Entomopathogenicity and Biological Attributes of Himalayan Treasured Fungus Ophiocordyceps sinensis (Yarsagumba)
Abstract
:1. Introduction
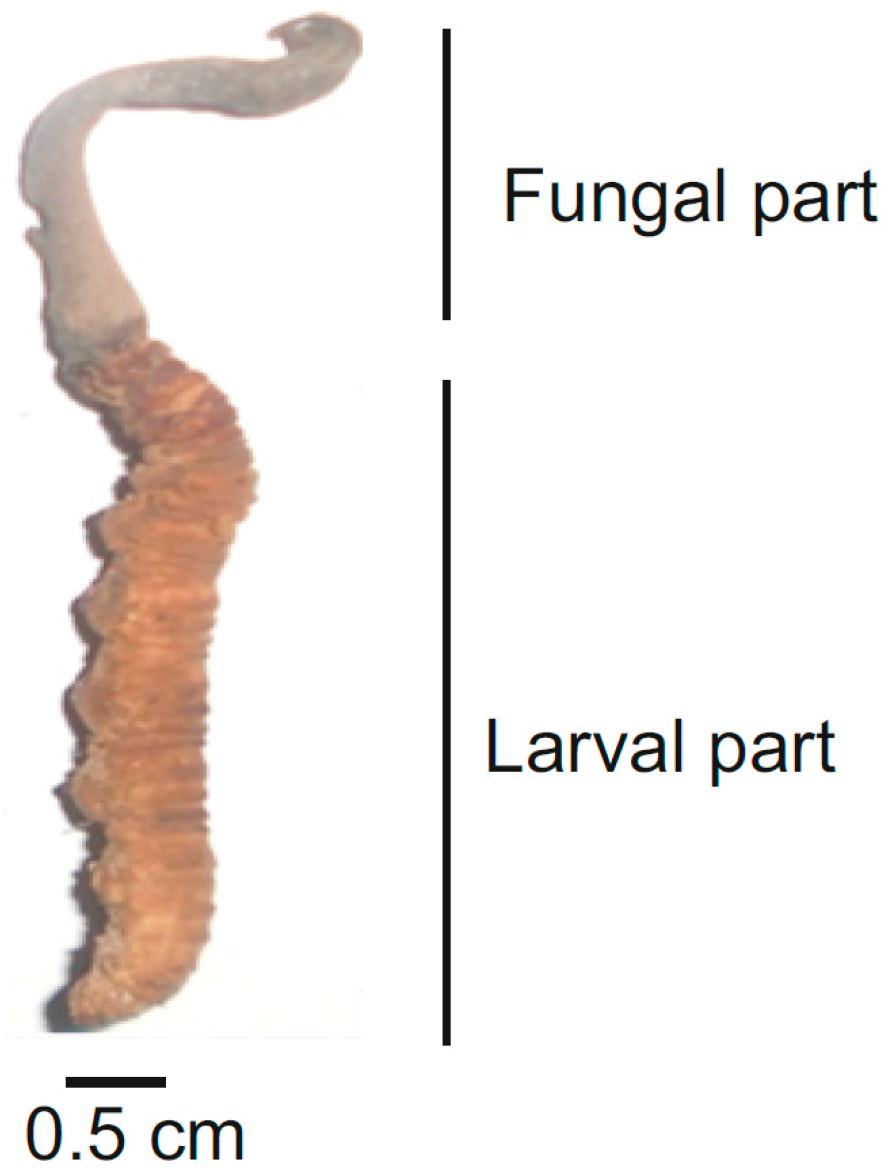
2. Cordyceps Ecology
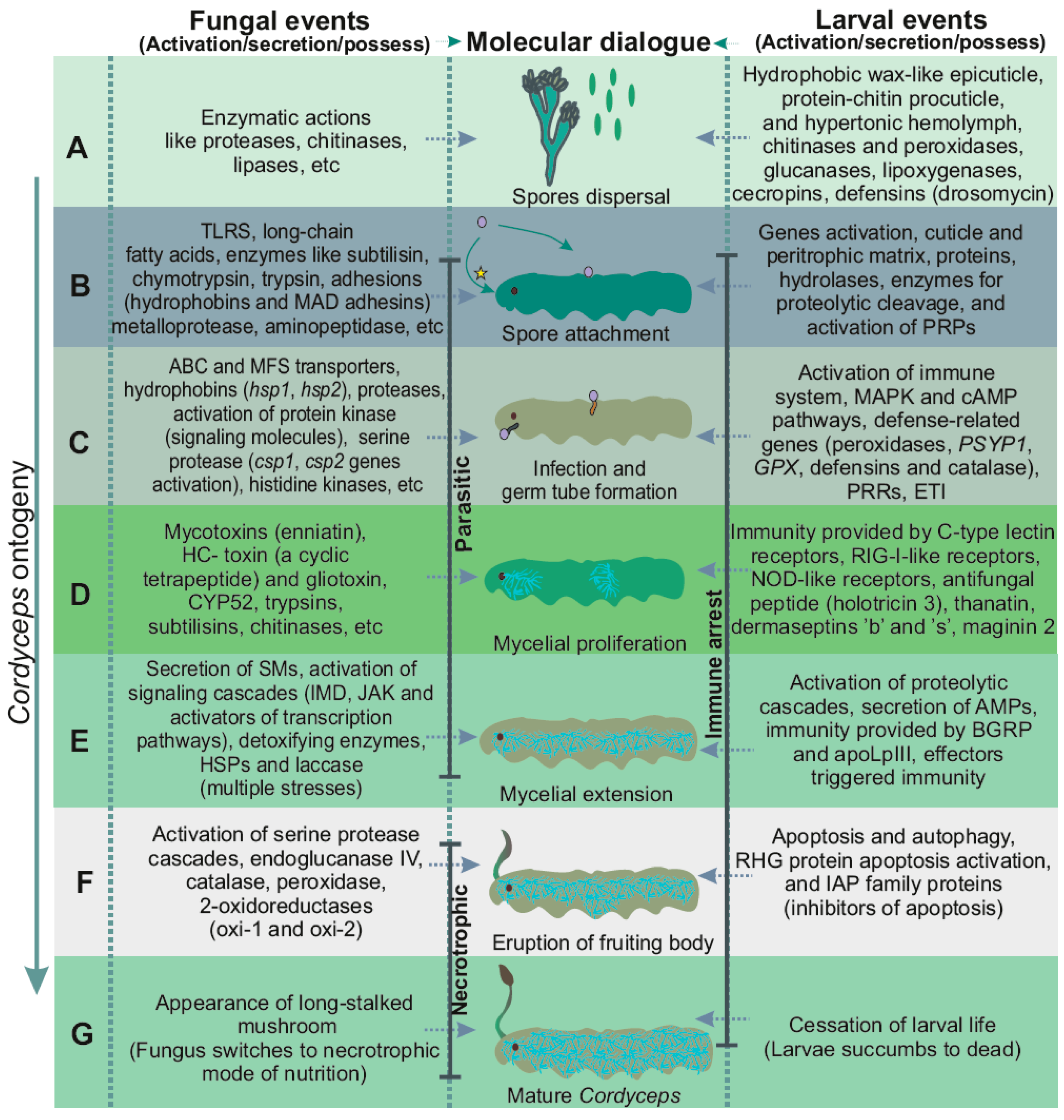
3. Cordyceps Ontogeny
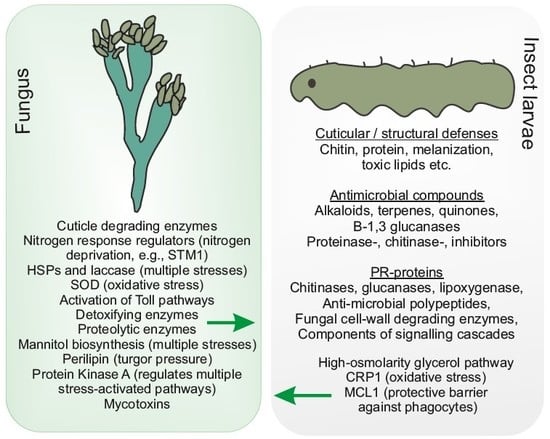
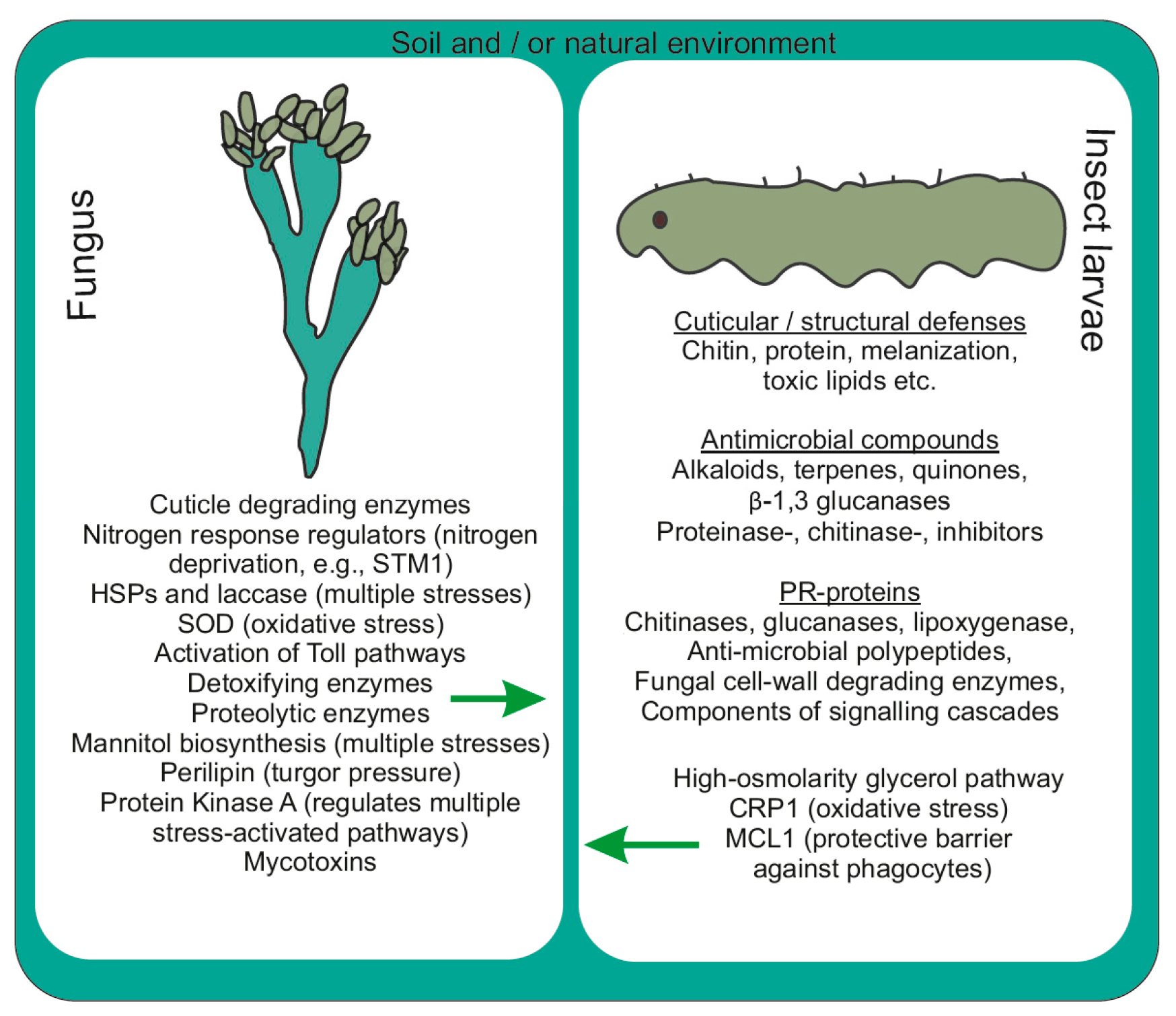
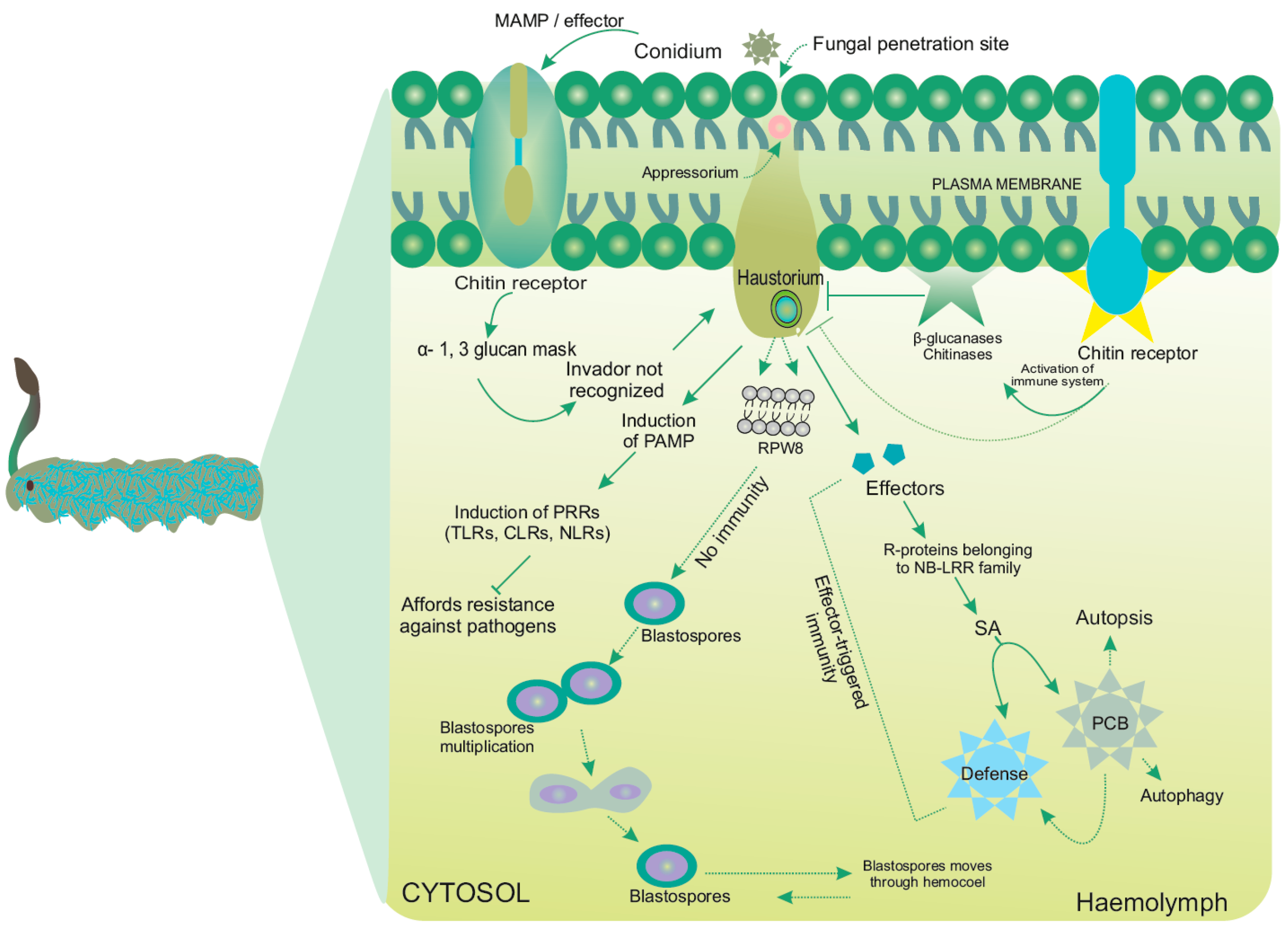
4. Events Incurred during Pathogenicity
4.1. Molecular Modality Displayed by OS
4.2. Antifungal Peptides in Insects: A Component of Defense Munitions
4.3. G-Protein–Coupled Receptors (GPCRs) in Fungus and Insects
4.4. MAP Kinase Cascades: Regulation of Transcription
4.5. Histidine Kinases: Osmosensing and Beyond
5. Enzymes Involved in Fruiting Body Production
6. Genomic Information
7. In Vitro Fungal Culture and Characteristics
8. Secondary Metabolites Repertoire in OS
Chemical Constituents, Bioactive Compounds and Uses of Natural Cordyceps
9. Challenges and Opportunities
10. Concluding Thoughts
11. Summary Points
- With tremendous globalization in agriculture, tracing fungal infectivity and elucidating the exact mechanism of fungal dispersal has gained an increased interest.
- Endowed with chemical and pharmacological properties, drugs of high therapeutic importance can be prepared by cultivating OS.
- Fungus raised on synthetic substrates (either on silkworm or cereal substrates) may prove to be significantly valuable with reduced dependence on biotic and natural resources, thus reducing the escape pressure.
- Fungal infection of agricultural pests may be a breakthrough achievement and may serve as a promising biological control agent.
- MAPKs play vital roles in regulating fungal development, growth, and pathogenicity.
12. Future Issues and Insights
- Anamorphs of OS such as Metarhizium sp. are widely used as biocontrol agents. The introduction of other anamorphs of OS plus other entomopathogenic fungi could also be done to check the spread of agricultural pests. This could prove significantly useful and certainly provides a great revolution in replacing synthetic insecticides.
- Rigorous experimentation on larval cuticle-degrading enzymes plus other enzymes may successfully lead to an improved selection of an OS strain that could effectively be integrated for agricultural pest management.
- Transcriptional responses of insect larvae against fungal infection could provide much-needed information on genes induced during pathogen infection in insect larvae.
- Tremendous innovative technologies to hunt for gene-encoding secondary metabolites and chemical entities discovered through activating their silent and orphan gene clusters involved in secondary biosynthesis could also be exploited.
- A search for the gold-standard mycological media to increase this fungus in controlled conditions seems enigmatic, but is highly desired.
- With its enormous biological and clinical attributes, biotechnological inventions of OS (natural and lab-cultured strains) may prove very promising and deserve further attention.
Acknowledgements
Conflicts of Interest
References
- Winkler, D. Yartsa Gunbu (Cordyceps sinensis) and the fungal commodification of Tibet’s rural economy. Econ. Bot. 2008, 62, 291–305. [Google Scholar] [CrossRef]
- Prasain, J.K. Pharmacological effects of Cordyceps and its bioactive compounds. Stud. Nat. Prod. Chem. 2013, 40, 453. [Google Scholar]
- Sung, G.H.; Hywel-Jones, N.L.; Sung, J.M.; Luangsa-Ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol 2007, 57, 5–59. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xie, J.; Wang, L.Y.; Li, S.P. Advanced development in chemical analysis of Cordyceps. J. Pharm. Biomed. Anal. 2014, 87, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Stone, R. Last stand for the body snatcher of the Himalayas? Science 2008, 322, 1182. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Yao, Y.J. Host insect species of Ophiocordyceps sinensis: A review. Zookeys 2011, 127, 43–59. [Google Scholar]
- Chen, S.; Yin, D.; Li, L.; Zha, X.; Shuen, J.; Zhama, C. Resources and distribution of Cordyceps sinensis in Naqu Tibet. Zhong Yao Cai 2000, 23, 673–675. [Google Scholar] [PubMed]
- de Faria, M.R.; Wraight, S.P. Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control. 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Gao, Q.; Jin, K.; Ying, S.H.; Zhang, Y.; Xiao, G.; Shang, Y.; Duan, Z.; Hu, X.; Xie, X.Q.; Zhou, G. Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and M. acridum. PLoS Genet. 2011, 7, e1001264. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Hu, B.; Xu, F.; Zhang, W.; Zhou, H.; Qu, L.H. Genetic variation of Cordyceps sinensis, a fruit-body-producing entomopathogenic species from different geographical regions in China. FEMS Microbiol. Lett. 2004, 230, 153–158. [Google Scholar] [CrossRef]
- Kinjo, N.; Zang, M. Morphological and phylogenetic studies on Cordyceps sinensis distributed in southwestern China. Mycoscience 2001, 42, 567–574. [Google Scholar] [CrossRef]
- Boesi, A. The Dbyar Rtswa Dgun’Bu (Cordyceps sinensis Berk.): An important trade item for the Tibetan population of the Lithang County, Sichuan Province, China. Tibet J. 2003, 283, 29–42. [Google Scholar]
- Zhang, Y.; Li, E.; Wang, C.; Li, Y.; Liu, X. Ophiocordyceps sinensis, the flagship fungus of China: Terminology, life strategy and ecology. Mycology 2012, 3, 2–10. [Google Scholar]
- Baral, B.; Shrestha, B.; da Silva, J.A.T. A review of Chinese Cordyceps with special reference to Nepal, focusing on conservation. Environ. Exp. Biol. 2015, 13, 61–73. [Google Scholar]
- Humber, R.A. Fungal pathogens and parasites of insects. In Applied Microbial Systematics; Springer: Dordrecht, The Netherlands, 2000; pp. 203–230. [Google Scholar]
- Hywel-Jones, N.L. Multiples of eight in Cordyceps ascospores. Mycol. Res. 2002, 106, 2–3. [Google Scholar] [CrossRef]
- Muslim, N.; Rahman, H. A possible new record of Cordyceps species from Ginseng Camp, Maliau Basin, Sabah, Malaysia. J. Trop. Biol. Conserv. 2010, 6, 39–41. [Google Scholar]
- Zheng, P.; Xia, Y.; Xiao, G.; Xiong, C.; Hu, X.; Zhang, S.; Zheng, H.; Huang, Y.; Zhou, Y.; Wang, S.; et al. Genome sequence of the insect pathogenic fungus Cordyceps militaris, a valued traditional Chinese medicine. Genome Biol. 2011, 12, R116. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan, T.I.; Franco-Molano, A.E.; Kepler, R.M.; Spatafora, J.W.; Tabima, J.; Vasco-Palacios, A.M.; Restrepo, S. Five new species of entomopathogenic fungi from the Amazon and evolution of neotropical Ophiocordyceps. Fungal Biol. 2015, 119, 901–916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W. Genetic variation of Cordyceps militaris and its allies based on phylogenetic analysis of rDNA ITS sequence data. Fungal Divers. 2008, 31, 147–155. [Google Scholar]
- Zhang, Y.; Xu, L.; Zhang, S.; Liu, X.; An, Z.; Wang, M.; Guo, Y.; Sung, G.; Hywel-Jones, N.; Sung, J.; et al. Genetic diversity of Ophiocordyceps sinensis, a medicinal fungus endemic to the Tibetan Plateau: Implications for its evolution and conservation. BMC Evol. Biol. 2009, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Peng, Q.; Li, S.; Chen, H.; Sun, H.; Zhang, G. Detection of Ophiocordyceps sinensis in the roots of plants in alpine meadows by nested-touchdown polymerase chain reaction. Fungal Biol. 2014, 118, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Tai Lu, C.; Chia Chun, T.; Ching Lung, M. A preliminary study on the biology of the “insect herb”, Hepialus armoricanus Oberthur. Acta Entomol. Sin. 1973, 2, 11. [Google Scholar]
- Hu, X.; Zhang, Y.; Xiao, G.; Zheng, P.; Xia, Y.; Zhang, X.; St Leger, R.J.; Liu, X.; Wang, C. Genome survey uncovers the secrets of sex and lifestyle in caterpillar fungus. Chin. Sci. Bull. 2013, 58, 2846–2854. [Google Scholar] [CrossRef]
- Ng, T.B.; Wang, H.X. Pharmacological actions of Cordyceps, a prized folk medicine. J. Pharm. Pharmacol. 2005, 57, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hsiang, T.; Yang, R.H.; Hu, X.D.; Wang, K.; Wang, W.J.; Wang, X.L.; Jiao, L.; Yao, Y.J. Comparison of different sequencing and assembly strategies for a repeat-rich fungal genome, Ophiocordyceps sinensis. J. Microbiol. Methods 2016, 128, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang Yan, D.; Shen, F.; Dong, D. Studies on Hepialid larvae for being infected by Chinese “insect herb” fungus (Cordyceps sinensis). Zool. Res. 1989, 10, 227–231. [Google Scholar]
- Charnley, A.K. Fungal pathogens of insects: Cuticle degrading enzymes and toxins. Adv. Bot. Res. 2003, 40, 241–321. [Google Scholar]
- Shrestha, U.B.; Bawa, K.S. Impact of climate change on potential distribution of Chinese caterpillar fungus (Ophiocordyceps sinensis) in Nepal Himalaya. PLoS ONE 2014, 9, e106405. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.R. Cordyceps: A traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry 2008, 69, 1469–1495. [Google Scholar] [CrossRef] [PubMed]
- Leger, R.J.S.; May, B.; Allee, L.L.; Frank, D.C.; Staples, R.C.; Roberts, D.W. Genetic differences in allozymes and in formation of infection structures among isolates of the entomopathogenic fungus Metarhizium anisopliae. J. Invertebr. Pathol. 1992, 60, 89–101. [Google Scholar] [CrossRef]
- Chen, Q.T.; Xiao, S.R.; Shi, Z.Y. Paecilomyces sinensis sp. nov. and its connection with Cordyceps sinensis. Acta Mycol. Sin. 1984, 3, 24–28. [Google Scholar]
- Liu, X.J.; Guo, Y.L.; Yu, Y.X.; Zeng, W. Isolation and identification of the anamorphic state of Cordyceps sinensis (Berk.) Sacc. Acta Mycol. Sin. 1989, 8, 35–40. [Google Scholar]
- Chen, S.J.; Yin, D.H.; Li, L.; Zhou, X.L.; Za, X. Studies on anamorph of Cordyceps sinensis (Berk) from Naqu Tibet. Zhongguo Zhong Yao Za Zhi 2001, 26, 453–454. [Google Scholar] [PubMed]
- Liu, Z.Q.; Lin, S.; Baker, P.J.; Wu, L.F.; Wang, X.R.; Wu, H.; Xu, F.; Wang, H.Y.; Brathwaite, M.E.; Zheng, Y.G. Transcriptome sequencing and analysis of the entomopathogenic fungus Hirsutella sinensis isolated from Ophiocordyceps sinensis. BMC Genom. 2015, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Boucias, D.G.; Pendland, J.C.; Latge, J.P. Nonspecific factors involved in attachment of entomopathogenic deuteromycetes to host insect cuticle. Appl. Environ. Microbiol. 1988, 54, 1795–1805. [Google Scholar] [PubMed]
- Butt, T.M.; Greenfield, B.P.J.; Greig, C.; Maffeis, T.G.G.; Taylor, J.W.D.; Piasecka, J.; Dudley, E.; Abdulla, A.; Dubovskiy, I.M.; Garrido-Jurado, I.; et al. Metarhizium anisopliae pathogenesis of mosquito larvae: A verdict of accidental death. PLoS ONE 2013, 8, e81686. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Leger, R.J.S. The MAD1 adhesin of Metarhizium anisopliae links adhesion with blastospore production and virulence to insects, and the MAD2 adhesin enables attachment to plants. Eukaryot. Cell. 2007, 6, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.U.; Yongjie, Z.; Guohua, X.; Peng, Z.; Yongliang, X.I.A. Genome survey uncovers the secrets of sex and lifestyle in caterpillar. Chin. Sci. Bull. 2013, 58, 2846–2854. [Google Scholar]
- Hoffmann, J.A. Innate immunity of insects. Curr. Opin. Immunol. 1995, 7, 4–10. [Google Scholar] [CrossRef]
- Boguś, M.I.; Kędra, E.; Bania, J.; Szczepanik, M.; Czygier, M.; Jabłoński, P.; Pasztaleniec, A.; Samborski, J.; Mazgajska, J.; Polanowski, A. Different defense strategies of Dendrolimus pini, Galleria mellonella, and Calliphora vicina against fungal infection. J. Insect Physiol. 2007, 53, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.E.; Tobin, P.C. Introduced pathogens follow the invasion front of a spreading alien host. J. Anim. Ecol. 2011, 80, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, J.L. Oomycetes: Lagenidium giganteum. J. Am. Mosq. Control. Assoc. 2007, 23, 50–57. [Google Scholar] [CrossRef]
- Patwardhan, A.; Gandhe, R.; Ghole, V.; Mourya, D. Larvicidal activity of the fungus Aphanomyces (oomycetes: Saprolegniales) against Culex quinquefasciatus. J. Commun. Dis. 2005, 37, 269–274. [Google Scholar] [PubMed]
- Souza-Neto, J.A.; Sim, S.; Dimopoulos, G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc. Natl. Acad. Sci. USA 2009, 106, 17841–17846. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Ramirez, J.L.; Dimopoulos, G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008, 4, e1000098. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.W.; Kokoza, V.; Bian, G.; Cheon, H.-M.; Kim, Y.J.; Raikhel, A.S. REL1, a homologue of Drosophila dorsal, regulates toll antifungal immune pathway in the female mosquito Aedes aegypti. J. Biol. Chem. 2005, 280, 16499–16507. [Google Scholar] [CrossRef] [PubMed]
- Parsa, S.; Ortiz, V.; Vega, F.E. Establishing fungal entomopathogens as endophytes: Towards endophytic biological control. J. Vis. Exp. 2013, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Turrà, D.; Segorbe, D.; Di Pietro, A. Protein kinases in plant-pathogenic fungi: Conserved regulators of infection. Annu. Rev. Phytopathol. 2014, 52, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Wichadakul, D.; Kobmoo, N.; Ingsriswang, S.; Tangphatsornruang, S.; Chantasingh, D.; Luangsa-Ard, J.J.; Eurwilaichitr, L. Insights from the genome of Ophiocordyceps polyrhachis-furcata to pathogenicity and host specificity in insect fungi. BMC Genom. 2015, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhong, X.; Kan, X.; Gu, L.; Sun, H.; Zhang, G.; Liu, X. De novo transcriptome analysis of Thitarodes jiachaensis before and after infection by the caterpillar fungus, Ophiocordyceps sinensis. Gene 2016, 580, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Baral, B.; Kovalchuk, A.; Asiegbu, F.O. Genome organisation and expression profiling of ABC protein-encoding genes in Heterobasidion annosum s.l. complex. Fungal Biol. 2016, 120, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.; Hui, S.; Choi, J.; Asiegbu, F.O.; Valkonen, J.P.T.; Lee, Y. Secret lifestyles of Neurospora crassa. Sci. Rep. 2014, 4, 5135. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yu, J.; Wu, W.; Zhang, G. Molecular characterization and gene expression of apolipophorin III from the ghost moth, Thitarodes pui (Lepidoptera, Hepialidae). Arch. Insect Biochem. Physiol. 2012, 80, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fahrbach, S.E.; Nambu, J.R.; Schwartz, L.M. Programmed cell death in insects. In Insect Molecular Biology and Biochemistry; Gilbert, L.I., Ed.; Academic: Waltham, MA, USA, 2012. [Google Scholar]
- Leger, R.J.S.; Goettel, M.; Roberts, D.W.; Staples, R.C. Prepenetration events during infection of host cuticle by Metarhizium anisopliae. J. Invertebr. Pathol. 1991, 58, 168–179. [Google Scholar] [CrossRef]
- Leger, R.J.S.; Charnley, A.K.; Cooper, R.M. Cuticle-degrading enzymes of entomopathogenic fungi: Synthesis in culture on cuticle. J. Invertebr. Pathol. 1986, 48, 85–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, M. Cloning, expression, and characterization of two novel cuticle-degrading serine proteases from the entomopathogenic fungus Cordyceps sinensis. Res. Microbiol. 2008, 159, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Lovett, B.; Leger, R.J.S. Stress is the rule rather than the exception for Metarhizium. Curr. Genet. 2014, 61, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ren, S.; Huang, Z.; Wu, J. Purification of enzymes related to host penetration and pathogenesis from entomopathogenic fungi. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2010; pp. 15–22. [Google Scholar]
- Wang, B.; Won, S.; Yu, Z.; Su, C. Free radical scavenging and apoptotic effects of Cordyceps sinensis fractionated by supercritical carbon dioxide. Food Chem. Toxicol. 2005, 43, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Ying, S.H.; Zheng, P.; Wang, Z.L.; Zhang, S.; Xie, X.Q.; Shang, Y.; St Leger, R.J.; Zhao, G.P.; Wang, C.; Feng, M.G. Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci. Rep. 2012, 2, 483. [Google Scholar] [CrossRef] [PubMed]
- Leger, R.J.S.; Butt, T.M.; Goettel, M.S.; Staples, R.C.; Roberts, D.W. Production in vitro of appressoria by the entomopathogenic fungus Metarhizium anisopliae. Exp. Mycol. 1989, 13, 274–288. [Google Scholar] [CrossRef]
- Inbar, M.; Mayer, R.T.; Doostdar, H. Induced activity of pathogenesis related (PR) proteins in aphid galls. Symbiosis-Rehovot 2003, 34, 293–300. [Google Scholar]
- Ganz, T.; Selsted, M.E.; Lehrer, R.I. Defensins. Eur. J. Haematol. 1990, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Segal, G.P.; Lehrer, R.I.; Selsted, M.E. In vitro effect of phagocyte cationic peptides on Coccidioides immitis. J. Infect. Dis. 1985, 151, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Stciner, H.; Hultmark, D.; Engstrom, A.; Bennich, H.I.; Barman, H.G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 1981, 292, 246–248. [Google Scholar] [CrossRef]
- De Lucca, A.J.; Walsh, T.J. Antifungal peptides: Novel therapeutic compounds against emerging pathogens. Antimicrob. Agents Chemother. 1999, 43, 1–11. [Google Scholar] [PubMed]
- Michaut, L.; Fehlbaum, P.; Moniatte, M.; Van Dorsselaer, A.; Reichhart, J.M.; Bulet, P. Determination of the disulfide array of the first inducible antifungal peptide from insects: Drosomycin from Drosophila melanogaster. FEBS Lett. 1996, 395, 6–10. [Google Scholar] [CrossRef]
- Iijima, R.; Kurata, S.; Natori, S. Purification, characterization, and cDNA cloning of an antifungal protein from the hemolymph of Sarcophaga peregrina (flesh fly) larvae. J. Biol. Chem. 1993, 268, 12055–12061. [Google Scholar] [PubMed]
- Fehlbaum, P.; Bulet, P.; Chernysh, S.; Briand, J.P.; Roussel, J.P.; Letellier, L.; Hetru, C.; Hoffmann, J.A. Structure-activity analysis of thanatin, a 21-residue inducible insect defense peptide with sequence homology to frog skin antimicrobial peptides. Proc. Natl. Acad. Sci. USA 1996, 93, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.V.R.; Yedery, R.D.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Faruck, M.O.; Yusof, F.; Chowdhury, S. An overview of antifungal peptides derived from insect. Peptides 2015, 80, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Hane, J.K.; Lowe, R.G.T.; Solomon, P.S.; Tan, K.C.; Schoch, C.L.; Spatafora, J.W.; Crous, P.W.; Kodira, C.; Birren, B.W.; Galagan, J.E. Dothideomycete–plant interactions illuminated by genome sequencing and EST analysis of the wheat pathogen Stagonospora nodorum. Plant. Cell. 2007, 19, 3347–3368. [Google Scholar] [CrossRef] [PubMed]
- Kämper, J.; Kahmann, R.; Bölker, M.; Ma, L.-J.; Brefort, T.; Saville, B.J.; Banuett, F.; Kronstad, J.W.; Gold, S.E.; Müller, O. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 2006, 444, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; St Leger, R.J. RNA binding proteins mediate the ability of a fungus to adapt to the cold. Environ. Microbiol. 2010, 12, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Sasan, R.K.; Bidochka, M.J. The insect-pathogenic fungus Metarhizium robertsii (Clavicipitaceae) is also an endophyte that stimulates plant root development. Am. J. Bot. 2012, 99, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Keyhani, N.O.; Yu, X.; He, Z.; Luo, Z.; Pei, Y.; Zhang, Y. The MAP kinase Bbslt2 controls growth, conidiation, cell wall integrity, and virulence in the insect pathogenic fungus Beauveria bassiana. Fungal Genet. Biol. 2012, 49, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Erwig, L.P.; Gow, N.A.R. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Mehrabi, R.; Xu, J.R. Mitogen-activated protein kinase pathways and fungal pathogenesis. Eukaryot. Cell. 2007, 6, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Gustin, M.C.; Albertyn, J.; Alexander, M.; Davenport, K. MAP kinase pathways in the Yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 1998, 62, 1264–1300. [Google Scholar] [PubMed]
- Ortiz-Urquiza, A.; Keyhani, N.O. Stress response signaling and virulence: Insights from entomopathogenic fungi. Curr. Genet. 2014, 61, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Bushley, K.; Li, Y.; Wang, W.; Wang, X.; Jiao, L. Isolation of the MAT1–1 mating type idiomorph and evidence for selfing in the Chinese medicinal fungus Ophiocordyceps sinensis. Fungal Biol. 2013, 117, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Han, L.; Xia, Y. MaMk1, a FUS3/KSS1-type mitogen-activated protein kinase gene, is required for appressorium formation, and insect cuticle penetration of the entomopathogenic fungus Metarhizium acridum. J. Invertebr. Pathol. 2014, 115, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Jiang, X.; Wang, G.; Luo, Z.; Fan, Y.; Wu, Z.; Pei, Y. Requirement of a mitogen-activated protein kinase for appressorium formation and penetration of insect cuticle by the entomopathogenic fungus Beauveria bassiana. Appl. Environ. Microbiol. 2010, 76, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, J.; Fang, W.; Zhang, J.; Luo, Z.; Zhang, M.; Fan, Y.; Pei, Y. Mitogen-activated protein kinase hog1 in the entomopathogenic fungus Beauveria bassiana regulates environmental stress responses and virulence to insects. Appl. Environ. Microbiol. 2009, 75, 3787–3795. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Pava-Ripoll, M.; Wang, S.; Leger, R.S. Protein kinase A regulates production of virulence determinants by the entomopathogenic fungus, Metarhizium anisopliae. Fungal Genet. Biol. 2009, 46, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Dixon, K.P.; Xu, J.R.; Smirnoff, N.; Talbot, N.J. Independent signaling pathways regulate cellular turgor during hyperosmotic stress and appressorium-mediated plant infection by Magnaporthe grisea. Plant. Cell. 1999, 11, 2045–2058. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, L.; Sánchez, O.; Shiozaki, K.; Aguirre, J. SakA MAP kinase is involved in stress signal transduction, sexual development and spore viability in Aspergillus nidulans. Mol. Microbiol. 2002, 45, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Takano, Y.; Yoshimi, A.; Tanaka, C.; Kikuchi, T.; Okuno, T. Fungicide activity through activation of a fungal signalling pathway. Mol. Microbiol. 2004, 53, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lamm, R.; Pillonel, C.; Lam, S.; Xu, J.R. Osmoregulation and fungicide resistance: The Neurospora crassa os-2 gene encodes a HOG1 mitogen-activated protein kinase homologue. Appl. Environ. Microbiol. 2002, 68, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Selitrennikoff, C.P. Antifungal Proteins. Appl. Environ. Microbiol. 2001, 67, 2883–2894. [Google Scholar] [CrossRef] [PubMed]
- Fargues, J.; Goettel, M.S.; Smits, N.; Ouedraogo, A.; Rougier, M. Effect of temperature on vegetative growth of Beauveria bassiana isolates from different origins. Mycologia 1997, 89, 383–392. [Google Scholar] [CrossRef]
- Smits, N.; Briere, J.F.; Fargues, J. Comparison of non-linear temperature-dependent development rate models applied to in vitro growth of entomopathogenic fungi. Mycol. Res. 2003, 107, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bravo, M.; Garrido-Jurado, I.; Valverde-García, P.; Enkerli, J.; Quesada-Moraga, E. Responses to abiotic environmental stresses among phylloplane and soil isolates of Beauveria bassiana from two holm oak ecosystems. J. Invertebr. Pathol. 2016, 141, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jin, H.; Fu, M.; Shen, J.; Li, Q. Sequence analysis and blue light-influenced expression of histidine kinase gene (CmHK) from fungus Cordyceps militaris. Chin. J. Appl. Environ. Biol. 2014, 1, 50–55. [Google Scholar]
- Zheng, Z.; Qiu, X.; Han, R. Identification of the genes involved in the fruiting body production and cordycepin formation of Cordyceps militaris fungus. Mycobiology 2015, 43, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Guo, M.; Yang, H.; Guo, S.; Dong, C. The blue-light receptor CmWC-1 mediates fruit body development and secondary metabolism in Cordyceps militaris. Appl. Microbiol. Biotechnol. 2016, 100, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Cha, W.S.; Park, N.; Kim, H.W.; Lee, J.H.; Park, J.S.; Park, S.S. Purification and characterization of a novel fibrinolytic enzyme from fruiting bodies of Korean Cordyceps militaris. Bioresour. Technol. 2011, 102, 3279–3285. [Google Scholar] [CrossRef] [PubMed]
- Won, S.Y.; Park, E.H. Anti-inflammatory and related pharmacological activities of cultured mycelia and fruiting bodies of Cordyceps militaris. J. Ethnopharmacol. 2005, 96, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.M.; Choi, Y.S.; Lee, H.K.; Kim, S.H.; Kim, Y.O.; Sung, G.H. Production of fruiting body using cultures of entomopathogenic fungal species. Korean J. Mycol. 1999, 27, 15–19. [Google Scholar]
- Baral, B.; Maharjan, J. In vitro culture of Ophiocordyceps sinensis (Yarsagumba) and their associated endophytic fungi of Nepal Himalaya. Sci. World 2012, 10, 38–42. [Google Scholar] [CrossRef]
- Li, Q.S.; Zeng, W.; Yi, D.H.; Huang, T.F. Studies on the alternation of generations in Cordyceps sinensis. ChungKuoYaoTsaChil 1998, 23, 210–212. [Google Scholar]
- Hamburger, M. Comment on comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. J. Agric. Food Chem. 2007, 55, 7213–7216. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Yao, Y. In vitro evaluation of antioxidant activities of aqueous extracts from natural and cultured mycelia of Cordyceps sinensis. LWT-Food Sci. Technol. 2008, 41, 669–677. [Google Scholar] [CrossRef]
- Au, D.; Wang, L.; Yang, D.; Mok, D.K.; Chan, A.S.; Xu, H. Application of microscopy in authentication of valuable Chinese medicine I- Cordyceps sinensis, its counterfeits, and related products. Microsc. Res. Tech. 2012, 75, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Hywel-Jones, N. Cordyceps myrmecophila-like fungi infecting ants in the leaf litter of tropical forest in Thailand. Mycol. Res. 1996, 100, 613–619. [Google Scholar] [CrossRef]
- Buenz, E.J.; Bauer, B.A.; Osmundson, T.W.; Motley, T.J. The traditional Chinese medicine Cordyceps sinensis and its effects on apoptotic homeostasis. J. Ethnopharmacol. 2005, 96, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.G.L.; Bik-San Lau, C.; Fung, K.P.; Leung, P.C.; Ko, W.H. Effects of Cordyceps sinensis, Cordyceps militaris and their isolated compounds on ion transport in Calu-3 human airway epithelial cells. J. Ethnopharmacol. 2008, 117, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, J.; Qiu, S.; Chen, J.; Zheng, Y. Effects of the exopolysaccharide fraction (EPSF) from a cultivated Cordyceps sinensis on immunocytes of H22 tumor bearing mice. Fitoterapia 2008, 79, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; He, Y.; Zhang, D.; He, Z.; Chen, S.; Tu, Y.; Li, L.; Liu, F.; Zeng, W. Review on natural enemies and diseases in the artificial cultivation of Chinese caterpillar mushroom, Ophiocordyceps sinensis (Ascomycetes). Int. J. Med. Mushrooms 2015, 17, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Liu, T. A comparison of hypoglycemic activity of three species of basidiomycetes rich in vanadium. Biol. Trace Elem. Res. 2009, 127, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, P.; Zhao, D.; Tang, H.; Guo, J. Protective effect of extract of Cordyceps sinensis in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Behav. Brain Funct. 2010, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.H.; Shiao, L.H.; Hsieh, C.; Chang, D.M. A comparison of the chemical composition and bioactive ingredients of the Chinese medicinal mushroom DongChongXiaCao, its counterfeit and mimic, and fermented mycelium of Cordyceps sinensis. Food Chem. 2002, 78, 463–469. [Google Scholar] [CrossRef]
- Lindequist, U.; Niedermeyer, T.H.J.; Jülich, W.D. The pharmacological potential of mushrooms. Evidence-Based Complement. Altern. Med. 2005, 2, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.P.; Kang, P.D.; Kim, K.Y.; Nam, S.H.; Lee, M.Y.; Choi, Y.S.; Kim, N.S.; Kim, H.K.; Lee, K.G.; Humber, R.A. Fruit body formation on silkworm by Cordyceps militaris. Mycobiology 2010, 38, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Holliday, J.; Cleaver, M.; Wasser, S.P. Encyclopedia of Dietary Supplements; Taylor and Francis Publishing: London, UK, 2005; pp. 1–13. [Google Scholar]
- Yan, J.K.; Wang, W.Q.; Wu, J.Y. Recent advances in Cordyceps sinensis polysaccharides: Mycelial fermentation, isolation, structure, and bioactivities: A review. J. Funct. Foods 2014, 6, 33–47. [Google Scholar] [CrossRef]
- Jordan, J.L.; Sullivan, A.M.; Lee, T.D.G. Immune activation by a sterile aqueous extract of Cordyceps sinensis: Mechanism of action. Immunopharmacol. Immunotoxicol. 2008, 30, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.J. An application of Cordyceps sinensis tonic preparation in medicinal wines. J. Chin. Med. Mater. 1993, 16, 41–43. [Google Scholar]
- Halpern, G.M. Cordyceps: China’s healing mushroom. Avery Publishing Group: New York, NY, USA, 1999. [Google Scholar]
- Li, S.P.; Su, Z.R.; Dong, T.T.; Tsim, K.W. The fruiting body and its caterpillar host of Cordyceps sinensis show close resemblance in main constituents and anti-oxidation activity. Phytomedicine 2002, 9, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Bi-Cheng, L.; Al-Assaf, S.; Phillips, G.O.; Phillips, A.O. Cordyceps sinensis decreases TGF-β1 dependent epithelial to mesenchymal transdifferentiation and attenuates renal fibrosis. Food Hydrocoll. 2012, 28, 200–212. [Google Scholar] [CrossRef]
- Chen, S.; Chu, J. NMR and IR studies on the characterization of cordycepin and 2’-deoxyadenosine. Chin. J. Antibiot. 1996, 21, 9–12. [Google Scholar]
- de Sio, F.; Laratta, B.; Giovane, A.; Quagliuolo, L.; Castaldo, D.; Servillo, L. Analysis of free and esterified ergosterol in tomato products. J. Agric. Food Chem. 2000, 48, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, H.; Lv, X.; Wang, Y.; Gao, H.; Wang, M. Protection of chronic renal failure by a polysaccharide from Cordyceps sinensis. Fitoterapia 2010, 81, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Bok, J.W.; Lermer, L.; Chilton, J.; Klingeman, H.G.; Towers, G.H. Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry 1999, 51, 891–898. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, Y.; Bian, Y.; Wong, J.; Ng, T. Hypoglycemic activity of the fungi Cordyceps militaris, Cordyceps sinensis, Tricholoma mongolicum, and Omphalia lapidescens in streptozotocin-induced diabetic rats. Appl. Microbiol. 2006, 72, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Kadota, S.; Shima, T.; Kikuchi, T. Steroidal components of I-TIAM-HONG and Cordyceps sinensis- separation and identification by high-performance liquid chromatography. J. Pharm. Soc. Jpn. 1986, 106, 1092–1097. [Google Scholar]
- Czub, J.; Baginski, M. Comparative molecular dynamics study of lipid membranes containing cholesterol and ergosterol. Biophys. J. 2006, 90, 2368–2382. [Google Scholar] [CrossRef] [PubMed]
- Shiao, M.S.; Wang, Z.N.; Lin, L.J.; Lien, J.Y.; Wang, J.J. Profiles of nucleosides and nitrogen bases in Chinese medicinal fungus Cordyceps sinensis and related species. Bot. Bulll.-Acad. Sin. 1994, 35, 261–267. [Google Scholar]
- Li, S.P.; Li, P.; Dong, T.T.X.; Tsim, K.W.K. Anti-oxidation activity of different types of natural Cordyceps sinensis and cultured Cordyceps mycelia. Phytomedicine 2001, 8, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Shiao, M.S.; Lee, S.S.; Wang, S.Y. Effect of Cordyceps sinensis on the proliferation and differentiation of human leukemic U937 cells. Life Sci. 1997, 60, 2349–2359. [Google Scholar] [CrossRef]
- Kawas, C.; Resnick, S.; Morrison, A.; Brookmeyer, R. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease. Neurology 1997, 48, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Luo, L.; Dressel, W.; Shadier, G.; Krumbiegel, D.; Schmidtke, P.; Zepp, F.; Meyer, C.U. Cordycepin is an immunoregulatory active ingredient of Cordyceps sinensis. Am. J. Chin. Med. 2008, 36, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Canney, S. Cordyceps sinensis animal, vegetable or both? J. Chin. Med. 2006, 80, 43–49. [Google Scholar]
- Wu, D.T.; Meng, L.Z.; Wang, L.Y.; Lv, G.P.; Cheong, K.L.; Hu, D.J.; Guan, J.; Zhao, J.; Li, S.P. Chain conformation and immunomodulatory activity of a hyperbranched polysaccharide from Cordyceps sinensis. Carbohydr. Polym. 2014, 110, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, B.B. Can estrogen keep you smart? Evidence from clinical studies. J. Psychiatry Neurosci. 1999, 24, 315–321. [Google Scholar] [PubMed]
- Li, S.P.; Yang, F.Q.; Tsim, K.W. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J. Pharm. Biomed. Anal. 2006, 41, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Siu, K.M.; Mak, D.H.; Chiu, P.Y.; Poon, M.K.; Du, Y.; Ko, K.M. Pharmacological basis of “Yin-nourishing” and “Yang-invigorating” actions of Cordyceps, a Chinese tonifying herb. Life Sci. 2004, 76, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.K.; Guo, S.X. The structure and histochemistry of sclerotia of Ophiocordyceps sinensis. Mycologia 2008, 100, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Negi, C.S.; Koranga, P.R.; Ghinga, H.S. Yar tsa Gumba (Cordyceps sinensis): A call for its sustainable exploitation. Int. J. Sustain. Dev. World Ecol. 2006, 13, 165–172. [Google Scholar] [CrossRef]
- Wang, S.M.; Lee, L.J.; Lin, W.W.; Chang, C.M. Effects of a water-soluble extract of Cordyceps sinensis on steroidogenesis and capsular morphology of lipid droplets in cultured rat adrenocortical cells. J. Cell. Biochem 1998, 69, 483–489. [Google Scholar] [CrossRef]
- Mizuno, T. Medicinal effects and utilization of Cordyceps (Fr.) Link (Ascomycetes) and Isaria Fr. (mitosporic fungi) Chinese caterpillar fungi. Int. J. Med. Mushrooms 1999, 1, 251–261. [Google Scholar] [CrossRef]
- Zhu, J.S.; Halpern, G.M.; Jones, K. The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis: Part I. J. Altern. Complement. Med. 1998, 4, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.M.; Wang, B.S.; Huang, S.C.; Duh, P.D. Comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. J. Agric. Food Chem. 2006, 54, 3132–3138. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.M.; Tao, H.H.; Feng, B.M. Cordyceamides A and B from the culture liquid of Cordyceps sinensis (Berk.) sacc. Chem. Pharm. Bull. 2009, 57, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.S.; Halpern, G.M.; Jones, K. The scientific rediscovery of a precious ancient Chinese herbal regimen: Cordyceps sinensis: Part II. J. Altern. Complement. Med. 1998, 4, 429–457. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.H.; Kim, J.M.; Chang, U.J.; Suh, H.J. Hypocholesterolemic effect of hot-water extract from mycelia of Cordyceps sinensis. Biol. Pharm. Bull. 2003, 26, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Kiho, T.; Yamane, A.; Hui, J.; Usui, S.; Ukai, S. Polysaccharides in fungi. XXXVI. Hypoglycemic activity of a polysaccharide (CS-F30) from the cultural mycelium of Cordyceps sinensis and its effect on glucose metabolism in mouse liver. Biol. Pharm. Bull. 1996, 19, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.F.; Chen, C.C.; Luo, Y.H.; Huang, R.Y.; Chuang, W.J.; Sheu, C.C.; Lin, Y.S. Cordyceps sinensis mycelium protects mice from group A streptococcal infection. J. Med. Microbiol. 2005, 54, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Konoha, K.; Yamaguchi, Y.; Kagota, S.; Shinozuka, K.; Kunitomo, M. Combined effects of Cordyceps sinensis and methotrexate on hematogenic lung metastasis in mice. Recept. Channels 2003, 9, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Borel, J.F. History of the discovery of cyclosporin and of its early pharmacological development. Wien. Klin. Wochenschr. 2002, 114, 433–437. [Google Scholar] [PubMed]
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baral, B. Entomopathogenicity and Biological Attributes of Himalayan Treasured Fungus Ophiocordyceps sinensis (Yarsagumba). J. Fungi 2017, 3, 4. https://doi.org/10.3390/jof3010004
Baral B. Entomopathogenicity and Biological Attributes of Himalayan Treasured Fungus Ophiocordyceps sinensis (Yarsagumba). Journal of Fungi. 2017; 3(1):4. https://doi.org/10.3390/jof3010004
Chicago/Turabian StyleBaral, Bikash. 2017. "Entomopathogenicity and Biological Attributes of Himalayan Treasured Fungus Ophiocordyceps sinensis (Yarsagumba)" Journal of Fungi 3, no. 1: 4. https://doi.org/10.3390/jof3010004
APA StyleBaral, B. (2017). Entomopathogenicity and Biological Attributes of Himalayan Treasured Fungus Ophiocordyceps sinensis (Yarsagumba). Journal of Fungi, 3(1), 4. https://doi.org/10.3390/jof3010004