Recent Findings in Onychomycosis and Their Application for Appropriate Treatment
Abstract
1. Introduction
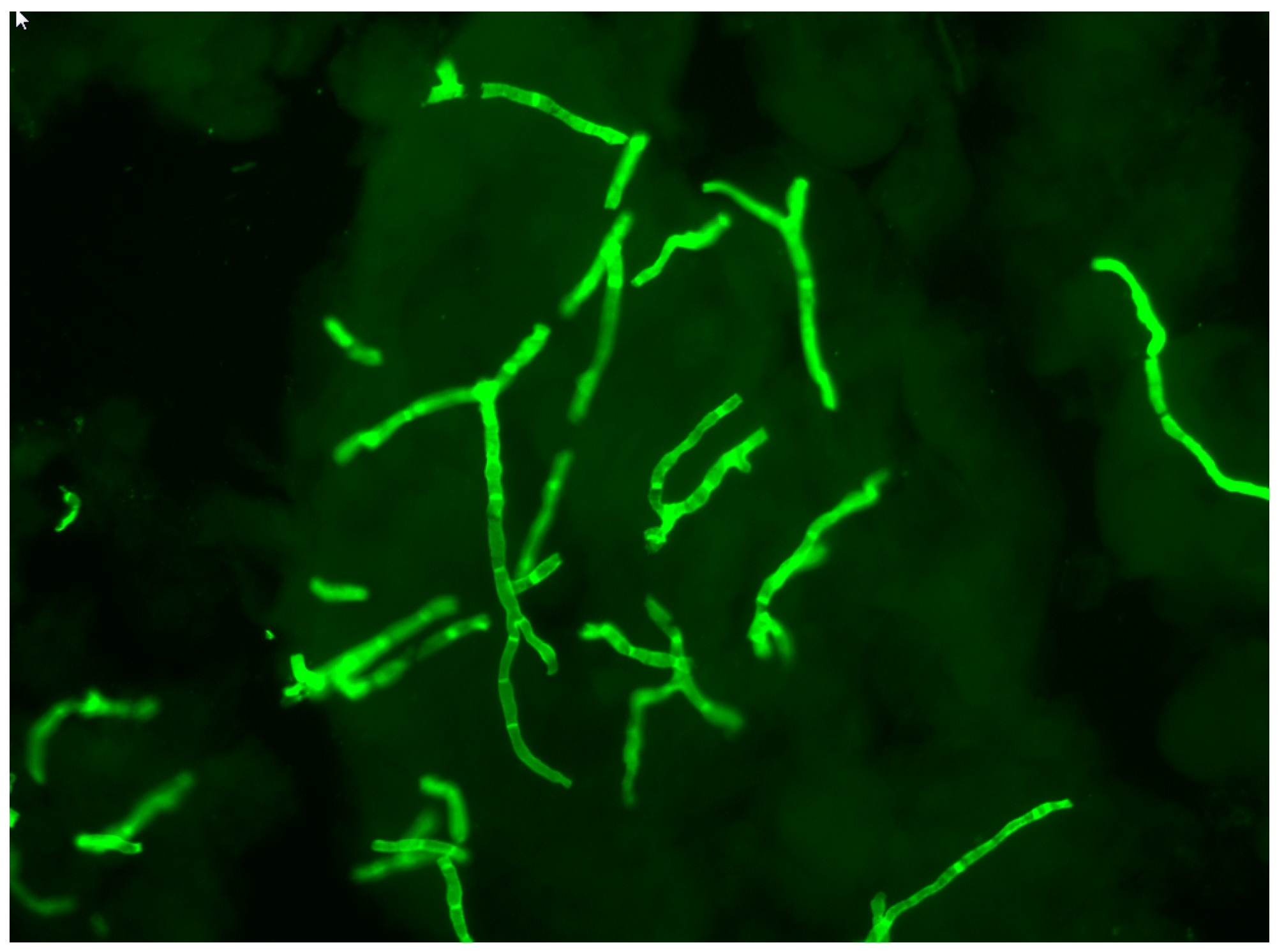
2. Direct Mycological Examination of Nail Samples
3. Fungal Cultures from Nail Samples
4. Identification of Fungi In Situ in Nail Samples
5. Mechanisms of Nail Invasion
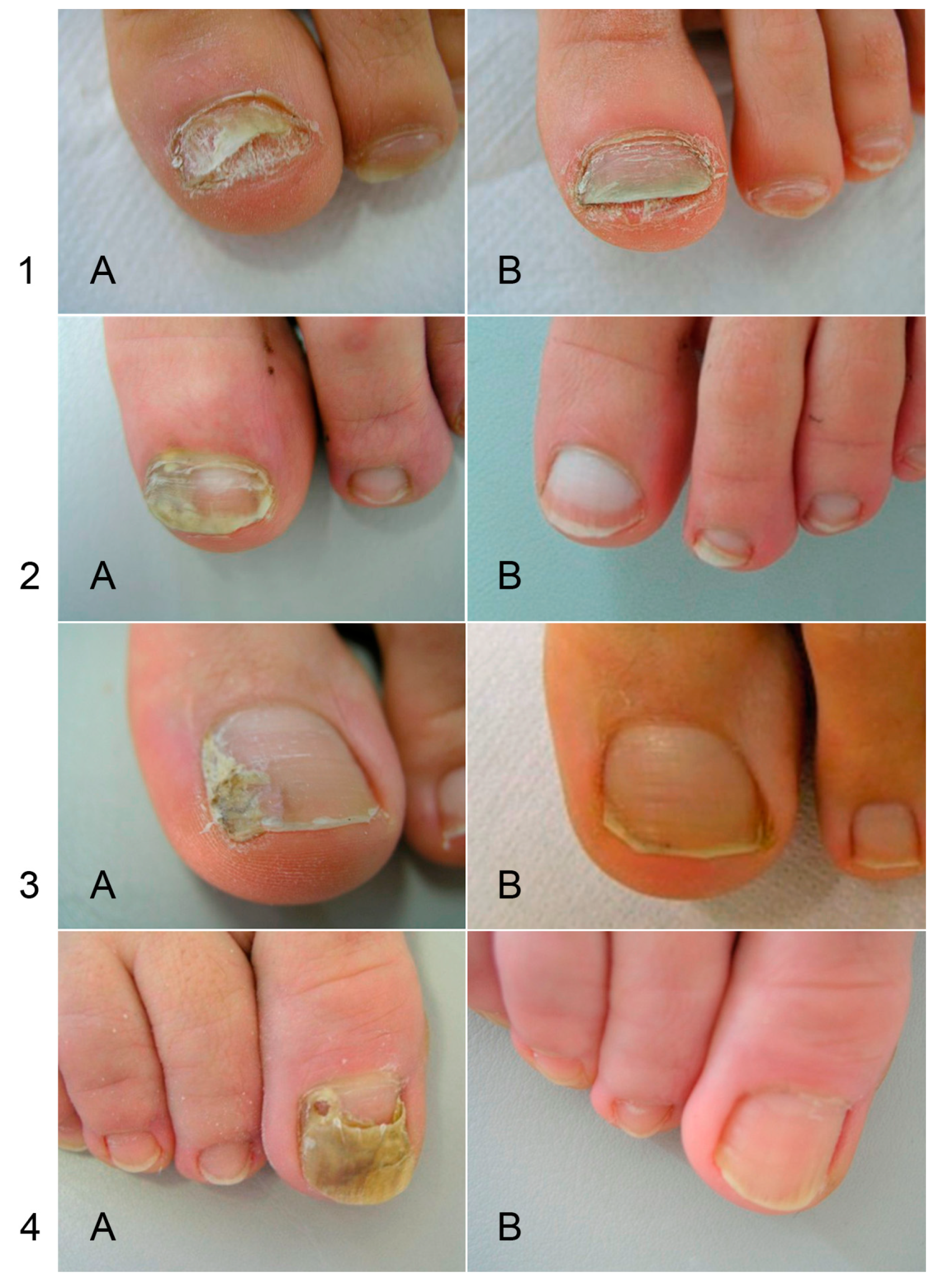
6. Resistance of Onychomycoses to Standard Treatment
6.1. Antifungal Susceptibility Testing
6.2. Resistance of Dermatophytes to Standard Treatments
6.3. Insensitivity of NDM Onychomycosis to Standard Treatments Used for T. unguium
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Baran, R.; Hay, R.; Haneke, E.; Tosti, A. Onychomycosis, the Current Approach to Diagnosis and Therapy, 2nd ed.; Taylor and Francis: Abington, UK, 2006; pp. 1–145. [Google Scholar]
- Monod, M.; Baudraz-Rosselet, F.; Ramelet, A.A.; Frenk, E. Direct mycological examination in dermatology: A comparison of different methods. Dermatologica 1989, 179, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Monheit, J.E.; Cowan, D.F.; Moore, D.G. Rapid detection of fungi in tissues using calcofluor white and fluorescence microscopy. Arch. Pathol. Lab. Med. 1984, 108, 616–618. [Google Scholar] [PubMed]
- Holländer, H.; Keilig, W.; Bauer, J.; Rothemund, E. A reliable fluorescent stain for fungi in tissue sections and clinical specimens. Mycopathologia 1984, 88, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Gip, L.; Abelin, J. Differential staining of fungi in clinical specimens using fluorescent whitening agent (Blankophor). Mykosen 1987, 30, 21–24. [Google Scholar] [CrossRef] [PubMed]
- English, M.P. Nails and fungi. Br. J. Dermatol. 1976, 94, 697–701. [Google Scholar] [CrossRef]
- Brillowska-Dabrowska, A.; Saunte, D.M.; Arendrup, M.C. Five-hour diagnosis of dermatophyte nail infections with specific detection of Trichophyton rubrum. J. Clin. Microbiol. 2007, 45, 1200–1204. [Google Scholar] [CrossRef]
- Brillowska-Dabrowska, A.; Nielsen, S.S.; Nielsen, H.V.; Arendrup, M.C. Optimized 5-hour multiplex PCR test for the detection of tinea unguium: Performance in a routine PCR laboratory. Med. Mycol. 2010, 48, 828–831. [Google Scholar] [CrossRef]
- Brasch, J.; Beck-Jendroschek, V.; Gläser, R. Fast and sensitive detection of Trichophyton rubrum in superficial tinea and onychomycosis by use of a direct polymerase chain reaction assay. Mycoses 2011, 54, e313–e317. [Google Scholar] [CrossRef]
- Monod, M.; Bontems, O.; Zaugg, C.; Léchenne, B.; Fratti, M.; Panizzon, R. Fast and reliable PCR/sequencing/RFLP assay for identification of fungi in onychomycoses. J. Med. Microbiol. 2006, 55, 1211–1216. [Google Scholar] [CrossRef]
- Verrier, J.; Pronina, M.; Peter, C.; Bontems, O.; Fratti, M.; Salamin, K.; Schürch, S.; Gindro, K.; Wolfender, J.L.; Harshman, K.; et al. Identification of infectious agents in onychomycoses by PCR-terminal restriction fragment length polymorphism. J. Clin. Microbiol. 2012, 50, 553–561. [Google Scholar] [CrossRef]
- Méhul, B.; Gu, Z.; Jomard, A.; Laffet, G.; Feuilhade, M.; Monod, M. Sub6 (Tri r 2), an onychomycosis marker revealed by proteomics analysis of Trichophyton rubrum secreted proteins in patient nail samples. J. Investig. Dermatol. 2016, 136, 331–333. [Google Scholar] [CrossRef]
- Méhul, B.; de Coi, N.; Grundt, P.; Genette, A.; Voegel, J.J.; Monod, M. Detection of Trichophyton rubrum and Trichophyton interdigitale in onychomycosis using monoclonal antibodies against Sub6 (Tri r 2). Mycoses 2018. [Google Scholar] [CrossRef] [PubMed]
- Noriki, S.; Ishida, H. Production of an anti-dermatophyte monoclonal antibody and its application: Immunochromatographic detection of dermatophytes. Med. Mycol. 2016, 54, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Miyoshi, H.; Takeda, K.; Saruwatari, H.; Kubo, H.; Sakaguchi, I.; Iwata, M.; Uchida, Y.; Tada, K.; Miyamoto, M.; et al. Evaluation of a newly-developed immunochromatography strip test for diagnosing dermatophytosis. Int. J. Dermatol. 2012, 51, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Tsunemi, Y.; Takehara, K.; Miura, Y.; Nakagami, G.; Sanada, H.; Kawashima, M. Screening for tinea unguium by Dermatophyte Test Strip. Br. J. Dermatol. 2014, 170, 328–331. [Google Scholar] [CrossRef]
- Tsunemi, Y.; Hiruma, M. Clinical study of Dermatophyte Test Strip; an immunochromatographic method; to detect tinea unguium dermatophytes. J. Dermatol. 2016, 43, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Wakamoto, H.; Miyamoto, M. Development of a new dermatophyte-detection device using immunochromatography. J. Med. Diagn. Methods 2016, 5, 1–9. [Google Scholar] [CrossRef]
- Verrier, J.; Bontems, O.; Baudraz-Rosselet, F.; Monod, M. Oral terbinafine and itraconazole treatments against dermatophytes appear not to favor the establishment of Fusarium spp. in nail. Dermatology 2014, 228, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Summerbell, R.C.; Cooper, E.; Bunn, U.; Jamieson, F.; Gupta, A.K. Onychomycosis: A critical study of techniques and criteria for confirming the etiologic significance of nondermatophytes. Med. Mycol. 2005, 43, 39–59. [Google Scholar] [CrossRef]
- Verrier, J.; Monod, M. Diagnosis of dermatophytosis using molecular biology. Mycopathologia 2017, 182, 193–202. [Google Scholar] [CrossRef]
- Scherer, W.P.; McCreary, J.P.; Hayes, W.W. The diagnosis of onychomycosis in a geriatric population: A study of 450 cases in South Florida. J. Am. Podiatr. Med. Assoc. 2001, 91, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Summerbell, R.C.; Kane, J.; Krajden, S. Onychomycosis; tinea pedis and tinea manuum caused by non-dermatophytic filamentous fungi. Mycoses 1989, 32, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Giddey, K.; Monod, M.; Barblan, J.; Potts, A.; Waridel, P.; Zaugg, C.; Quadroni, M. Comprehensive analysis of proteins secreted by Trichophyton rubrum and Trichophyton violaceum under in vitro conditions. J. Proteome Res. 2007, 6, 3081–3092. [Google Scholar] [CrossRef] [PubMed]
- Sriranganadane, D.; Waridel, P.; Salamin, K.; Feuermann, M.; Mignon, B.; Staib, P.; Neuhaus, J.M.; Quadroni, M.; Monod, M. Identification of novel secreted proteases during extracellular proteolysis by dermatophytes at acidic pH. Proteomics 2011, 11, 4422–4433. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.D.; De Coi, N.; Feuermann, M.; Schmid-Siegert, E.; Băguţ, E.T.; Mignon, B.; Waridel, P.; Peter, C.; Pradervand, S.; Pagni, M.; et al. RNA sequencing-based genome reannotation of the dermatophyte Arthroderma benhamiae and characterization of its secretome and whole gene expression profile during infection. mSystems 2016, 1, e00036-16. [Google Scholar] [CrossRef] [PubMed]
- Woodfolk, J.A.; Wheatley, L.M.; Piyasena, R.V.; Benjamin, D.C.; Platts-Mills, T.A. Trichophyton antigens associated with IgE antibodies and delayed type hypersensitivity. Sequence homology to two families of serine proteinases. J. Biol. Chem. 1998, 273, 29489–29496. [Google Scholar] [CrossRef] [PubMed]
- Ward, G.W., Jr.; Karlsson, G.; Rose, G.; Platts-Mills, T.A. Trichophyton asthma: Sensitisation of bronchi and upper airways to dermatophyte antigen. Lancet 1989, 1, 859–862. [Google Scholar] [CrossRef]
- Matsuoka, H.; Niimi, A.; Matsumoto, H.; Ueda, T.; Takemura, M.; Yamaguchi, M.; Jinnai, M.; Otsuka, K.; Oguma, T.; Takeda, T.; et al. Specific IgE response to trichophyton and asthma severity. Chest 2009, 135, 898–903. [Google Scholar] [CrossRef]
- Ward, G.W., Jr.; Woodfolk, J.A.; Hayden, M.L.; Jackson, S.; Platts-Mills, T.A. Treatment of late-onset asthma with fluconazole. J. Allergy Clin. Immunol. 1999, 104, 541–546. [Google Scholar] [CrossRef]
- Hürlimann, A.; Fäh, J. Asthma; rhinitis and dermatitis triggered by fungal infection: Therapeutic effects of terbinafine. Dermatology 2001, 202, 330–332. [Google Scholar]
- Jessup, C.J.; Wamer, J.; Isham, N.; Hasan, I.; Ghannoum, M.A. Antifungal susceptibility testing of dermatophytes: Establishing a medium for inducing conidial growth and evaluation of susceptibility of clinical isolates. J. Clin. Microbiol. 2000, 38, 341–344. [Google Scholar] [PubMed]
- National Committee for Clinical Laboratory Standards. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 2nd ed.; Approved standard M38-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Chin, B.; Knight, S. Growth of Trichophyton mentagrophytes and Trichophyton rubrum in increased carbon dioxide tensions. J. Gen. Microbiol. 1957, 16, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Monod, M. Production of Trichophyton rubrum microspores in large quantities and its application to evaluate amorolfine/azole compound interactions in vitro. Mycoses 2017, 60, 581–586. [Google Scholar] [CrossRef]
- Takami, T.; Fang, Y.; Zhou, X.; Jaiseng, W.; Ma, Y.; Kuno, T. A genetic and pharmacological analysis of isoprenoid pathway by LC-MS/MS in fission yeast. PLoS ONE 2012, 7, e49004. [Google Scholar] [CrossRef]
- Odds, F.C.; Brown, A.J.; Gow, N.A. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Reiss, E.; Shadomy, H.J.; Lyon, G.M. Fundamental Medical Mycology; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 75–105. [Google Scholar]
- Yamada, T.; Maeda, M.; Alshahni, M.M.; Tanaka, R.; Yaguchi, T.; Bontems, O.; Salamin, K.; Fratti, M.; Monod, M. Terbinafine resistance of Trichophyton clinical isolates caused by specific point mutations in the squalene epoxidase gene. Antimicrob. Agents Chemother. 2017, 61, e00115-17. [Google Scholar] [CrossRef] [PubMed]
- Schøsler, L.; Andersen, L.K.; Arendrup, M.C.; Sommerlund, M. Recurrent terbinafine resistant Trichophyton rubrum infection in a child with congenital ichthyosis. Pediatr. Dermatol. 2018, 35, 259–260. [Google Scholar] [CrossRef]
- Digby, S.S.; Hald, M.; Arendrup, M.C.; Hjort, S.V.; Kofoed, K. Darier disease complicated by terbinafine-resistant Trichophyton rubrum: A case report. Acta Derm. Venereol. 2017, 97, 139–140. [Google Scholar] [CrossRef]
- Singh, A.; Masih, A.; Khurana, A.; Singh, P.K.; Gupta, M.; Hagen, F.; Meis, J.F.; Chowdhary, A. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi; India harbouring mutations in the squalene epoxidase gene. Mycoses 2018, 61, 477–484. [Google Scholar] [CrossRef]
- Osborne, C.S.; Leitner, I.; Favre, B.; Ryder, N.S. Amino acid substitution in Trichophyton rubrum squalene epoxidase associated with resistance to terbinafine. Antimicrob. Agents Chemother. 2005, 49, 2840–2844. [Google Scholar] [CrossRef]
- Osborne, C.S.; Leitner, I.; Hofbauer, B.; Fielding, C.A.; Favre, B.; Ryder, N.S. Biological, biochemical, and molecular characterization of a new clinical Trichophyton rubrum isolate resistant to terbinafine. Antimicrob. Agents Chemother. 2006, 50, 2234–2236. [Google Scholar] [CrossRef]
- Sanglard, D.; Kuchler, K.; Ischer, F.; Pagani, J.L.; Monod, M.; Bille, J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 1995, 39, 2378–2386. [Google Scholar] [CrossRef]
- Morschhäuser, J. Regulation of multidrug resistance in pathogenic fungi. Fungal Genet. Biol. 2010, 47, 94–106. [Google Scholar] [CrossRef]
- Cervelatti, E.P.; Fachin, A.L.; Ferreira-Nozawa, M.S.; Martinez-Rossi, N.M. Molecular cloning and characterization of a novel ABC transporter gene in the human pathogen Trichophyton rubrum. Med. Mycol. 2006, 44, 141–147. [Google Scholar] [CrossRef]
- Fachin, A.L.; Ferreira-Nozawa, M.S.; Maccheroni, W., Jr.; Martinez-Rossi, N.M. Role of the ABC transporter TruMDR2 in terbinafine; 4-nitroquinoline N-oxide and ethidium bromide susceptibility in Trichophyton rubrum. J. Med. Microbiol. 2006, 55, 1093–1099. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Shankarnarayan, S.A.; Dogra, S.; Shaw, D.; Mushtaq, K.; Paul, R.A.; Narang, T.; Chakrabarti, A. Mutation in the squalene epoxidase gene of Trichophyton interdigitale and Trichophyton rubrum associated with allylamine resistance. Antimicrob. Agents Chemother. 2018, 62, e02522-17. [Google Scholar] [CrossRef]
- Baudraz-Rosselet, F.; Ruffieux, C.; Lurati, M.; Bontems, O.; Monod, M. Onychomycosis insensitive to systemic terbinafine and azole treatments reveals non-dermatophyte moulds as infectious agents. Dermatology 2010, 220, 164–168. [Google Scholar] [CrossRef]
- Lurati, M.; Baudraz-Rosselet, F.; Vernez, M.; Spring, P.; Bontems, O.; Fratti, M.; Monod, M. Efficacious treatment of non-dermatophyte mould onychomycosis with topical amphotericin B. Dermatology 2011, 223, 289–292. [Google Scholar] [CrossRef]
- Tosti, A.; Piraccini, B.M.; Lorenzi, S. Onychomycosis caused by nondermatophytic molds: Clinical features and response to treatment of 59 cases. J. Am. Acad. Dermatol. 2000, 42, 217–224. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gregurek-Novak, T.; Konnikov, N.; Lynde, C.W.; Hofstader, S.; Summerbell, R.C. Itraconazole and terbinafine treatment of some nondermatophyte molds causing onychomycosis of the toes and a review of the literature. J. Cutan. Med. Surg. 2001, 5, 206–210. [Google Scholar] [CrossRef]
- Tosti, A.; Piraccini, B.M.; Lorenzi, S.; Iorizzo, M. Treatment of nondermatophyte mold and Candida onychomycosis. Dermatol. Clin. 2003, 21, 491–497. [Google Scholar] [CrossRef]
- Brasch, J.; Köppl, G. Persisting onychomycosis caused by Fusarium solani in an immunocompetent patient. Mycoses 2009, 52, 285–286. [Google Scholar] [CrossRef]
- Baginski, M.; Czub, J. Amphotericin B and its new derivatives—Mode of action. Curr. Drug Metab. 2009, 10, 459–469. [Google Scholar] [CrossRef]
- Selleslag, D. A case of fusariosis in an immunocompromised patient successfully treated with liposomal amphotericin B. Acta Biomed. 2006, 77 (Suppl. 2), 32–35. [Google Scholar]
- Wisselink, G.J.; van Zanten, E.; Kooistra-Smid, A.M. Trapped in keratin; a comparison of dermatophyte detection in nail; skin and hair samples directly from clinical samples using culture and real-time PCR. J. Microbiol. Methods 2011, 85, 62–66. [Google Scholar] [CrossRef]
| Methods | Target | Usage § | Time | Specificity # | References |
|---|---|---|---|---|---|
| Direct Mycological Examination | Hyphae and spores in nails | PP, RL | 2–5 min | Fungi (Trichophyton, NDMs, Yeasts) | [2,3,4,5] |
| Cultures | Growing fungi | RL | 7–14 days | Tru, Tint NDMs if repeated isolations Yeasts if repeated isolations | [6] |
| PCR with Specific Primers | Nail fungal DNA | RL | 5 h | Tru | [7,8,9] |
| PCR with Pan-Fungal Primers /Sequencing | Nail fungal DNA | RL | 24 h | Trichophyton NDMs Yeasts | [10,11] |
| Mass Spectrometry (MRM) | Sub6 | RD | Tru, Tint | [12] | |
| Western Blotting | Sub6 | RD | 24 h | Trichophyton | [13] |
| ELISA | Sub6 | ND | 24 h | Trichophyton | [13] |
| Strip Tests | Polysaccharides | PP | 15 min | Trichophyton | [14,15,16,17,18] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monod, M.; Méhul, B. Recent Findings in Onychomycosis and Their Application for Appropriate Treatment. J. Fungi 2019, 5, 20. https://doi.org/10.3390/jof5010020
Monod M, Méhul B. Recent Findings in Onychomycosis and Their Application for Appropriate Treatment. Journal of Fungi. 2019; 5(1):20. https://doi.org/10.3390/jof5010020
Chicago/Turabian StyleMonod, Michel, and Bruno Méhul. 2019. "Recent Findings in Onychomycosis and Their Application for Appropriate Treatment" Journal of Fungi 5, no. 1: 20. https://doi.org/10.3390/jof5010020
APA StyleMonod, M., & Méhul, B. (2019). Recent Findings in Onychomycosis and Their Application for Appropriate Treatment. Journal of Fungi, 5(1), 20. https://doi.org/10.3390/jof5010020




