Candidemia in a General Hospital in Kuwait: Epidemiology, Species Distribution, Risk Factors, and Antifungal Susceptibility Patterns over a 10-Year Period (2015–2024)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Case Identification and Classification of Candidemia Episodes
2.3. Isolation, Species-Specific Identification, and Antifungal Susceptibility Testing of Yeast Isolates
2.4. Statistical Analyses
3. Results
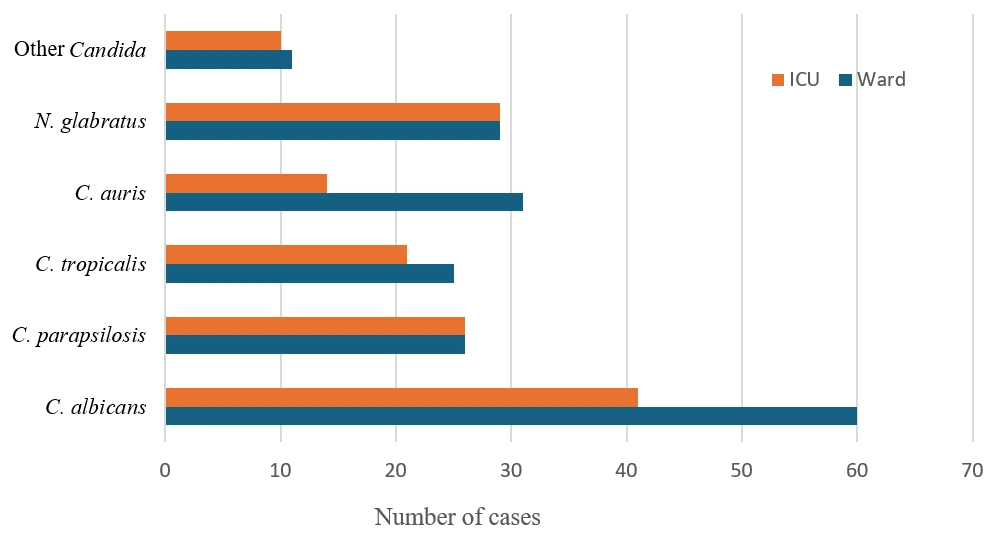
3.1. Demographic and Epidemiological Trends
3.2. Distribution of Candida spp. and Related Species
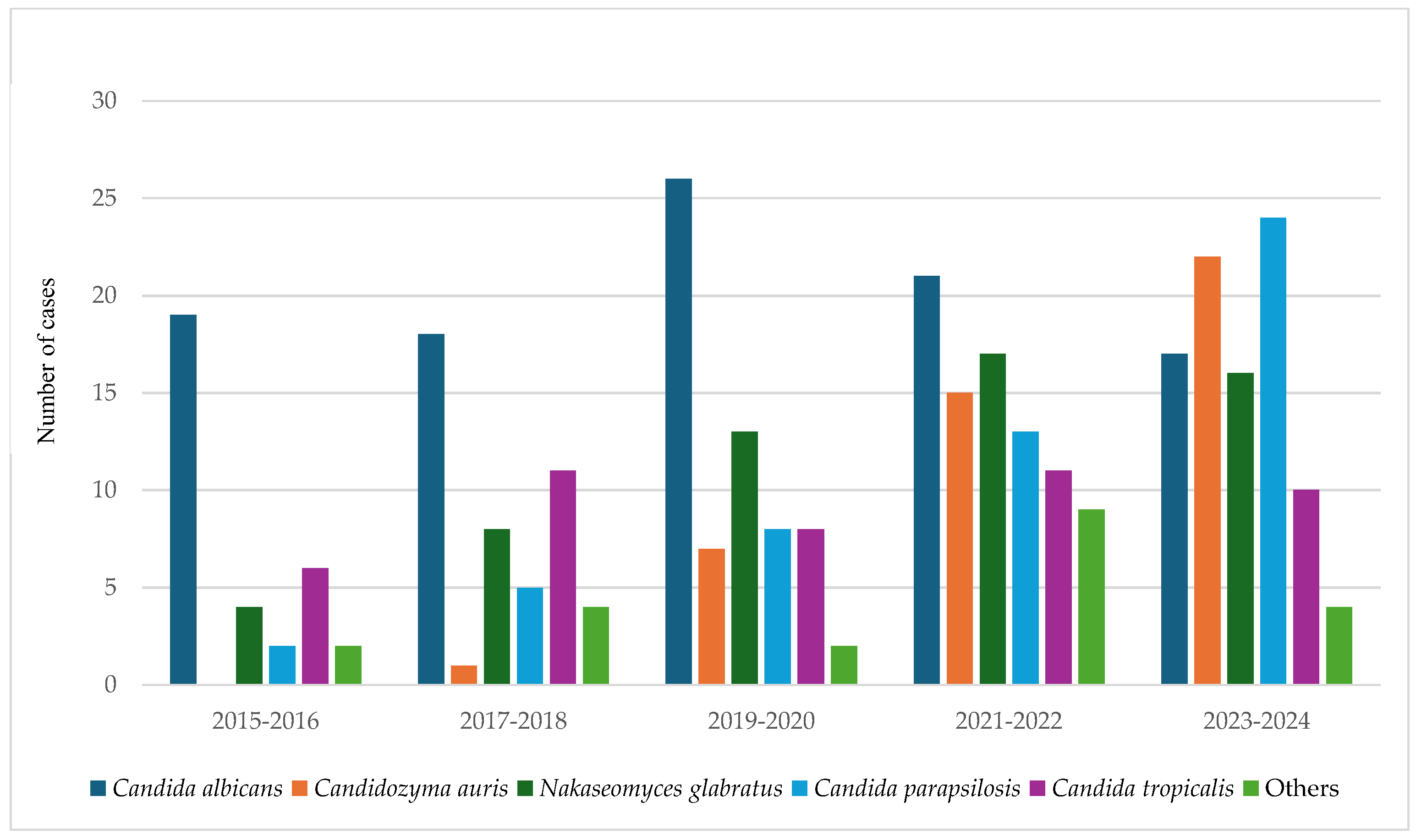
3.3. Shifting Trends in Candida Species over Time
3.4. Antifungal Susceptibility Patterns of Candida Bloodstream Isolates
3.5. Multivariate Analysis of Risk Factors for Species-Specific Candidemia and Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MIC | Minimum inhibitory concentration |
| NAC | Non-albicans Candida |
References
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Parslow, B.Y.; Thornton, C.R. Continuing Shifts in Epidemiology and Antifungal Susceptibility Highlight the Need for Improved Disease Management of Invasive Candidiasis. Microorganisms 2022, 10, 1208. [Google Scholar] [CrossRef]
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L.; Calandra, T. Changes in the epidemiological landscape of invasive candidiasis. J. Antimicrob. Chemother. 2018, 73 (Suppl. S1), i4–i13. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef]
- Alobaid, K.; Ahmad, S.; Asadzadeh, M.; Mokaddas, E.; Al-Sweih, N.; Albenwan, K.; Alfouzan, W.; Al-Obaid, I.; Jeragh, A.; Al-Roomi, E.; et al. Epidemiology of Candidemia in Kuwait: A Nationwide, Population-Based Study. J. Fungi 2021, 7, 673. [Google Scholar] [CrossRef] [PubMed]
- Alfouzan, W.; Al-Wathiqi, F.; Altawalah, H.; Asadzadeh, M.; Khan, Z.; Denning, D.W. Human Fungal Infections in Kuwait-Burden and Diagnostic Gaps. J. Fungi 2020, 6, 306. [Google Scholar] [CrossRef]
- Khan, Z.; Ahmad, S.; Al-Sweih, N.; Mokaddas, E.; Al-Banwan, K.; Alfouzan, W.; Al-Obaid, I.; Al-Obaid, K.; Asadzadeh, M.; Jeragh, A.; et al. Changing trends in epidemiology and antifungal susceptibility patterns of six bloodstream Candida species isolates over a 12-year period in Kuwait. PLoS ONE 2019, 14, e0216250. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, A.; Klamer, K.; Sharma, M.; Ortiz, D.; Saravolatz, L. Candida auris: A Continuing Threat. Microorganisms 2025, 13, 652. [Google Scholar] [CrossRef]
- Jamal, W.; Ahmad, S.; Khan, Z.U.; Rotimi, V.O. Comparative evaluation of two matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) systems for the identification of clinically significant yeasts. Int. J. Infect. Dis. 2014, 26, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaid, K.; Asadzadeh, M.; Ahmad, S.; Khan, Z. Population structure and molecular genetic characterization of clinical Candida tropicalis isolates from a tertiary-care hospital in Kuwait reveal infections with unique strains. PLoS ONE 2017, 12, e0182292. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.U.; Ahmad, S.; Al-Sweih, N.; Joseph, L.; Alfouzan, F.; Asadzadeh, M. Increasing prevalence, molecular characterization and antifungal drug susceptibility of serial Candida auris isolates in Kuwait. PLoS ONE 2018, 13, e0195743. [Google Scholar] [CrossRef]
- Asadzadeh, M.; Ahmad, S.; Hagen, F.; Meis, J.F.; Al-Sweih, N.; Khan, Z. Simple, low-cost detection of Candida parapsilosis complex isolates and molecular fingerprinting of Candida orthopsilosis strains in Kuwait by ITS region sequencing and amplified fragment length polymorphism analysis. PLoS ONE 2015, 10, e0142880. [Google Scholar] [CrossRef]
- Khan, Z.; Ahmad, S.; Al-Sweih, N.; Khan, S.; Joseph, L. Candida lusitaniae in Kuwait: Prevalence, antifungal susceptibility and role in neonatal fungemia. PLoS ONE 2019, 14, e0213532. [Google Scholar] [CrossRef]
- Khan, Z.U.; Ahmad, S.; Hagen, F.; Fell, J.W.; Kowshik, T.; Chandy, R.; Boekhout, T. Cryptococcus randhawai sp. nov., a novel anamorphic basidiomycetous yeast isolated from tree trunk hollow of Ficus religiosa (peepal tree) from New Delhi, India. Antonie Leeuwenhoek 2010, 97, 253–259. [Google Scholar] [CrossRef]
- TREK Diagnostic Systems. Sensititre YeastOne® Susceptibility Testing: Manufacturer’s Instructions; TREK Diagnostic Systems: East Grinstead, UK, 2018. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antifungal Susceptibility Testing of Yeasts, 3rd ed.; CLSI Supplement M27M44S; CLSI: Wayne, PA, USA, 2022. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Antifungal Susceptibility Testing and Interpretation for Candida auris. 2023. Available online: http://www.cdc.gov/candida-auris/hcp/laboratories/antifungal-susceptibility-testing.html (accessed on 1 June 2025).
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; CLSI Standard M27; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Soriano, A.; Honore, P.M.; Puerta-Alcalde, P.; Garcia-Vidal, C.; Pagotto, A.; Gonçalves-Bradley, D.C.; Verweij, P.E. Invasive candidiasis: Current clinical challenges and unmet needs in adult populations. J. Antimicrob. Chemother. 2023, 78, 1569–1585. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef]
- Alkhalifa, W.; Alhawaj, H.; Alamri, A.; Alturki, F.; Alshahrani, M.; Alnimr, A. Clinical and Microbiological Characteristics of Candidemia Cases in Saudi Arabia. Infect. Drug Resist. 2023, 16, 4489–4503. [Google Scholar] [CrossRef]
- Saeed, N.K.; Almusawi, S.; Al-Beltagi, M. Candidemia chronicles: Retrospective analysis of candidemia epidemiology, species distribution, and antifungal susceptibility patterns in Bahrain. World J. Virol. 2024, 13, 98839. [Google Scholar] [CrossRef]
- Egger, M.; Hoenigl, M.; Thompson, G.R., 3rd; Carvalho, A.; Jenks, J.D. Let’s talk about sex characteristics-As a risk factor for invasive fungal diseases. Mycoses 2022, 65, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Williams, S.R.; Berkow, E.L.; Farley, M.M.; Harrison, L.H.; Bonner, L.; Marceaux, K.M.; Hollick, R.; Zhang, A.Y.; Schaffner, W.; et al. Population-Based Active Surveillance for Culture-Confirmed Candidemia—Four Sites, United States, 2012-2016. MMWR Surveill. Summ. 2019, 68, 1–15. [Google Scholar] [CrossRef]
- Tang, H.J.; Liu, W.L.; Lin, H.L.; Lai, C.C. Epidemiology and prognostic factors of candidemia in elderly patients. Geriatr. Gerontol. Int. 2015, 15, 688–693. [Google Scholar] [CrossRef]
- Poissy, J.; Damonti, L.; Bignon, A.; Khanna, N.; Von Kietzell, M.; Boggian, K.; Neofytos, D.; Vuotto, F.; Coiteux, V.; Artru, F.; et al. Risk factors for candidemia: A prospective matched case-control study. Crit. Care 2020, 24, 109. [Google Scholar] [CrossRef]
- Bilal, H.; Zhang, D.; Shafiq, M.; Khan, M.N.; Chen, C.; Khan, S.; Wang, Q.; Cai, L.; Islam, R.; Hu, H.; et al. Six-Year Retrospective Analysis of Epidemiology, Risk Factors, and Antifungal Susceptibilities of Candidiasis from a Tertiary Care Hospital in South China. Microbiol. Spectr. 2023, 11, e00708-28. [Google Scholar] [CrossRef]
- Mazzanti, S.; Brescini, L.; Morroni, G.; Orsetti, E.; Pocognoli, A.; Donati, A.; Cerutti, E.; Munch, C.; Montalti, R.; Barchiesi, F. Candidemia in intensive care units over nine years at a large Italian university hospital: Comparison with other wards. PLoS ONE 2021, 16, e0252165. [Google Scholar] [CrossRef]
- Kim, E.J.; Lee, E.; Kwak, Y.G.; Yoo, H.M.; Choi, J.Y.; Kim, S.R.; Shin, M.J.; Yoo, S.Y.; Cho, N.H.; Choi, Y.H. Trends in the Epidemiology of Candidemia in Intensive Care Units From 2006 to 2017: Results From the Korean National Healthcare-Associated Infections Surveillance System. Front. Med. 2020, 7, 606976. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997–2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Patterson, T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216 (Suppl. S3), S445–S451. [Google Scholar] [CrossRef]
- Yamin, D.H.; Husin, A.; Harun, A. Risk Factors of Candida parapsilosis Catheter-Related Bloodstream Infection. Front. Public Health 2021, 9, 631865. [Google Scholar] [CrossRef]
- Al-Siyabi, T.; Al Busaidi, I.; Balkhair, A.; Al-Muharrmi, Z.; Al-Salti, M.; Al’Adawi, B. First report of Candida auris in Oman: Clinical and microbiological description of five candidemia cases. J. Infect. 2017, 75, 373–376. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef]
- Bassetti, M.; Righi, E.; Montravers, P.; Cornely, O.A. What has changed in the treatment of invasive candidiasis? A look at the past 10 years and ahead. J. Antimicrob. Chemother. 2018, 73 (Suppl. S1), i14–i25. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.F. Candida auris: Epidemiology Update and a Review of Strategies to Prevent Spread . J. Clin. Med. 2024, 13, 6675. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Eachempati, S.R.; Hydo, L.; Barie, P.S. Gender-based differences in outcome in patients with sepsis. Arch. Surg. 1999, 134, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Vallabhaneni, S.; Jackson, B.R.; Chiller, T.M. Candida auris: An Emerging Antimicrobial Resistance Threat. Ann. Intern. Med. 2019, 171, 432–433. [Google Scholar] [CrossRef] [PubMed]
| Year | Total No. of Patients | Gender | No. Candidemia of Patients of Different Age (Years) | Hospital Unit | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | ≤1 | 1–19 | 20–49 | 50–64 | ≥65 | ICU | Ward | ||
| 2015–2016 | 33 | 12 | 21 | - | - | 6 | 8 | 19 | 12 (36%) | 21 (64%) |
| 2017–2018 | 47 | 22 | 25 | - | - | 4 | 8 | 35 | 19 (40%) | 28 (60%) |
| 2019–2020 | 64 | 37 | 27 | - | - | 14 | 20 | 30 | 25 (38%) | 39 (62%) |
| 2021–2022 | 86 | 42 | 44 | - | 2 | 14 | 19 | 51 | 40 (47%) | 46 (53%) |
| 2023–2024 | 93 | 48 | 45 | - | - | 14 | 24 | 55 | 36 (39%) | 57 (61%) |
| Total | 323 | 161 (49.8%) | 162 (50.2%) | - | 2 | 52 | 79 | 190 | 132 (41%) | 191 (59%) |
| Age of Candidemia Patients (Years) | Candida spp. and Related Species Isolates Identified as | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. albicans, n = 101 | C. parapsilosis, n = 52 | C. tropicalis, n = 46 | C. auris, n = 45 | N. glabratus, n = 58 | Others n = 21 | ||||||||
| M | F | M | F | M | F | M | F | M | F | M | F | ||
| ≤1 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1–19 | - | - | - | - | 2 | - | - | - | - | - | - | - | 2 |
| 20–49 | 2 | 8 | 5 | 5 | 7 | 2 | 8 | 1 | 7 | 6 | - | 1 | 52 |
| 50–64 | 17 | 6 | 6 | 9 | 3 | 4 | 10 | 5 | 7 | 7 | 3 | 2 | 79 |
| ≥65 | 32 | 36 | 11 | 16 | 12 | 16 | 8 | 13 | 14 | 17 | 7 | 8 | 190 |
| Total | 51 | 50 | 22 | 30 | 24 | 22 | 26 | 19 | 28 | 30 | 10 | 11 | 323 |
| Organism | 2015–2016 | 2017–2018 | 2019–2020 | 2021–2022 | 2023–2024 |
|---|---|---|---|---|---|
| Candida albicans | 19 | 18 | 26 | 21 | 17 |
| Candidozyma auris | 0 | 1 | 7 | 15 | 22 |
| Nakaseomyces glabratus | 4 | 8 | 13 | 17 | 16 |
| Candida parapsilosis | 2 | 5 | 8 | 13 | 24 |
| Candida tropicalis | 6 | 11 | 8 | 11 | 10 |
| Others * | 2 | 4 | 2 | 9 | 4 |
| Total | 33 | 47 | 64 | 86 | 93 |
| Organism | Antifungal | Range | GM | MIC50 | MIC90 | No. Resistant Isolates (%) |
|---|---|---|---|---|---|---|
| Candida albicans (14) | ||||||
| Fluconazole | 0.25–256 | 0.862 | 0.5 | 16 | 2 (14.3%) | |
| Posaconazole | 0.008–0.03 | 0.011 | 0.008 | 0.015 | 0 (0.0%) | |
| Voriconazole | 0.008–0.03 | 0.011 | 0.008 | 0.015 | 0 (0.0%) | |
| Amphotericin B | 0.12–1 | 0.247 | 0.25 | 0.5 | 0 (0.0%) | |
| Anidulafungin | 0.015–0.12 | 0.042 | 0.06 | 0.06 | 0 (0.0%) | |
| Caspofungin | 0.06–0.25 | 0.121 | 0.12 | 0.25 | 0 (0.0%) | |
| Micafungin | 0.008–8 | 0.067 | 0.03 | 1 | 0 (0.0%) | |
| 5-Flucytosine | <0.06–0.25 | 0.06 | 0.06 | 0.06 | 0 (0.0%) | |
| Candidozyma auris * (39) | ||||||
| Fluconazole | 0.25–256 | 70.28 | 64 | 256 | 38 (97.4%) | |
| Posaconazole | 0.008–1 | 0.048 | 0.06 | 0.12 | N/A | |
| Voriconazole | 0.06–8 | 0.401 | 0.5 | 1 | 5 (12.8%) | |
| Amphotericin B | 0.25–2 | 1 | 1 | 2 | 1 (2.6%) | |
| Anidulafungin | 0.06–2 | 0.162 | 0.12 | 0.12 | 3 (7.7%) | |
| Caspofungin | 0.12–8 | 0.265 | 0.25 | 1 | 2 (5.1%) | |
| Micafungin | 0.015–4 | 0.083 | 0.06 | 0.12 | 2 (5.1%) | |
| 5-Flucytosine | <0.06–64 | 0.121 | 0.06 | 0.12 | 1 (2.6%) | |
| Nakaseomyces glabratus (16) | ||||||
| Fluconazole | 0.25–64 | 3.67 | 4 | 64 | 4 (25.0%) | |
| Posaconazole | 0.25–8 | 1.382 | 1 | 8 | N/A | |
| Voriconazole | 0.12–4 | 0.499 | 0.25 | 4 | 0 (0.0%) | |
| Amphotericin B | 0.25–0.5 | 0.439 | 0.5 | 0.5 | 0 (0.0%) | |
| Anidulafungin | 0.03–0.12 | 0.063 | 0.06 | 0.12 | 0 (0.0%) | |
| Caspofungin | 0.12–64 | 0.627 | 0.12 | 8 | 0 (0.0%) | |
| Micafungin | 0.015–1 | 0.031 | 0.015 | 0.5 | 0 (0.0%) | |
| 5-Flucytosine | <0.06 | 0.06 | 0.06 | 0.06 | 0 (0.0%) | |
| Candida parapsilosis (12) | ||||||
| Fluconazole | 0.12–128 | 0.791 | 0.5 | 2 | 1 (8.3%) | |
| Posaconazole | 0.015–0.12 | 0.048 | 0.045 | 0.12 | N/A | |
| Voriconazole | 0.008–0.06 | 0.015 | 0.008 | 0.06 | 0 (0.0%) | |
| Amphotericin B | 0.12–2 | 0.311 | 0.25 | 1 | 0 (0.0%) | |
| Anidulafungin | 0.06–2 | 0.442 | 0.5 | 2 | 0 (0.0%) | |
| Caspofungin | 0.12–2 | 0.498 | 0.5 | 2 | 0 (0.0%) | |
| Micafungin | 0.008–2 | 0.332 | 0.5 | 1 | 0 (0.0%) | |
| 5-Flucytosine | <0.06–0.5 | 0.091 | 0.06 | 0.5 | 0 (0.0%) | |
| Candida tropicalis (16) | ||||||
| Fluconazole | 0.5–4 | 1.587 | 1 | 4 | 0 (0.0%) | |
| Posaconazole | 0.03–0.5 | 0.108 | 0.12 | 0.5 | N/A | |
| Voriconazole | 0.015–0.25 | 0.08 | 0.09 | 0.25 | 0 (0.0%) | |
| Amphotericin B | 0.5 | 0.5 | 0.5 | 0.5 | 0 (0.0%) | |
| Anidulafungin | 0.03–0.5 | 0.096 | 0.06 | 0.5 | 0 (0.0%) | |
| Caspofungin | 0.06–0.25 | 0.174 | 0.25 | 0.25 | 0 (0.0%) | |
| Micafungin | 0.03–0.5 | 0.068 | 0.03 | 0.5 | 0 (0.0%) | |
| 5-Flucytosine | <0.06 | 0.06 | 0.06 | 0.06 | 0 (0.0%) | |
| Risk Factor | C. albicans OR (95% CI) | p-Value | N. glabratus OR (95% CI) | p-Value | C. auris OR (95% CI) | p-Value | C. parapsilosis OR (95% CI) | p-Value | Mortality OR (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender, male | 1.04 (0.65–1.66) | 0.905 | 0.95 (0.54–1.67) | 0.886 | 1.45 (0.77–2.74) | 0.265 | 1.05 (0.58–1.89) | 0.870 | 0.60 (0.38–0.93) | 0.025 |
| Age (per 10-year) | 75 (32–88) | 69 (39–92) | 58 (28–92) | 68 (16–104) | 1.17 (0.96–1.42) | 0.033 | ||||
| Cancer | 5.94 (2.86–12.90) | <0.001 | 3.32 (1.68–6.50) | <0.001 | 0.33 (0.10–0.87) | 0.016 | 0.75 (0.40–1.41) | 0.370 | 0.23 (0.12–0.41) | <0.001 |
| Chronic kidney disease | 3.97 (1.62–10.21) | 0.001 | 0.88 (0.43–1.74) | 0.749 | 0.09 (0.02–0.31) | <0.001 | 0.92 (0.48–1.78) | 0.810 | 2.62 (1.62–4.27) | <0.001 |
| Cerebrovascular accident | 2.73 (0.68–11.61) | 0.105 | 0.50 (0.12–1.50) | 0.263 | 1.36 (0.51–3.28) | 0.490 | 1.32 (0.52–3.36) | 0.560 | 1.44 (0.77–2.74) | 0.232 |
| Ischemic heart disease | 4.43 (1.76–11.85) | <0.001 | 0.95 (0.46–1.88) | 1.000 | 0.77 (0.35–1.60) | 0.501 | 1.14 (0.63–2.07) | 0.670 | 3.03 (1.86–5.00) | <0.001 |
| Hypertension | 2.79 (1.33–5.91) | 0.005 | 2.92 (1.57–5.54) | <0.001 | 0.31 (0.14–0.64) | <0.001 | 1.22 (0.68–2.19) | 0.510 | 1.71 (1.07–2.75) | 0.024 |
| Diabetes mellitus | 2.38 (1.17–4.82) | 0.012 | 1.69 (0.92–3.15) | 0.081 | 0.44 (0.22–0.88) | 0.014 | 1.09 (0.60–1.99) | 0.780 | 1.26 (0.77–2.05) | 0.351 |
| Dyslipidemia | 2.79 (1.18–6.68) | 0.010 | 2.06 (1.10–3.86) | 0.017 | 0.29 (0.11–0.65) | <0.001 | 1.31 (0.73–2.36) | 0.370 | 1.58 (0.99–2.53) | 0.043 |
| Surgery | 1.56 (0.77–3.13) | 0.224 | 6.08 (2.95–13.61) | <0.001 | 0.69 (0.35–1.38) | 0.323 | 2.91 (1.63–5.19) | 0.008 | 0.41 (0.24–0.68) | <0.001 |
| Mechanical ventilation | 3.36 (1.12–10.75) | 0.016 | 0.29 (0.07–0.84) | 0.014 | 0.68 (0.26–1.58) | 0.448 | 1.18 (0.66–2.11) | 0.580 | 1.91 (1.12–3.29) | 0.015 |
| Total parenteral nutrition | 2.67 (0.75–9.90) | 0.122 | 0.85 (0.30–2.07) | 0.834 | 1.04 (0.39–2.45) | 1.000 | 3.71 (1.93–7.14) | 0.002 | 1.41 (0.78–2.55) | 0.261 |
| Central venous catheter | 2.99 (0.96–9.77) | 0.048 | 10.57 (5.34–21.38) | <0.001 | 0.75 (0.29–1.76) | 0.564 | 4.18 (2.34–7.49) | <0.001 | 1.64 (0.95–2.84) | 0.068 |
| Prolonged IV therapy (>7 d) | – | – | – | – | – | – | 2.43 (1.36–4.34) | 0.015 | – | – |
| Urinary catheter | 3.35 (0.38–40.77) | 0.179 | 0 (0–1.08) | 0.050 | 0 (0–0.98) | 0.034 | 1.55 (0.84–2.86) | 0.160 | 1.76 (0.71–4.53) | 0.213 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Benwan, K.; Ahmed, S.; Al Banwan, D.; John, M. Candidemia in a General Hospital in Kuwait: Epidemiology, Species Distribution, Risk Factors, and Antifungal Susceptibility Patterns over a 10-Year Period (2015–2024). J. Fungi 2025, 11, 670. https://doi.org/10.3390/jof11090670
Al Benwan K, Ahmed S, Al Banwan D, John M. Candidemia in a General Hospital in Kuwait: Epidemiology, Species Distribution, Risk Factors, and Antifungal Susceptibility Patterns over a 10-Year Period (2015–2024). Journal of Fungi. 2025; 11(9):670. https://doi.org/10.3390/jof11090670
Chicago/Turabian StyleAl Benwan, Khalifa, Sarah Ahmed, Dalal Al Banwan, and Maria John. 2025. "Candidemia in a General Hospital in Kuwait: Epidemiology, Species Distribution, Risk Factors, and Antifungal Susceptibility Patterns over a 10-Year Period (2015–2024)" Journal of Fungi 11, no. 9: 670. https://doi.org/10.3390/jof11090670
APA StyleAl Benwan, K., Ahmed, S., Al Banwan, D., & John, M. (2025). Candidemia in a General Hospital in Kuwait: Epidemiology, Species Distribution, Risk Factors, and Antifungal Susceptibility Patterns over a 10-Year Period (2015–2024). Journal of Fungi, 11(9), 670. https://doi.org/10.3390/jof11090670






