Healthcare Resource Utilization, Treatment Costs, and Mortality in Patients with Malignancies or Transplantation Who Develop Invasive Aspergillosis
Abstract
1. Introduction
2. Methods
3. Results
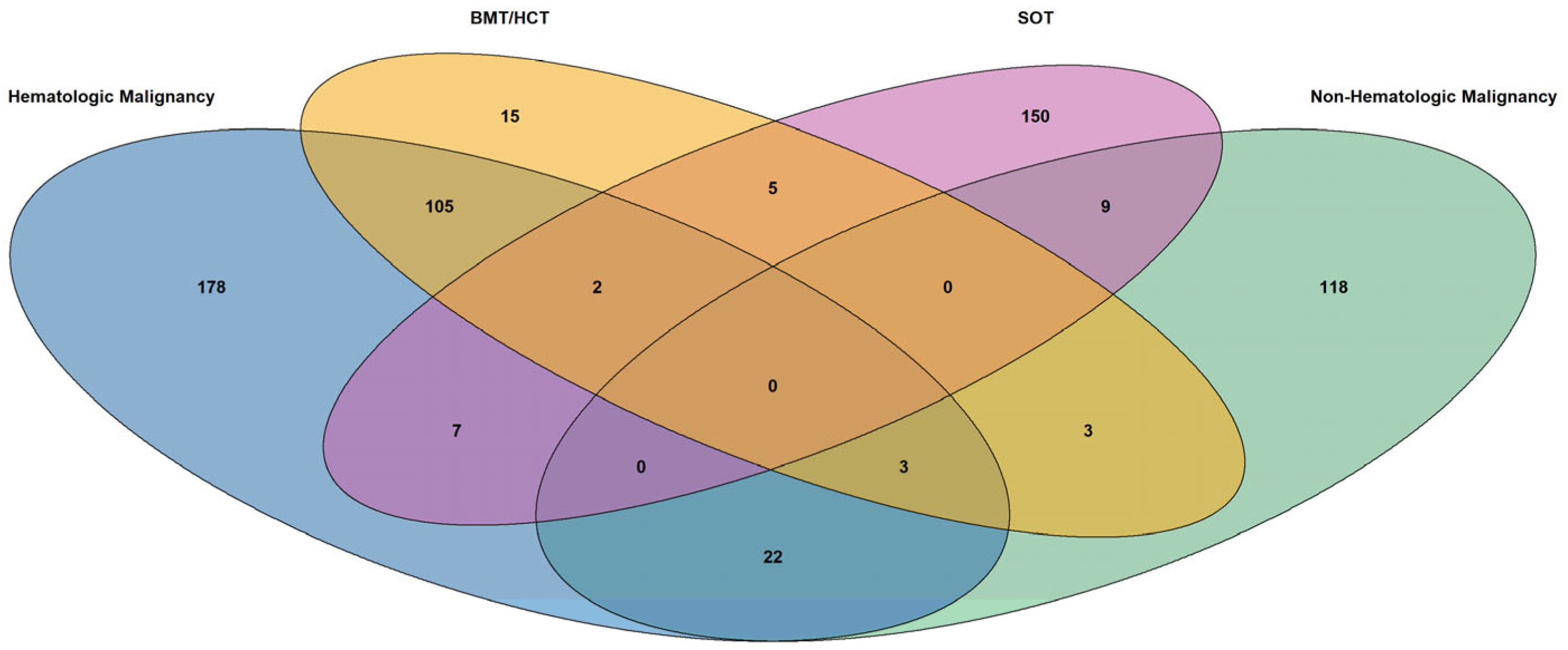
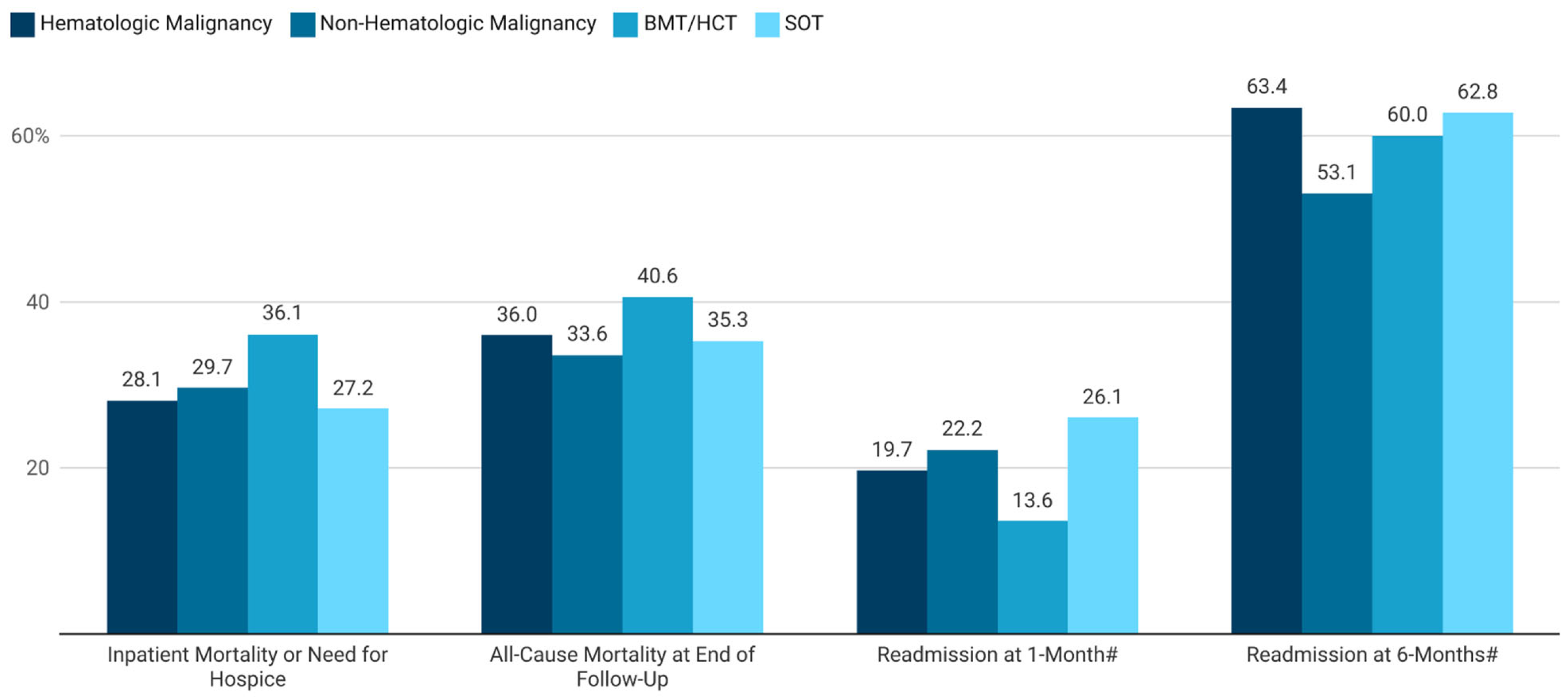
3.1. Overall Population
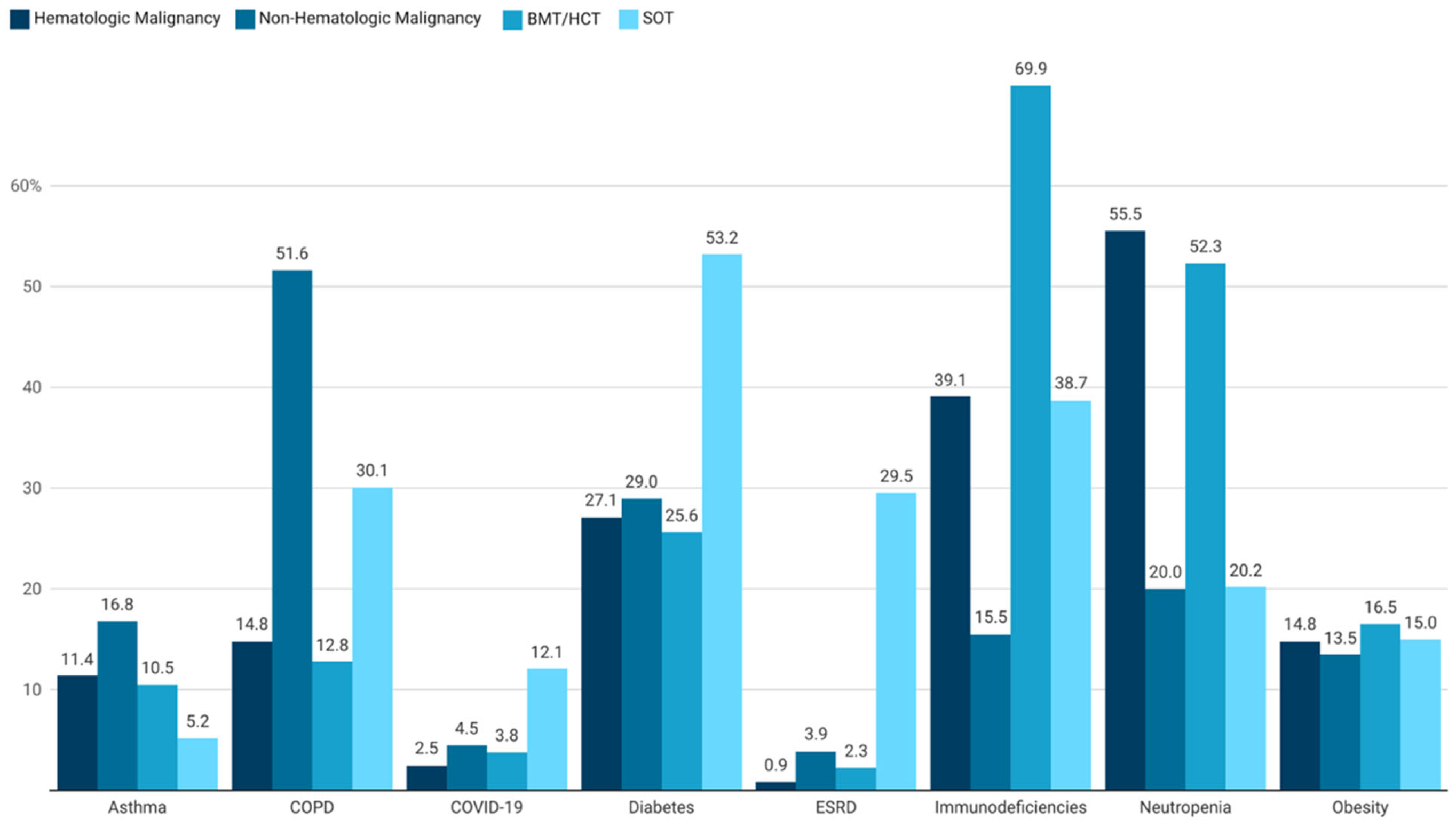
3.2. Hematologic Malignancies
3.3. Non-Hematologic Malignancies
3.4. BMT/HCT Recipients
3.5. SOT Recipients
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, G.R.; Young, J.H. Aspergillus Infections. N. Engl. J. Med. 2021, 385, 1496–1509. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development, and Public Health Action; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240060241 (accessed on 5 September 2024).
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.; Whitham, H.K.; Jackson, B.R. Economic burden of fungal diseases in the United States. Open Forum Infect. Dis. 2022, 9, ofac097. [Google Scholar] [CrossRef] [PubMed]
- Rayens, E.; Norris, K.A. Prevalence and healthcare burden of fungal infections in the United States, 2018. Open Forum Infect. Dis. 2022, 9, ofab593. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, M.D.; Nathanson, B.H.; Harrington, R.; Spalding, J.R.; Shorr, A.F. Epidemiology and Outcomes of Hospitalizations With Invasive Aspergillosis in the United States, 2009–2013. Clin. Infect. Dis. 2018, 67, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.B.; Lau, C.J.; Murtagh, K.; Layton, A.J.; Seifeldin, R. The economic impact of aspergillosis: Analysis of hospital expenditures across patient subgroups. Int. J. Infect. Dis. 2009, 13, 24–36. [Google Scholar] [CrossRef] [PubMed]
- IQVIA Available IQVIA Data. Available online: https://www.iqvia.com/insights/the-iqvia-institute/available-iqvia-data (accessed on 23 May 2024).
- Khalil, A.; Singh, P.; Mir, T.; Uddin, M.; Soubani, A.O. Invasive Pulmonary Aspergillosis in Hospitalized Hematopoietic Stem Cell Transplantation Recipients: Outcomes Based on the United States National Readmission Database. Hematol. Oncol. Stem Cell Ther. 2023, 17, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Gerson, S.L.; Talbot, G.H.; Hurwitz, S.; Strom, B.L.; Lusk, E.J.; Cassileth, P.A. Prolonged granulocytopenia: The major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann. Intern. Med. 1984, 100, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Neofytos, D.; Treadway, S.; Ostrander, D.; Alonso, C.D.; Dierberg, K.L.; Nussenblatt, V.; Durand, C.M.; Thompson, C.B.; Marr, K.A. Epidemiology, outcomes, and mortality predictors of invasive mold infections among transplant recipients: A 10-year, single-center experience. Transpl. Infect. Dis. 2013, 15, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive fungal infections among organ transplant recipients: Results of the transplant-associated infection surveillance network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: Overview of the TRANSNET database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Otu, A.; Kosmidis, C.; Mathioudakis, A.G.; Ibe, C.; Denning, D.W. The clinical spectrum of aspergillosis in chronic obstructive pulmonary disease. Infection 2023, 51, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Ohmagari, N.; Raad, I.I.; Hachem, R.; Kontoyiannis, D.P. Invasive aspergillosis in patients with solid tumors. Cancer 2004, 101, 2300–2302. [Google Scholar] [CrossRef] [PubMed]
- Herrera, S.; Magyar, U.; Husain, S. Invasive Aspergillosis in the Current Era. Infect. Dis. Clin. N. Am. 2025, 39, e33–e60. [Google Scholar] [CrossRef] [PubMed]
- Zubovskaia, A.; Vazquez, J.A. Invasive Aspergillosis in the Intensive Care Unit. J. Fungi 2025, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.K.; Salmon, J.W.; Kong, S.X.; Zhao, S.Z. Administrative databases and outcomes assessment: An overview of issues and potential utility. J. Manag. Care Pharm. 1999, 5, 215–222. [Google Scholar] [CrossRef]
- Cochran, G.L.; Cochran, H.C.; Ernst, M.E. Research and scholarly methods: Mitigating information bias. J. Am. Coll. Clin. Pharm. 2025, 1–8. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Calandra, T. Pulmonary aspergillosis: Diagnosis and treatment. Eur. Respir. Rev. 2022, 31, 220114. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | Hematologic Malignancy N = 317 | Non-Hematologic Malignancy N = 155 | BMT/HCT N = 133 | SOT N = 173 |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Hematologic malignancy | 317 (100.0) | 25 (16.1) | 115 (86.5) | 9 (5.2) |
| Leukemia | 260 (82.0) | 21 (13.5) | 96 (72.2) | 6 (3.5) |
| Non-Hodgkin lymphoma | 71 (22.4) | 5 (3.2) | 21 (15.8) | 2 (1.2) |
| Non-hematologic malignancy | 25 (7.9) | 155 (100.0) | 6 (4.5) | 10 (5.8) |
| Breast | 9 (2.8) | 25 (16.1) | 3 (2.3) | 1 (0.6) |
| Colon, rectum, anus | 4 (1.3) | 17 (11.0) | 1 (0.8) | 2 (1.2) |
| Lung, trachea, bronchus | 8 (2.5) | 84 (54.2) | 0 (0.0) | 2 (1.2) |
| Ovary, uterus, cervix | 1 (0.3) | 6 (3.9) | 0 (0.0) | 0 (0.0) |
| Pancreas | 0 (0.0) | 2 (1.3) | 0 (0.0) | 0 (0.0) |
| Prostate | 2 (0.6) | 11 (7.1) | 1 (0.8) | 3 (1.7) |
| Urinary | 2 (0.6) | 22 (14.2) | 1 (0.8) | 2 (1.2) |
| BMT/HCT history † | 110 (34.7) | 6 (3.9) | 125 (94.0) | 6 (3.5) |
| BMT | 67 (21.1) | 4 (2.6) | 77 (57.9) | 4 (2.3) |
| HCT | 100 (31.5) | 6 (3.9) | 111 (83.5) | 4 (2.3) |
| SOT history † | 9 (2.8) | 9 (5.8) | 7 (5.3) | 170 (98.3) |
| Heart | 0 (0.0) | 2 (1.3) | 0 (0.0) | 16 (9.3) |
| Heart and lung | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (1.7) |
| Kidney | 2 (0.6) | 3 (1.9) | 1 (0.8) | 74 (42.8) |
| Liver | 0 (0.0) | 0 (0.0) | 0 (0.0) | 21 (12.1) |
| Lung | 1 (0.3) | 4 (2.6) | 0 (0.0) | 74 (42.8) |
| Other | 6 (1.9) | 1 (0.6) | 6 (4.5) | 17 (9.8) |
| Characteristics | Hematologic Malignancy N = 317 | Non-Hematologic Malignancy N = 155 | BMT/HCT N = 133 | SOT N = 173 |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Age group | ||||
| 18–44 years | 44 (13.9) | 6 (3.9) | 26 (19.6) | 18 (10.4) |
| 45–64 years | 138 (43.5) | 44 (28.4) | 57 (42.9) | 77 (44.5) |
| 65+ years | 135 (42.6) | 105 (67.7) | 50 (37.6) | 78 (45.1) |
| Gender | ||||
| Female | 116 (36.6) | 73 (47.1) | 52 (39.1) | 62 (35.8) |
| Male | 201 (63.4) | 82 (52.9) | 81 (60.9) | 111 (64.2) |
| Geographic region † | ||||
| Northeast | 23 (7.3) | 20 (12.9) | 9 (6.8) | 12 (6.9) |
| Midwest | 31 (9.8) | 13 (8.4) | 11 (8.3) | 4 (2.3) |
| South | 36 (11.4) | 30 (19.4) | 5 (3.8) | 64 (37.0) |
| West | 227 (71.6) | 92 (59.4) | 108 (81.2) | 93 (53.8) |
| Payer type | ||||
| Commercial | 122 (38.5) | 49 (31.6) | 52 (39.1) | 53 (30.6) |
| Medicaid | 26 (8.2) | 4 (2.6) | 14 (10.5) | 5 (2.9) |
| Medicare | 111 (35.0) | 87 (56.1) | 43 (32.3) | 96 (55.5) |
| Unknown | 58 (18.3) | 15 (9.7) | 24 (18.1) | 19 (11.0) |
| HCRU/Costs (USD) | Hematologic Malignancy N = 317 | Non-Hematologic Malignancy N = 155 | BMT/HCT N = 133 | SOT N = 173 |
|---|---|---|---|---|
| Index IA Hospitalization | ||||
| LOS, days, median (Q1, Q3) | 18 (9, 33) | 12 (7, 20) | 18 (8, 33) | 15 (8, 28) |
| ICU LOS, days, median (Q1, Q3) | 12 (5, 21) | 10 (6, 16) | 12 (5, 22) | 13 (6, 23) |
| Total costs, 2023 USD, median (Q1, Q3) | USD 151,489 (USD 72,916, USD 372,649) | USD 79,058 (USD 43,016, USD 186,103) | USD 172,342 (USD 80,686, USD 507,860) | USD 124,753 (USD 54,999, USD 339,078) |
| All-Cause Total Treatment Costs During Follow-Up | ||||
| 1 Month, 2023 USD, median (Q1, Q3) | USD 236,325 (USD 126,282, USD 520,513) | USD 117,438 (USD 62,439, USD 249,349) | USD 226,676 (USD 115,488, USD 599,601) | USD 132,624 (USD 70,155, USD 443,311) |
| 6 Months, 2023 USD, median (Q1, Q3) | USD 397,857 (USD 237,651, USD 697,059) | USD 213,378 (USD 108,048, USD 420,493) | USD 389,296 (USD 172,248, USD 781,760 | USD 246,717 (USD 130,457, USD 444,578) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walsh, T.J.; Coleman, C.I.; Johnson, M.; Lovelace, B.; Alexander, B.D. Healthcare Resource Utilization, Treatment Costs, and Mortality in Patients with Malignancies or Transplantation Who Develop Invasive Aspergillosis. J. Fungi 2025, 11, 657. https://doi.org/10.3390/jof11090657
Walsh TJ, Coleman CI, Johnson M, Lovelace B, Alexander BD. Healthcare Resource Utilization, Treatment Costs, and Mortality in Patients with Malignancies or Transplantation Who Develop Invasive Aspergillosis. Journal of Fungi. 2025; 11(9):657. https://doi.org/10.3390/jof11090657
Chicago/Turabian StyleWalsh, Thomas J., Craig I. Coleman, Melissa Johnson, Belinda Lovelace, and Barbara D. Alexander. 2025. "Healthcare Resource Utilization, Treatment Costs, and Mortality in Patients with Malignancies or Transplantation Who Develop Invasive Aspergillosis" Journal of Fungi 11, no. 9: 657. https://doi.org/10.3390/jof11090657
APA StyleWalsh, T. J., Coleman, C. I., Johnson, M., Lovelace, B., & Alexander, B. D. (2025). Healthcare Resource Utilization, Treatment Costs, and Mortality in Patients with Malignancies or Transplantation Who Develop Invasive Aspergillosis. Journal of Fungi, 11(9), 657. https://doi.org/10.3390/jof11090657






