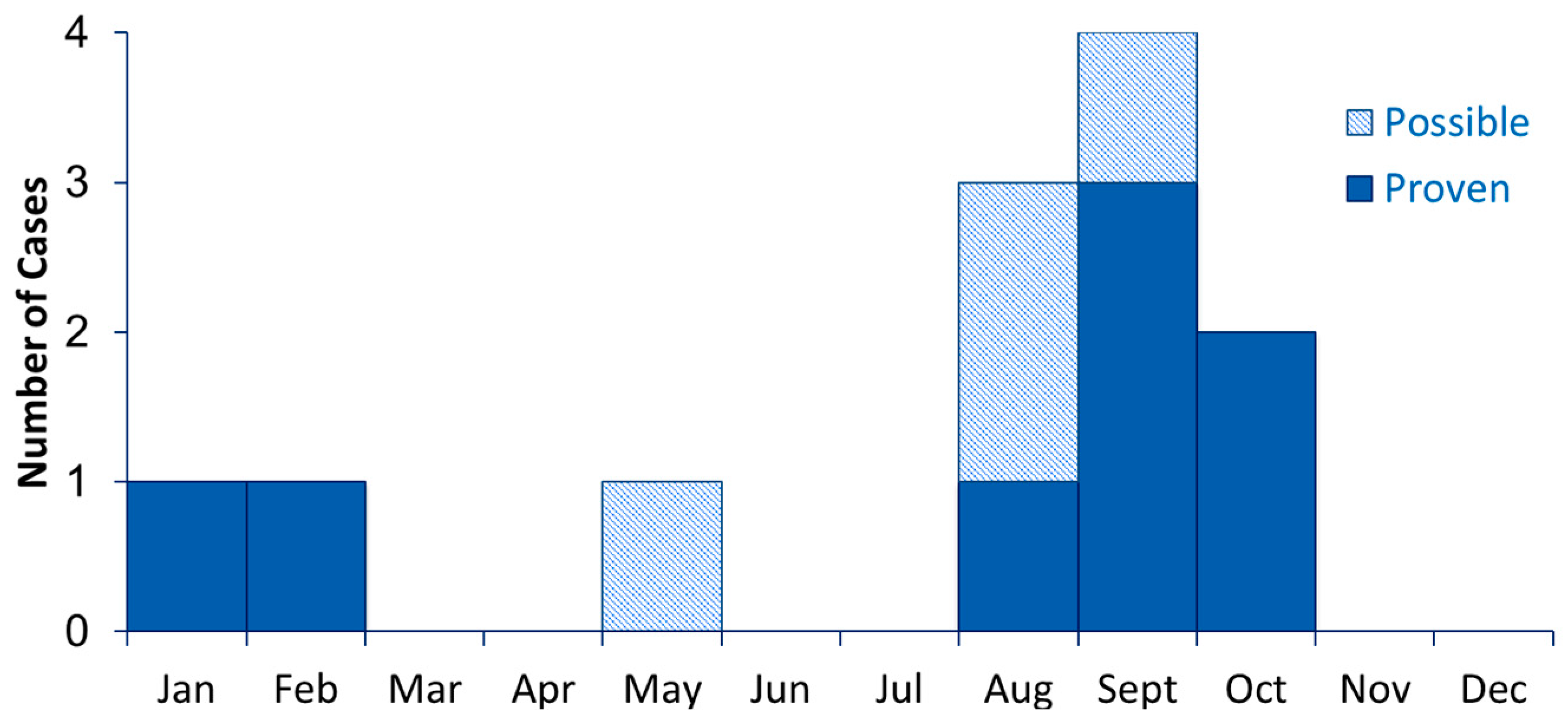
2.2.3. Seasonality
2.3. Notify and Involve Key Partners
- Encourage patients to seek care early if they have concerning symptoms, potentially leading to more timely diagnosis;
- Improve case-finding;
- Provide opportunity to educate patients on ways to avoid exposure to mold, both in the hospital and community;
- Remind patients, visitors, and clinicians about important of infection control interventions;
- Build patient and public trust through transparency.
2.4. Determine Exposures of Interest and Relevant Time Period
2.4.1. Epidemic Curve
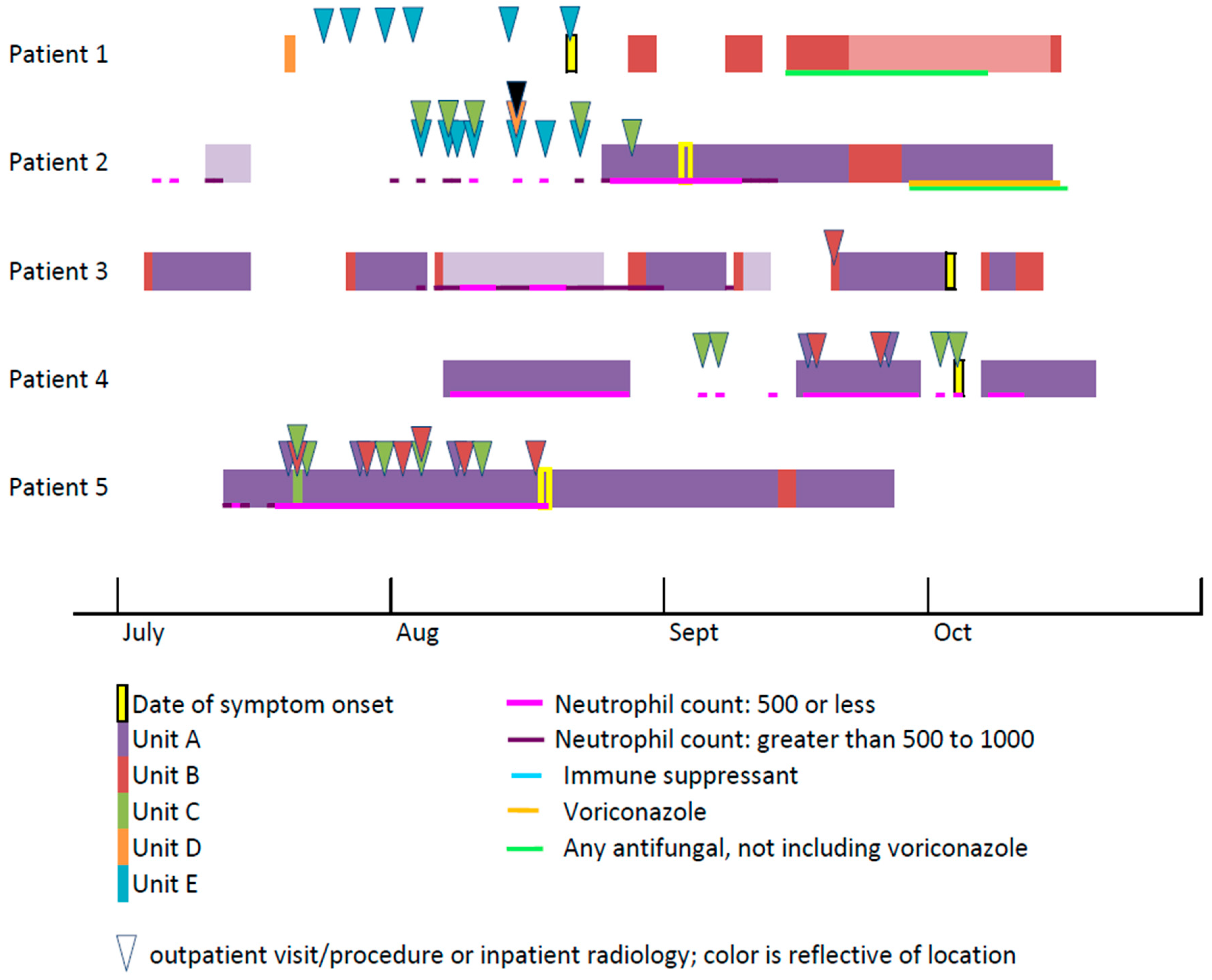
2.4.2. Line List and Case Reviews
- Age;
- Admission date(s), as well as discharge dates for previous hospitalizations within several months of suspected onset;
- Prior admissions and outpatient visits;
- Patient risk factors and underlying conditions (e.g., diabetes mellitus, hematologic disease, hematopoietic stem cell transplant, and prolonged neutropenia);
- Immunosuppressive medications (e.g., corticosteroids, cancer chemotherapy, biologics, and other immunotherapy);
- Signs and symptoms of fungal infection, with approximate dates of onset;
- Histopathology specimen collection date(s) and findings;
- Culture date(s), specimen source, and species;
- Antifungal medications given with dates and indication (prophylaxis versus treatment);
- Unit and room assignments with dates;
- Invasive disease versus colonization by EORTC/MSG definition or clinician determination;
- Devices, procedures, lines, and surgeries;
- Outcomes, including date of discharge or death.
2.4.3. Hospital Map
2.4.4. Timeline of Patient Locations
2.5. Develop Hypotheses
2.5.1. Non-Sterile Products
Bandages, Patches, Tape, and Adhesives:
Linen:
Wooden Tongue Depressors:
Medications, Supplements, and Food:
2.5.2. Procedures, Devices, and Organs
Catheters and Tubes:
Dental Procedures:
Cardiac Surgery:
Insulin Pumps and Finger Sticks:
Medical Devices:
Organ Transplant:
2.5.3. Environmental Sources: Air, Dust, Water, Soil
Air Filtration and Intakes:
Negative Pressure Rooms:
Construction:
Water Leaks and Water Damage:
Plants:
2.5.4. Other Exposures
2.6. Conduct Environmental Assessments
- General inspection for mold, leaks, dirt
- HVAC systems
- Environmental cleaning
- Construction and maintenance
- Linen
- Facility work orders for routine maintenance and repairs
- Infection Control Risk Assessments (ICRAs) conducted before starting construction
- Indoor air temperature and humidity logs, including any days when humidity exceeded 60%
- Dates of air filter changes
- HVAC system, filters, and fans maintained according to manufacturer instructions
- Frequency and method of filter performance
- Air flow monitoring records
Protective Environments
2.7. Evaluate Hypotheses Epidemiologically
2.8. Consider Whether Environmental Sampling Could Aid the Investigation
2.9. Implement Control and Prevention Measures
2.10. Initiate and Maintain Surveillance
3. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Richardson, M. The ecology of the Zygomycetes and its impact on environmental exposure. Clin. Microbiol. Infect. 2009, 15, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef] [PubMed]
- Rammaert, B.; Lanternier, F.; Zahar, J.R.; Dannaoui, E.; Bougnoux, M.E.; Lecuit, M.; Lortholary, O. Healthcare-associated mucormycosis. Clin. Infect. Dis. 2012, 54, S44–S54. [Google Scholar] [CrossRef] [PubMed]
- Vallabhaneni, S.; Benedict, K.; Derado, G.; Mody, R.K. Trends in hospitalizations related to invasive Aspergillosis and mucormycosis in the United States, 2000–2013. Open Forum Infect. Dis. 2017, 4, ofw268. [Google Scholar] [CrossRef] [PubMed]
- Neofytos, D.; Treadway, S.; Ostrander, D.; Alonso, C.D.; Dierberg, K.L.; Nussenblatt, V.; Durand, C.M.; Thompson, C.B.; Marr, K.A. Epidemiology, outcomes, and mortality predictors of invasive mold infections among transplant recipients: A 10-year, single-center experience. Transpl. Infect. Dis. 2013, 15, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Imhof, A.; Balajee, S.A.; Fredricks, D.N.; Englund, J.A.; Marr, K.A. Breakthrough fungal infections in stem cell transplant recipients receiving voriconazole. Clin. Infect. Dis. 2004, 39, 743–746. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Lionakis, M.S.; Lewis, R.E.; Chamilos, G.; Healy, M.; Perego, C.; Safdar, A.; Kantarjian, H.; Champlin, R.; Walsh, T.J.; et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: A case-control observational study of 27 recent cases. J. Infect. Dis. 2005, 191, 1350–1360. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Wessel, V.C.; Bodey, G.P.; Rolston, K.V. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin. Infect. Dis. 2000, 30, 851–856. [Google Scholar] [CrossRef]
- Marr, K.A.; Carter, R.A.; Crippa, F.; Wald, A.; Corey, L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 2002, 34, 909–917. [Google Scholar] [CrossRef]
- Marty, F.M.; Cosimi, L.A.; Baden, L.R. Breakthrough zygomycosis after voriconazole treatment in recipients of hematopoietic stem-cell transplants. N. Engl. J. Med. 2004, 350, 950–952. [Google Scholar] [CrossRef]
- Siwek, G.T.; Dodgson, K.J.; de Magalhaes-Silverman, M.; Bartelt, L.A.; Kilborn, S.B.; Hoth, P.L.; Diekema, D.J.; Pfaller, M.A. Invasive zygomycosis in hematopoietic stem cell transplant recipients receiving voriconazole prophylaxis. Clin. Infect. Dis. 2004, 39, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Vigouroux, S.; Morin, O.; Moreau, P.; Méchinaud, F.; Morineau, N.; Mahé, B.; Chevallier, P.; Guillaume, T.; Dubruille, V.; Harousseau, J.L.; et al. Zygomycosis after prolonged use of voriconazole in immunocompromised patients with hematologic disease: Attention required. Clin. Infect. Dis. 2005, 40, e35–e37. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.; Jackson, B.R.; Chiller, T.; Beer, K.D. Estimation of direct healthcare costs of fungal diseases in the United States. Clin. Infect. Dis. 2019, 68, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Azie, N.; Franks, B.; Horn, D.L. Prospective antifungal therapy (PATH) alliance (®): Focus on mucormycosis. Mycoses 2014, 57, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Lass-Florl, C.; Bouza, E.; Klimko, N.; Gaustad, P.; et al. Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European confederation of medical mycology (ECMM) working group on zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011, 17, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Lewis, R.E.; Lortholary, O.; Spellberg, B.; Petrikkos, G.; Roilides, E.; Ibrahim, A.; Walsh, T.J. Future directions in mucormycosis research. Clin. Infect. Dis. 2012, 54 (Suppl. 1), S79–S85. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.; Harris, J.; Gade, L.; Sehulster, L.; Newhouse, E.; O’Connell, H.; Noble-Wang, J.; Rao, C.; Balajee, S.A.; Chiller, T. Mucormycosis outbreak associated with hospital linens. Pediatr. Infect. Dis. J. 2014, 33, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.C.; Chen, J.H.K.; Wong, S.C.Y.; Leung, S.S.M.; So, S.Y.C.; Lung, D.C.; Lee, W.M.; Trendell-Smith, N.J.; Chan, W.M.; Ng, D.; et al. Hospital outbreak of pulmonary and cutaneous zygomycosis due to contaminated linen items from substandard laundry. Clin. Infect. Dis. 2016, 62, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Pappas, P.G.; Wannemuehler, K.A.; Alexander, B.D.; Anaissie, E.J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; Brumble, L.M.; Freifeld, A.G.; et al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001–2006. Emerg. Infect. Dis. 2011, 17, 1855–1864. [Google Scholar] [CrossRef]
- Schuster, M.G.; Cleveland, A.A.; Dubberke, E.R.; Kauffman, C.A.; Avery, R.K.; Husain, S.; Paterson, D.L.; Silveira, F.P.; Chiller, T.M.; Benedict, K.; et al. Infections in hematopoietic cell transplant recipients: Results from the organ transplant infection project, a multicenter, prospective, cohort study. Open Forum Infect. Dis. 2017, 4, ofx050. [Google Scholar] [CrossRef]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Castrejόn-Pérez, A.D.; Welsh, E.C.; Miranda, I.; Ocampo-Candiani, J.; Welsh, O. Cutaneous mucormycosis. Bras. Derm. 2017, 92, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Notes from the field: Fatal fungal soft-tissue infections after a tornado―Joplin, Missouri, 2011. Morb. Mortal. Wkly. Rep. 2011, 60, 992. [Google Scholar]
- Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Division of Foodborne, Waterborne, and Environmental Diseases. Fungal Diseases Laboratory Submission Information. Available online: https://www.cdc.gov/fungal/lab_submission.html (accessed on 29 May 2019).
- Gholinejad-Ghadi, N.; Shokohi, T.; Seifi, Z.; Aghili, S.R.; Roilides, E.; Nikkhah, M.; Pormosa, R.; Karami, H.; Larjani, L.V.; Ghasemi, M.; et al. Identification of Mucorales in patients with proven invasive mucormycosis by polymerase chain reaction in tissue samples. Mycoses 2018, 61, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.; Shute, T.; Lysen, C.; Lockhart, S.R.; Keating, M.K.; Custer, P.; Orscheln, R. Saksenaea vasiformis orbital cellulitis in an immunocompetent child treated with posaconazole. J. Pediatr. Infect. Dis. Soc. 2018, 7, e169–e171. [Google Scholar] [CrossRef]
- Legrand, M.; Gits-Muselli, M.; Boutin, L.; Garcia-Hermoso, D.; Maurel, V.; Soussi, S.; Benyamina, M.; Ferry, A.; Chaussard, M.; Hamane, S.; et al. Detection of circulating Mucorales DNA in critically ill burn patients: Preliminary report of a screening strategy for early diagnosis and treatment. Clin. Infect. Dis. 2016, 63, 1312–1317. [Google Scholar] [CrossRef]
- Millon, L.; Larosa, F.; Lepiller, Q.; Legrand, F.; Rocchi, S.; Daguindau, E.; Scherer, E.; Bellanger, A.P.; Leroy, J.; Grenouillet, F. Quantitative polymerase chain reaction detection of circulating DNA in serum for early diagnosis of mucormycosis in immunocompromised patients. Clin. Infect. Dis. 2013, 56, e95–e101. [Google Scholar] [CrossRef]
- Jung, J.; Park, Y.S.; Sung, H.; Song, J.S.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Kim, S.H. Using immunohistochemistry to assess the accuracy of histomorphologic diagnosis of Aspergillosis and mucormycosis. Clin. Infect. Dis. 2015, 61, 1664–1670. [Google Scholar] [CrossRef]
- Talmi, Y.P.; Goldschmied-Reouven, A.; Bakon, M.; Barshack, I.; Wolf, M.; Horowitz, Z.; Berkowicz, M.; Keller, N.; Kronenberg, J. Rhino-orbital and rhino-orbito-cerebral mucormycosis. Otolaryngol. Head Neck Surg. 2002, 127, 22–31. [Google Scholar] [CrossRef]
- Shpitzer, T.; Keller, N.; Wolf, M.; Goldschmied-Reouven, A.; Bahar, G.; Bahar, I.; Kronenberg, J.; Feinmesser, R.; Talmi, Y.P. Seasonal variations in rhino-cerebral Mucor infection. Ann. Otol. Rhinol. Laryngol. 2005, 114, 695–698. [Google Scholar] [CrossRef]
- Al-Ajam, M.R.; Bizri, A.R.; Mokhbat, J.; Weedon, J.; Lutwick, L. Mucormycosis in the Eastern Mediterranean: A seasonal disease. Epidemiol. Infect. 2006, 134, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Sivagnanam, S.; Sengupta, D.J.; Hoogestraat, D.; Jain, R.; Stednick, Z.; Fredricks, D.N.; Hendrie, P.; Whimbey, E.; Podczervinski, S.T.; Krantz, E.M.; et al. Seasonal clustering of sinopulmonary mucormycosis in patients with hematologic malignancies at a large comprehensive cancer center. Antimicrob. Resist. Infect. Control 2017, 6, 123. [Google Scholar] [CrossRef] [PubMed]
- Dudzinski, D.M.; Hébert, P.C.; Foglia, M.B.; Gallagher, T.H. The disclosure dilemma―Large-scale adverse events. N. Engl. J. Med. 2010, 363, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Kachalia, A.; Sands, K.; Van Niel, M.; Dodson, S.; Roche, S.; Novack, V.; Yitshak-Sade, M.; Folcarelli, P.; Benjamin, E.M.; Woodward, A.C.; et al. Effects of a communication-and-resolution program on hospitals’ malpractice claims and costs. Health Aff. 2018, 37, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Division of Healthcare Quality Promotion. Patient Notification Toolkit. Available online: https://www.cdc.gov/injectionsafety/pntoolkit/index.html (accessed on 29 May 2019).
- Llata, E.; Blossom, D.B.; Khoury, H.J.; Rao, C.Y.; Wannemuehler, K.A.; Noble-Wang, J.; Langston, A.A.; Ribner, B.S.; Lyon, G.M.; Arnold, K.E.; et al. A cluster of mucormycosis infections in hematology patients: Challenges in investigation and control of invasive mold infections in high-risk patient populations. Diagn. Microbiol. Infect. Dis. 2011, 71, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.M.; Lee, J.; Mody, R.K. Determining the incubation time of mucormycosis: A systematic review. Open Forum Infect. Dis. 2015, 2, 461. [Google Scholar] [CrossRef]
- Neblett Fanfair, R.; Benedict, K.; Bos, J.; Bennett, S.D.; Lo, Y.C.; Adebanjo, T.; Etienne, K.; Deak, E.; Derado, G.; Shieh, W.J.; et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N. Engl. J. Med. 2012, 367, 2214–2225. [Google Scholar] [CrossRef]
- LeMaile-Williams, M.; Burwell, L.A.; Salisbury, D.; Noble-Wang, J.; Arduino, M.; Lott, T.; Brandt, M.E.; Iiames, S.; Srinivasan, A.; Fridkin, S.K. Outbreak of cutaneous Rhizopus arrhizus infection associated with karaya ostomy bags. Clin. Infect. Dis. 2006, 43, e83–e88. [Google Scholar] [CrossRef]
- Alexander, B.D.; Schell, W.A.; Siston, A.M.; Rao, C.Y.; Bower, W.A.; Balajee, S.A.; Howell, D.N.; Moore, Z.S.; Noble-Wang, J.; Rhyne, J.A.; et al. Fatal Apophysomyces elegans infection transmitted by deceased donor renal allografts. Am. J. Transpl. 2010, 10, 2161–2167. [Google Scholar] [CrossRef]
- Novosad, S.A.; Vasquez, A.M.; Nambiar, A.; Arduino, M.J.; Christensen, E.; Moulton-Meissner, H.; Keckler, M.S.; Miller, J.; Perz, J.F.; Lockhart, S.R.; et al. Notes from the Field: Probable mucormycosis among adult solid organ transplant recipients at an acute care hospital―Pennsylvania, 2014–2015. Morb. Mortal. Wkly. Rep. 2016, 65, 481–482. [Google Scholar] [CrossRef][Green Version]
- Vallabhaneni, S.; Walker, T.A.; Lockhart, S.R.; Ng, D.; Chiller, T.; Melchreit, R.; Brandt, M.E.; Smith, R.M. Fatal gastrointestinal mucormycosis in a premature infant associated with a contaminated dietary supplement―Connecticut, 2014. Morb. Mortal. Wkly. Rep. 2015, 64, 155–156. [Google Scholar]
- Torres-R, J.R.; Maki, N.J.; Taddonio, R.F.; Sanders, C.V. Cutaneous Rhizopus infection associated with Elastoplast wound dressing. Orthopedics 1979, 2, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Nosocomial outbreak of Rhizopus infections associated with Elastoplast wound dressings—Minnesota. Morb. Mortal. Wkly. Rep. 1978, 27, 33–34. [Google Scholar]
- Centers for Disease Control and Prevention. Follow-up on Rhizopus infections associated with Elastoplast bandages—United States. Morb. Mortal. Wkly. Rep. 1978, 27, 243–244. [Google Scholar]
- Dennis, J.E.; Rhodes, K.H.; Cooney, D.R.; Roberts, G.D. Nosocomical Rhizopus infection (zygomycosis) in children. J. Pediatr. 1980, 96, 824–828. [Google Scholar] [CrossRef]
- Gartenberg, G.; Bottone, E.J.; Keusch, G.T.; Weitzman, I. Hospital-acquired mucormycosis (Rhizopus rhizopodiformis) of skin and subcutaneous tissue: Epidemiology, mycology and treatment. N. Engl. J. Med. 1978, 299, 1115–1118. [Google Scholar] [CrossRef]
- Everett, E.D.; Pearson, S.; Rogers, W. Rhizopus surgical wound infection with elasticized adhesive tape dressings. Arch. Surg. 1979, 114, 738–739. [Google Scholar] [CrossRef]
- Mead, J.H.; Lupton, G.P.; Dillavou, C.L.; Odom, R.B. Cutaneous Rhizopus infection: Occurrence as a postoperative complication associated with an elasticized adhesive dressing. JAMA 1979, 242, 272–274. [Google Scholar] [CrossRef]
- Christiaens, G.; Hayette, M.P.; Jacquemin, D.; Melin, P.; Mutsers, J.; De Mol, P. An outbreak of Absidia corymbifera infection associated with bandage contamination in a burns unit. J. Hosp. Infect. 2005, 61, 88. [Google Scholar] [CrossRef]
- Shakoor, S.; Jabeen, K.; Idrees, R.; Jamil, B.; Irfan, S.; Zafar, A. Necrotising fasciitis due to Absidia corymbifera in wounds dressed with non sterile bandages. Int. Wound J. 2011, 8, 651–655. [Google Scholar] [CrossRef]
- Dickinson, M.; Kalayanamit, T.; Yang, C.A.; Pomper, G.J.; Franco-Webb, C.; Rodman, D. Cutaneous zygomycosis (mucormycosis) complicating endotracheal intubation: Diagnosis and successful treatment. Chest 1998, 114, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Alsuwaida, K. Primary cutaneous mucormycosis complicating the use of adhesive tape to secure the endotracheal tube. Can. J. Anesth. 2002, 49, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, P.J.; Wentzel, L.F.; Delport, S.D.; van Damme, E. Zygomycotic necrotizing cellulitis in a premature infant. Dermatology 1997, 195, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.F.; Joshi, V.V.; Ellison, D.A.; Cedars, J.C. Zygomycosis in neonates. Pediatr. Infect. Dis. J. 1997, 16, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Petrikkos, G.; Skiada, A.; Sambatakou, H.; Toskas, A.; Vaiopoulos, G.; Giannopoulou, M.; Katsilambros, N. Mucormycosis: Ten-year experience at a tertiary-care center in Greece. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 753–756. [Google Scholar] [CrossRef]
- Johnson, A.S.; Ranson, M.; Scarffe, J.H.; Morgenstern, G.R.; Shaw, A.J.; Oppenheim, B.A. Cutaneous infection with Rhizopus oryzae and Aspergillus niger following bone marrow transplantation. J. Hosp. Infect. 1993, 25, 293–296. [Google Scholar] [CrossRef]
- Teal, L.J.; Schultz, K.M.; Weber, D.J.; Gergen, M.F.; Miller, M.B.; DiBiase, L.M.; Sickbert-Bennett, E.E.; Rutala, W.A. Invasive cutaneous Rhizopus infections in an immunocompromised patient population associated with hospital laundry carts. Infect. Control Hosp. Epidemiol. 2016, 37, 1251–1253. [Google Scholar] [CrossRef]
- Maravi-Poma, E.; Rodriguez-Tudela, J.L.; de Jalόn, J.G.; Manrique-Larralde, A.; Torroba, L.; Urtasun, J.; Salvador, B.; Montes, M.; Mellado, E.; Rodríguez-Albarrán, F.; et al. Outbreak of gastric mucormycosis associated with the use of wooden tongue depressors in critically ill patients. Intensive Care Med. 2004, 30, 724–728. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Gray, J.; Morgan, M.E.I.; Hocking, M.D.; Durbin, G.M. Nosocomial infection with Rhizopus microsporus in preterm infants: Association with wooden tongue depressors. Lancet 1996, 348, 441–443. [Google Scholar] [CrossRef]
- Oliver, M.R.; Van Voorhis, W.C.; Boeckh, M.; Mattson, D.; Bowden, R.A. Hepatic mucormycosis in a bone marrow transplant recipient who ingested naturopathic medicine. Clin. Infect. Dis. 1996, 22, 521–524. [Google Scholar] [CrossRef]
- Cheng, V.C.C.; Chan, J.F.W.; Ngan, A.H.Y.; To, K.K.W.; Leung, S.Y.; Tsoi, H.W.; Yam, W.C.; Tai, J.W.M.; Wong, S.S.Y.; Tse, H.; et al. Outbreak of intestinal infection due to R. microsporus. J. Clin. Microbiol. 2009, 47, 2834–2843. [Google Scholar] [CrossRef] [PubMed]
- Pérez de la Espejo, M.P.; Barrero Candau, R.; Chinchόn Espino, D.; Campoy Martínez, P. Bladder mucormycosis. Report of one case. Arch. Esp. Urol. 2004, 57, 67–69. [Google Scholar]
- Amin, S.B.; Ryan, R.M.; Metlay, L.A.; Watson, W.J. Absidia corymbifera infections in neonates. Clin. Infect. Dis. 1998, 26, 990–992. [Google Scholar] [CrossRef] [PubMed]
- Diven, S.C.; Angel, C.A.; Hawkins, H.K.; Rowen, J.L.; Shattuck, K.E. Intestinal zygomycosis due to Absidia corymbifera mimicking necrotizing enterocolitis in a preterm neonate. J. Perinatol. 2004, 24, 794–796. [Google Scholar] [CrossRef] [PubMed]
- Polo, J.R.; Luño, J.; Menarguez, C.; Gallego, E.; Robles, R.; Hernandez, P. Peritoneal mucormycosis in a patient receiving continuous ambulatory peritoneal dialysis. Am. J. Kidney Dis. 1989, 13, 237–239. [Google Scholar] [CrossRef]
- Branton, M.H.; Johnson, S.C.; Brooke, J.D.; Hasbargen, J.A. Peritonitis due to Rhizopus in a patient undergoing continuous ambulatory peritoneal dialysis. Rev. Infect. Dis. 1991, 13, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Nannini, E.C.; Paphitou, N.I.; Ostrosky-Zeichner, L. Peritonitis due to Aspergillus and zygomycetes in patients undergoing peritoneal dialysis: Report of 2 cases and review of the literature. Diagn. Microbiol. Infect. Dis. 2003, 46, 49–54. [Google Scholar] [CrossRef]
- Pimentel, J.D.; Dreyer, G.; Lum, G.D. Peritonitis due to Cunninghamella bertholletiae in a patient undergoing continuous ambulatory peritoneal dialysis. J. Med. Microbiol. 2006, 55, 115–118. [Google Scholar] [CrossRef][Green Version]
- Chopra, H.; Dua, K.; Bhatia, S.; Dua, N.; Mittal, V. Invasive rhino-orbital fungal sinusitis following dental manipulation. Mycoses 2009, 52, 368–371. [Google Scholar] [CrossRef]
- Szalai, G.; Fellegi, V.; Szabό, Z.; Vitéz, L.C. Mucormycosis mimicks sinusitis in a diabetic adult. Ann. N. Y. Acad. Sci. 2006, 1084, 520–530. [Google Scholar] [CrossRef]
- Kim, J.; Fortson, J.K.; Cook, H.E. A fatal outcome from rhinocerebral mucormycosis after dental extractions: A case report. J. Oral Maxillofac. Surg. 2001, 59, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Alqahtani, M.; Al Shehabi, M. A fatal case of rhinocerebral mucormycosis of the jaw after dental extractions and review of literature. J. Infect. Public Health 2018, 11, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, C.; Regennitter, F.; Viozzi, C.F. Invasive fungal infection of the maxilla following dental extractions in a patient with chronic obstructive pulmonary disease. J. Can. Dent. Assoc. 2006, 72, 149–152. [Google Scholar] [PubMed]
- Salisbury, P.L., 3rd; Caloss, R., Jr.; Cruz, J.M.; Powell, B.L.; Cole, R.; Kohut, R.I. Mucormycosis of the mandible after dental extractions in a patient with acute myelogenous leukemia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 83, 340–344. [Google Scholar] [CrossRef]
- De Repentigny, L.; St-Germain, G.; Charest, H.; Kokta, V.; Vobecky, S. Fatal zygomycosis caused by Mucor indicus in a child with an implantable left ventricular assist device. Pediatr. Infect. Dis. J. 2008, 27, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, R.; Venugopal, P.; Chopra, P. Prosthetic mitral valve mucormycosis caused by Mucor species. Int. J. Cardiol. 1987, 17, 333–335. [Google Scholar] [CrossRef]
- Sanchez-Recalde, A.; Merino, J.L.; Dominguez, F.; Mate, I.; Larrea, J.L.; Sobrino, J.A. Successful treatment of prosthetic aortic valve mucormycosis. Chest 1999, 116, 1818–1820. [Google Scholar] [CrossRef] [PubMed]
- Khicha, G.J.; Berroya, R.B.; Escano, F.B., Jr.; Lee, C.S. Mucormycosis in a mitral prosthesis. J. Thorac. Cardiovasc. Surg. 1972, 63, 903–905. [Google Scholar]
- Abter, E.I.; Lutwick, S.M.; Chapnick, E.K.; Chittivelu, S.; Lutwick, L.I.; Sabado, M.; Jacobowitz, I. Mucormycosis of a median sternotomy wound. Cardiovasc. Surg. 1994, 2, 474–477. [Google Scholar] [CrossRef]
- Wickline, C.L.; Cornitius, T.G.; Butler, T. Cellulitis caused by Rhizomucor pusillus in a diabetic patient receiving continuous insulin infusion pump therapy. South. Med. J. 1989, 82, 1432–1434. [Google Scholar] [CrossRef]
- Quinio, D.; Karam, A.; Leroy, J.P.; Moal, M.C.; Bourbigot, B.; Masure, O.; Sassolas, B.; Le Flohic, A.M. Zygomycosis caused by Cunninghamella bertholletiae in a kidney transplant recipient. Med. Mycol. 2004, 42, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Delie, A.; Vlummens, P.; Creytens, D.; Steel, E. Cutaneous mucormycosis as result of insulin administration in an AML patient: Case report and review of the literature. Acta Clin. Belg. 2017, 72, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Hampson, F.G.; Ridgway, E.J.; Feeley, K.; Reilly, J.T. A fatal case of disseminated zygomycosis associated with the use of blood glucose self-monitoring equipment. J. Infect. 2005, 51, e269–e272. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.S.; Franco, D.; Nanni, J.C.; Basaldúa, M.L.; Boleas, M.; Aphalo, G.; Feltes Silva, P.; Elgadban, M.C.; Emery, F.; Gammara, S.; et al. Control of an outbreak of postoperative bone mucormycosis: An intervention study of contiguous cohorts. Am. J. Infect. Control 2016, 44, 1715–1717. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Mandal, A.; Kumar, N. Mycotic prosthetic-valve endocarditis. J. Hosp. Infect. 1992, 20, 122–125. [Google Scholar] [CrossRef]
- Del Palacio Hernanz, A.; Fereres, J.; Larregla Garraus, S.; Rodriguez-Noriega, A.; Sanz Sanz, F. Nosocomial infection by Rhizomucor pusillus in a clinical haematology unit. J. Hosp. Infect. 1983, 4, 45–49. [Google Scholar] [CrossRef]
- Krasinski, K.; Holzman, R.S.; Hanna, B.; Greco, M.A.; Graff, M.; Bhogal, M. Nosocomial fungal infection during hospital renovation. Infect. Control Hosp. Epidemiol. 1985, 6, 278–282. [Google Scholar] [CrossRef]
- Garner, D.; Machin, K. Investigation and management of an outbreak of mucormycosis in a paediatric oncology unit. J. Hosp. Infect. 2008, 70, 53–59. [Google Scholar] [CrossRef]
- LeSueur, B.W.; Warschaw, K.; Fredrikson, L. Necrotizing cellulitis caused by Apophysomyces elegans at a patch test site. Am. J. Contact Dermat. 2002, 13, 140–142. [Google Scholar] [CrossRef]
- Fréalle, E.; Rocchi, S.; Bacus, M.; Bachelet, H.; Pasquesoone, L.; Tavernier, B.; Mathieu, D.; Millon, L.; Jeanne, M. Real-time polymerase chain reaction detection of Lichtheimia species in bandages associated with cutaneous mucormycosis in burn patients. J. Hosp. Infect. 2018, 99, 68–74. [Google Scholar] [CrossRef]
- Mullens, J.E.; Leers, W.D.; Smith, G.W. Phycomycosis involving the intestine and anterior abdominal wall: A case report. Ann. Surg. 1970, 171, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Sehulster, L.M. Healthcare laundry and textiles in the United States: Review and commentary on contemporary infection prevention issues. Infect. Control Hosp. Epidemiol. 2015, 36, 1073–1088. [Google Scholar] [CrossRef] [PubMed]
- Sundermann, A.J.; Clancy, C.J.; Pasculle, A.W.; Liu, G.; Cumbie, R.B.; Driscoll, E.; Ayres, A.; Donahue, L.; Pergam, S.A.; Abbo, L.; et al. How clean is the linen at my hospital? The Mucorales on unclean linen discovery study of large United States transplant and cancer centers. Clin. Infect. Dis. 2019, 68, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Voss, A.; Donnelly, J.P.; de Pauw, B.E.; Meis, J.F. Wooden sticks as the source of a pseudoepidemic of infection with Rhizopus microsporus var. rhizopodiformis among immunocompromised patients. J. Clin. Microbiol. 1997, 35, 2422–2423. [Google Scholar] [PubMed]
- Leeming, J.G.; Moss, H.A.; Elliott, T.S.J. Risk of tongue depressors to the immunocompromised. Lancet 1996, 348, 889. [Google Scholar] [CrossRef]
- Harper, J.J.; Coulter, C.; Lye, G.R.; Nimmo, G.R. Rhizopus and tongue depressors. Lancet 1996, 348, 1250. [Google Scholar] [CrossRef]
- Davari, H.R.; Malekhossini, S.A.; Salahi, H.A.; Bahador, A.; Saberifirozi, M.; Geramizadeh, B.; Lahsaee, S.M.; Khosravi, M.B.; Imanieh, M.H.; Bagheri, M.H. Outcome of mucormycosis in liver transplantation: Four cases and a review of literature. Exp. Clin. Transpl. 2003, 1, 147–152. [Google Scholar]
- Minz, M.; Sharma, A.; Kashyap, R.; Udgiri, N.; Heer, M.; Kumar, V.; Vaiphei, K. Isolated renal allograft arterial mucormycosis: An extremely rare complication. Nephrol. Dial. Transpl. 2003, 18, 1034–1035. [Google Scholar] [CrossRef]
- Marcό del Pont, J.; De Cicco, L.; Gallo, G.; Llera, J.; De Santibanez, E.; D’Agostino, D. Hepatic arterial thrombosis due to Mucor species in a child following orthotopic liver transplantation. Transpl. Infect. Dis. 2000, 2, 33–35. [Google Scholar] [CrossRef]
- Mitwalli, A.; Hassan Malik, G.; l-Wakeel, J.; Abu Aisha, H.; l-Mohaya, S.; l-Jaser, A.; Assaf, H.; l Gamal, H. Mucormycosis of the graft in a renal transplant recipient. Nephrol. Dial. Transpl. 1994, 9, 718–720. [Google Scholar] [CrossRef]
- Chkhotua, A.; Yussim, A.; Tovar, A.; Weinberger, M.; Sobolev, V.; Bar-Nathan, N.; Shaharabani, E.; Shapira, Z.; Mor, E. Mucormycosis of the renal allograft: Case report and review of the literature. Transpl. Int. 2001, 14, 438–441. [Google Scholar] [CrossRef] [PubMed]
- American Society of Heating, Refrigerating, and Air-Conditioning Engineers (ASHRAE). The ASHRAE Handbook. Available online: https://www.ashrae.org/technical-resources/ashrae-handbook (accessed on 30 May 2019).
- Chang, C.C.; Athan, E.; Morrissey, C.O.; Slavin, M.A. Preventing invasive fungal infection during hospital building works. Intern. Med. J. 2008, 38, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Weems, J.J., Jr.; Davis, B.J.; Tablan, O.C.; Kaufman, L.; Martone, W.J. Construction activity: An independent risk factor for invasive Aspergillosis and zygomycosis in patients with hematologic malignancy. Infect. Control Hosp. Epidemiol. 1987, 8, 71–75. [Google Scholar] [CrossRef]
- Weber, S.F.; Peacock, J.E., Jr.; Do, K.A.; Cruz, J.M.; Powell, B.L.; Capizzi, R.L. Interaction of granulocytopenia and construction activity as risk factors for nosocomial invasive filamentous fungal disease in patients with hematologic disorders. Infect. Control Hosp. Epidemiol. 1990, 11, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, H.; Rutala, W.A.; Sickbert-Bennett, E.E.; Weber, D.J. Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clin. Infect. Dis. 2015, 61, 433–444. [Google Scholar] [CrossRef]
- Hopkins, R.J.; Rothman, M.; Fiore, A.; Goldblum, S.E. Cerebral mucormycosis associated with intravenous drug use: Three case reports and review. Clin. Infect. Dis. 1994, 19, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.W.; Smith, J.M.; Hink, E.M.; Durairaj, V.D. Increased incidence of rhino-orbital-cerebral mucormycosis after Colorado flooding. Ophthalmic Plast. Reconstr. Surg. 2017, 33, S148–S151. [Google Scholar] [CrossRef]
- Skiada, A.; Petrikkos, G. Cutaneous mucormycosis. Skinmed 2013, 11, 155–159. [Google Scholar]
- Lee, S.C.; Billmyre, R.B.; Li, A.; Carson, S.; Sykes, S.M.; Huh, E.Y.; Mieczkowski, P.; Ko, D.C.; Cuomo, C.A.; Heitman, J. Analysis of a food-borne fungal pathogen outbreak: Virulence and genome of a Mucor circinelloides isolate from yogurt. mBio 2014, 5, e01390-14. [Google Scholar] [CrossRef]
- Lazar, S.P.; Lukaszewicz, J.M.; Persad, K.A.; Reinhardt, J.F. Rhinocerebral Mucor circinelloides infection in immunocompromised patient following yogurt ingestion. Del. Med. J. 2014, 86, 245–248. [Google Scholar]
- Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Division of Foodborne, Waterborne, and Environmental Diseases. Targeted Environmental Investigation Checklist for Outbreaks of Invasive Infections Caused by Environmental Fungi. Available online: https://www.cdc.gov/fungal/outbreaks/health-professionals.html (accessed on 30 May 2019).
- Healthcare Laundry Accreditation Council. Accreditation Standards for Processing Reusable Textiles for Use in Healthcare Facilities. Available online: https://www.hlacnet.org/standards-documents (accessed on 30 May 2019).
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L. Health care infection control practices advisory committee. 2007 guideline for isolation precautions: Preventing transmission of infectious agents in health care settings. Am. J. Infect. Control 2007, 35, S65–S164. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.J.; Daveson, K.; Slavin, M.A.; van Hal, S.J.; Sorrell, T.C.; Lee, A.; Marriott, D.J.; Chapman, B.; Halliday, C.L.; Hajkowicz, K.; et al. Mucormycosis in Australia: Contemporary epidemiology and outcomes. Clin. Microbiol. Infect. 2016, 22, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Sun, X. Characteristics of pulmonary mucormycosis and predictive risk factors for the outcome. Infection 2018, 46, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Samarei, R.; Gharebaghi, N.; Zayer, S. Evaluation of 30 cases of mucormycosis at a university hospital in Iran. Mycoses 2017, 60, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Pana, Z.D.; Seidel, D.; Skiada, A.; Groll, A.H.; Petrikkos, G.; Cornely, O.A.; Roilides, E. Invasive mucormycosis in children: An epidemiologic study in European and non-European countries based on two registries. BMC Infect. Dis. 2016, 16, 667. [Google Scholar] [CrossRef]
- Singh, N.; Aguado, J.M.; Bonatti, H.; Forrest, G.; Gupta, K.L.; Safdar, N.; John, G.T.; Pursell, K.J.; Muñoz, P.; Patel, R.; et al. Zygomycosis in solid organ transplant recipients: A prospective, matched case-control study to assess risks for disease and outcome. J. Infect. Dis. 2009, 200, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Lien, M.Y.; Chou, C.H.; Lin, C.C.; Bai, L.Y.; Chiu, C.F.; Yeh, S.P.; Ho, M.W. Epidemiology and risk factors for invasive fungal infections during induction chemotherapy for newly diagnosed acute myeloid leukemia: A retrospective cohort study. PLoS ONE 2018, 13, e0197851. [Google Scholar] [CrossRef] [PubMed]
- Wattier, R.L.; Dvorak, C.C.; Hoffman, J.A.; Brozovich, A.A.; Bin-Hussain, I.; Groll, A.H.; Castagnola, E.; Knapp, K.M.; Zaoutis, T.E.; Gustafsson, B.; et al. A prospective, international cohort study of invasive mold infections in children. J. Pediatr. Infect. Dis. Soc. 2015, 4, 313–322. [Google Scholar] [CrossRef]
- American Industrial Hygiene Association (AIHA). Mold Resource Center. Available online: https://www.aiha.org/publications-and-resources/TopicsofInterest/Hazards/Pages/Mold.aspx (accessed on 30 May 2019).
- American Industrial Hygiene Association. Recognition, Evaluation, and Control of Indoor Mold; Prezant, B., Weekes, D.M., Miller, J.D., Eds.; American Industrial Hygiene Association: Fairfax, VA, USA, 2008. [Google Scholar]
- Garcia-Hermoso, D.; Criscuolo, A.; Lee, S.C.; Legrand, M.; Chaouat, M.; Denis, B.; Lafaurie, M.; Rouveau, M.; Soler, C.; Schaal, J.V.; et al. Outbreak of invasive wound mucormycosis in a burn unit due to multiple strains of Mucor circinelloides f. circinelloides resolved by whole-genome sequencing. mBio 2018, 9, e00573-18. [Google Scholar] [CrossRef]
| Proven Invasive Mucormycosis: Presence confirmed by pathology and/or culture of sterile material. | |
| Microscopic evidence | Histopathologic, cytopathologic, or direct microscopic examination of a specimen obtained by needle aspiration or biopsy in which characteristic hyphae are seen accompanied by evidence of associated tissue damage/vascular invasion. Mucormycete-specific microscopic findings may include:
|
| Culture evidence | Recovery of a mold by culture of a specimen obtained by a sterile procedure from a normally sterile and clinically or radiologically abnormal site consistent with an infectious disease process. Pathogenic mucormycete genera include but are not limited to: Mucor, Rhizopus, Rhizomucor, Cunninghamella, Apophysomyces, Lichtheimia, Cokeromyces, Saksenaea. |
| Probable Invasive Mucormycosis: Probable disease requires a host factor, a clinical criterion, and a mycological criterion, in the absence of confirmed non-mucormycete mold (e.g., Aspergillus). | |
| Host factors |
|
| Clinical criteria | |
| Lower respiratory tract disease | Presence of one of the following three signs on CT:
|
| Tracheobronchitis | Tracheobronchial ulceration, nodule, pseudomembrane, plaque, or eschar seen on bronchoscopic analysis |
| Sinonasal infection | Imaging showing sinusitis plus at least one of the following three signs:
|
| CNS infection | One of the following two signs:
|
| Mycological criteria | Mold in sputum, bronchoalveolar lavage fluid, bronchial brush, or sinus aspirate samples indicated by one of the following:
|
| Possible Invasive Mucormycosis: Possible disease requires a host factor and a clinical criterion from Probable Invasive Mucormycosis above. | |
| Exposure Reported as Likely Cause of Infection | Number of Infections and Site(s) | Publication Year | Reference |
|---|---|---|---|
| Non-Sterile Products | |||
| Bandages, patches, tape, and adhesives | |||
| Elastic adhesive bandages | >20 cutaneous | 1978–1980 | [44,45,46,47,48,49,50] |
| Bandages | 5 wounds; 3 necrotizing fasciitis | 2005, 2011 | [51,52] |
| Tape used to secure endotracheal tube | 2 cutaneous | 1998, 2002 | [53,54] |
| Karaya gum adhesive used to secure ostomy bag | 2 cutaneous infections of stomas | 2006 | [40] |
| Adhesive used to secure heart monitor and temperature probe in preterm infants | 2 cutaneous | 1997 | [55,56] |
| Nitroglycerin patches applied at home | 1 cutaneous | 2003 | [57] |
| Fabric under a cast | 1 cutaneous | 1993 | [58] |
| Linen | |||
| Contamination of linen and linen delivery bins | 5 cutaneous | 2014 | [17] |
| Contamination of hospital laundry carts | 4 cutaneous | 2016 | [59] |
| Poor ventilation, high humidity, and dust at company supplying hospital linen | 3 pulmonary, 2 cutaneous, 1 pulmonary and cutaneous | 2016 | [18] |
| Wooden tongue depressors | |||
| Wooden tongue depressors used to prepare oral medications given through nasogastric catheter | 5 gastric | 2004 | [60] |
| Wooden tongue depressors used as splints | 4 cutaneous | 1996 | [61] |
| Medications, supplements, and food | |||
| Probiotic powdered supplement | 1 gastrointestinal | 2014 | [43] |
| Probiotic oral supplement | 1 hepatic | 1996 | [62] |
| Allopurinol tablets | 17 intestinal | 2009 | [63] |
| Procedures, Devices, and Organs | |||
| Catheters and tubes | |||
| Permanent bladder catheter | 1 bladder | 2004 | [64] |
| Periumbilical cutaneous catheter and chest tube | 1 cutaneous; 1 chest wall and pulmonary | 1998 | [65] |
| Orogastric tube | 1 gastrointestinal | 2004 | [66] |
| Catheter for continuous ambulatory peritoneal dialysis | 4 peritonitis | 1989, 1991, 2003, 2006 | [67,68,69,70] |
| Dental procedures | |||
| Unspecified dental procedure | 2 rhino-orbital; 1 rhinocerebral | 2006, 2009 | [71,72] |
| Tooth extraction | 1 mandible, 1 rhinocerebral, 1 maxilla, 1 jaw | 1997, 2001, 2006, 2018 | [73,74,75,76] |
| Cardiac surgery | |||
| Explantation of left ventricular assist device | 1 aortic | 2008 | [77] |
| Implantation of prosthetic valves | 3 prosthetic valves | 1972, 1987, 1999 | [78,79,80] |
| Catheterization and bypass surgery | 1 sternotomy wound | 1994 | [81] |
| Insulin pumps and finger sticks | |||
| Insulin infusion pump insertion | 1 cutaneous | 1989 | [82] |
| Daily insulin needle punctures | 2 cutaneous | 2004, 2017 | [83,84] |
| Finger sticks for blood glucose self-monitoring | 1 disseminated (initially cutaneous) | 2005 | [85] |
| Medical devices | |||
| Deficiencies in sterilization of screws used in ligament reconstruction | 3 bone | 2016 | [86] |
| Organ transplant | |||
| Kidneys donated by near-drowning victim after motor-vehicle crash | 2 renal | 2010 | [41] |
| Environmental Sources: Air, Dust, Water, Soil | |||
| Air filtration and intakes | |||
| Air filter insufficient to trap spores | 4 mycotic endocarditis, including one with Mucor sp. | 1992 | [87] |
| Air intake close to the ground in outdoor yard | 3 sinus | 1993 | [88] |
| Negative pressure room | |||
| Use of negative-pressure isolation room for transplant surgery patients | 1 disseminated, 1 cutaneous, 1 pulmonary | 2015 | [42] |
| Construction | |||
| Inadequate barriers during renovation | 2 pulmonary | 1985 | [89] |
| Leaks and water damage | |||
| Shower leak that led to mold growth on wall in linen closet near patient rooms | 1 brain and 1 cutaneous | 2008 | [90] |
| Plants | |||
| Petal, stem, and leaf applied to skin for allergy test | 1 cutaneous | 2002 | [91] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartnett, K.P.; Jackson, B.R.; Perkins, K.M.; Glowicz, J.; Kerins, J.L.; Black, S.R.; Lockhart, S.R.; Christensen, B.E.; Beer, K.D. A Guide to Investigating Suspected Outbreaks of Mucormycosis in Healthcare. J. Fungi 2019, 5, 69. https://doi.org/10.3390/jof5030069
Hartnett KP, Jackson BR, Perkins KM, Glowicz J, Kerins JL, Black SR, Lockhart SR, Christensen BE, Beer KD. A Guide to Investigating Suspected Outbreaks of Mucormycosis in Healthcare. Journal of Fungi. 2019; 5(3):69. https://doi.org/10.3390/jof5030069
Chicago/Turabian StyleHartnett, Kathleen P., Brendan R. Jackson, Kiran M. Perkins, Janet Glowicz, Janna L. Kerins, Stephanie R. Black, Shawn R. Lockhart, Bryan E. Christensen, and Karlyn D. Beer. 2019. "A Guide to Investigating Suspected Outbreaks of Mucormycosis in Healthcare" Journal of Fungi 5, no. 3: 69. https://doi.org/10.3390/jof5030069
APA StyleHartnett, K. P., Jackson, B. R., Perkins, K. M., Glowicz, J., Kerins, J. L., Black, S. R., Lockhart, S. R., Christensen, B. E., & Beer, K. D. (2019). A Guide to Investigating Suspected Outbreaks of Mucormycosis in Healthcare. Journal of Fungi, 5(3), 69. https://doi.org/10.3390/jof5030069





