Blue Light Receptor WC-2 Regulates Ganoderic Acid Biosynthesis in Ganoderma lingzhi
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Culture Conditions, and Light Treatment
2.2. Plasmid Construction
2.3. G. lingzhi Strain Construction
2.4. Determination of Mycelial Growth, GA Content, Intermediate Accumulation, and Sporulation Analysis
2.5. Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR) Analysis
2.6. Statistical Analysis
3. Results
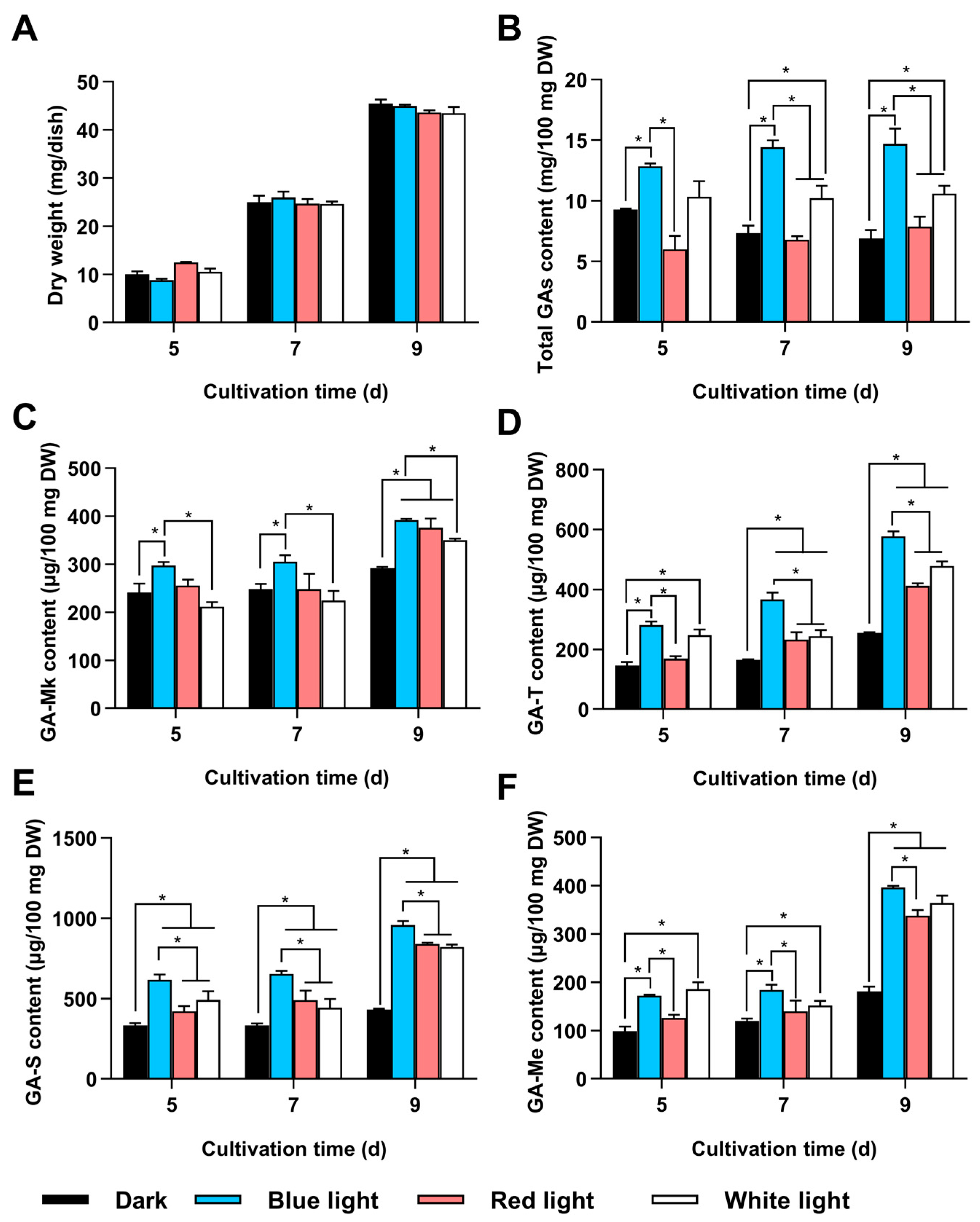
3.1. GA Accumulation in G. lingzhi Under Different Light Conditions
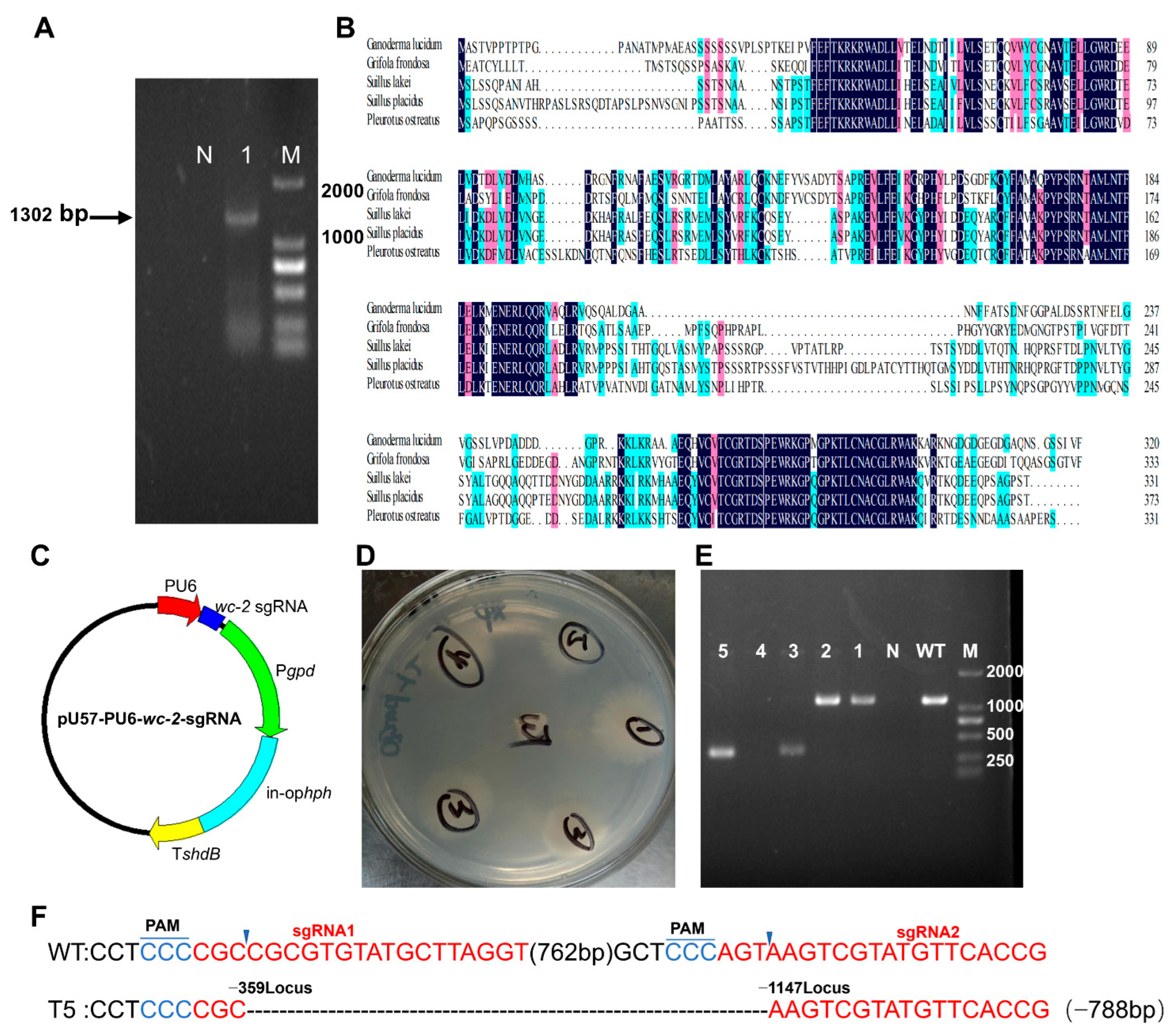
3.2. Identification and Deletion of wc-2 in G. lingzhi
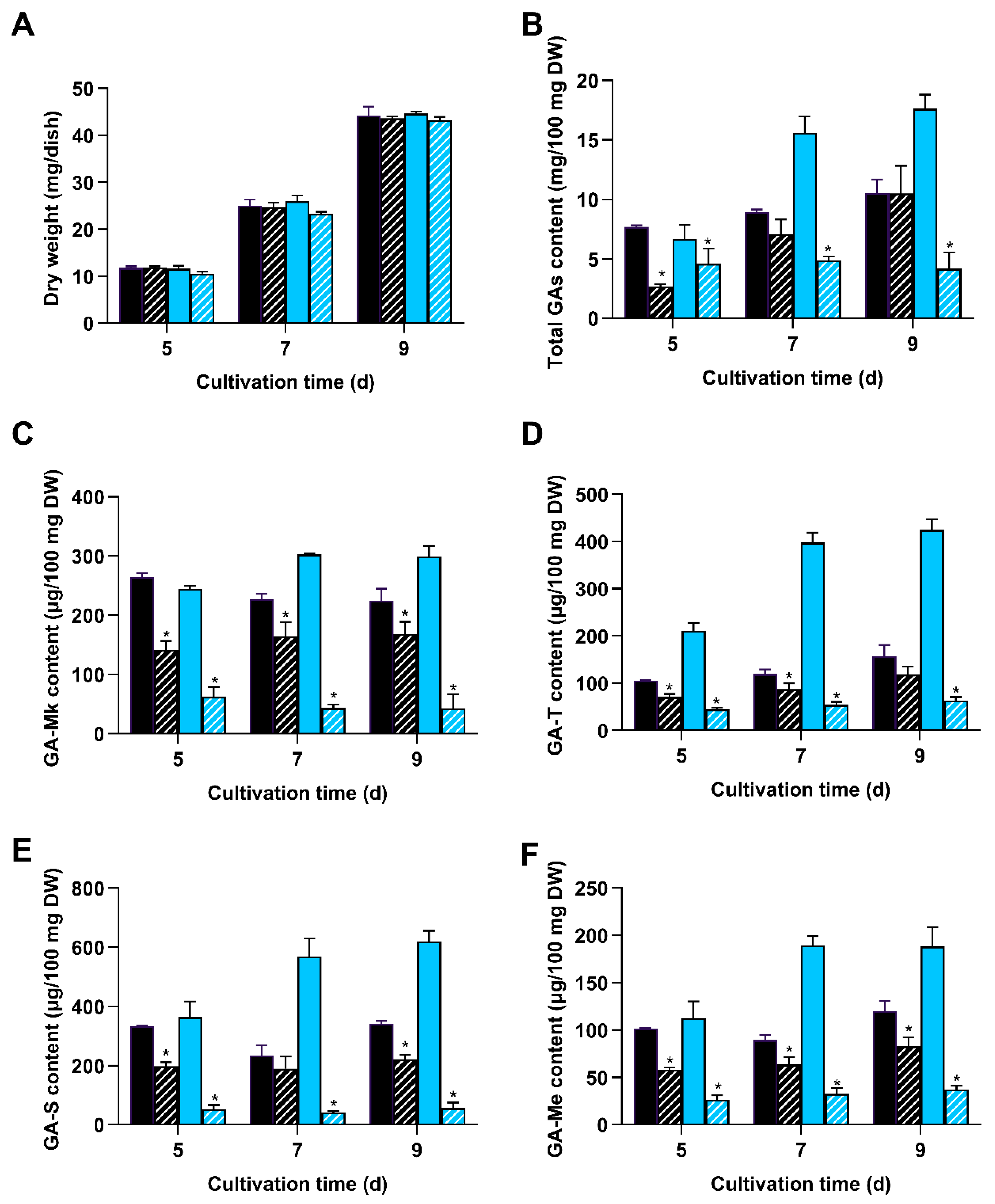
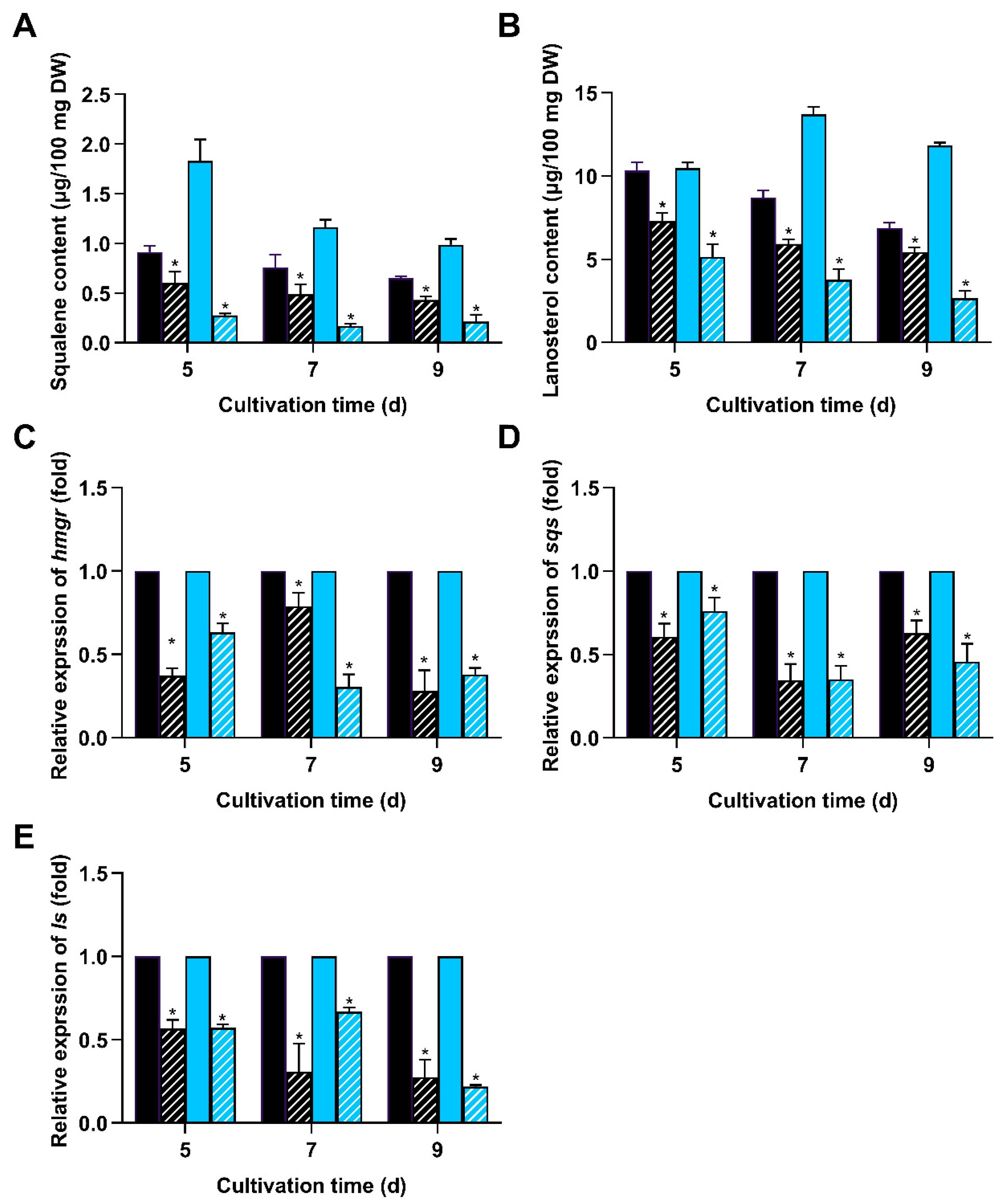
3.3. Deletion of wc-2 Significantly Reduced GA Biosynthesis
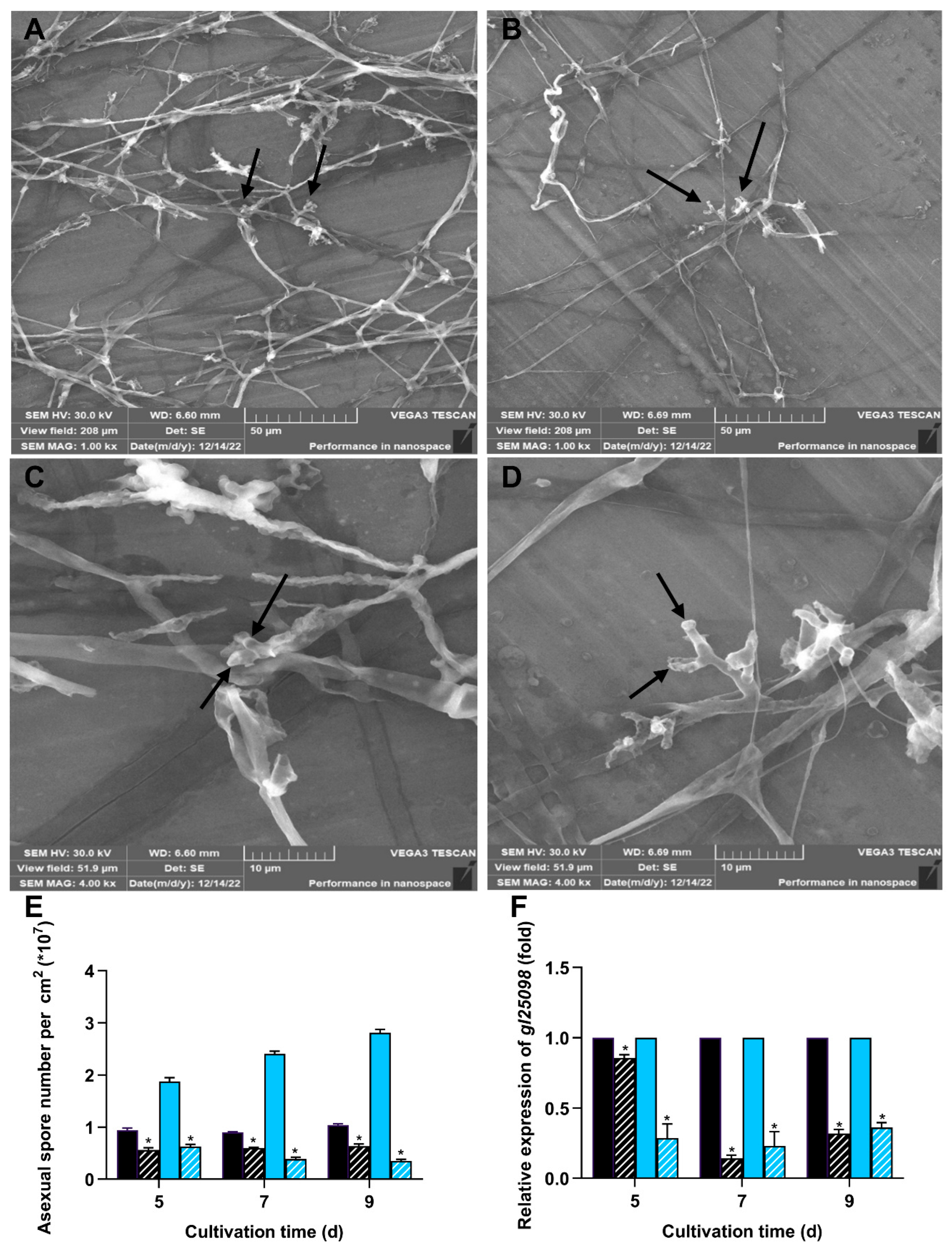
3.4. Deletion of wc-2 Significantly Impaired Sporulation
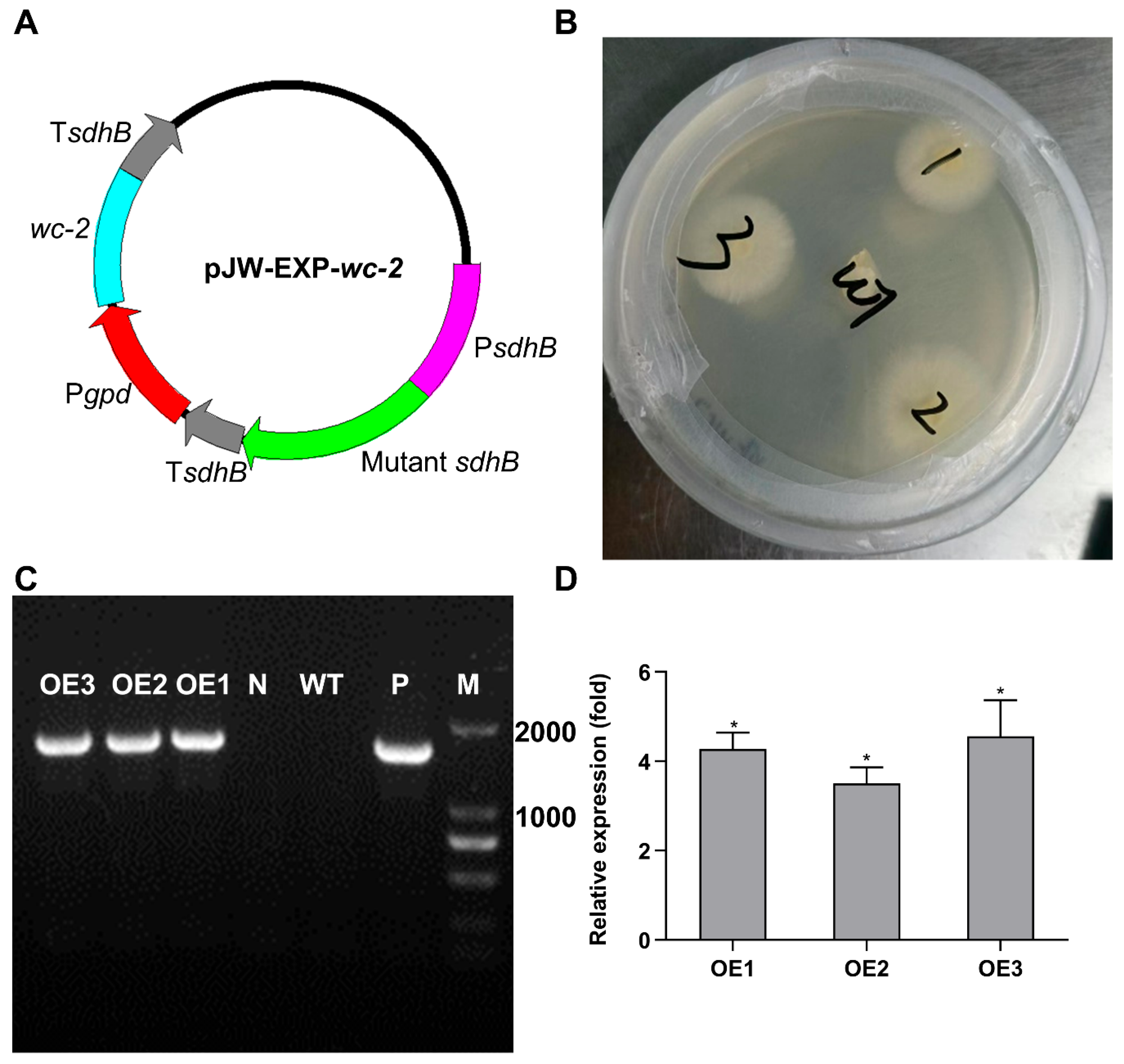
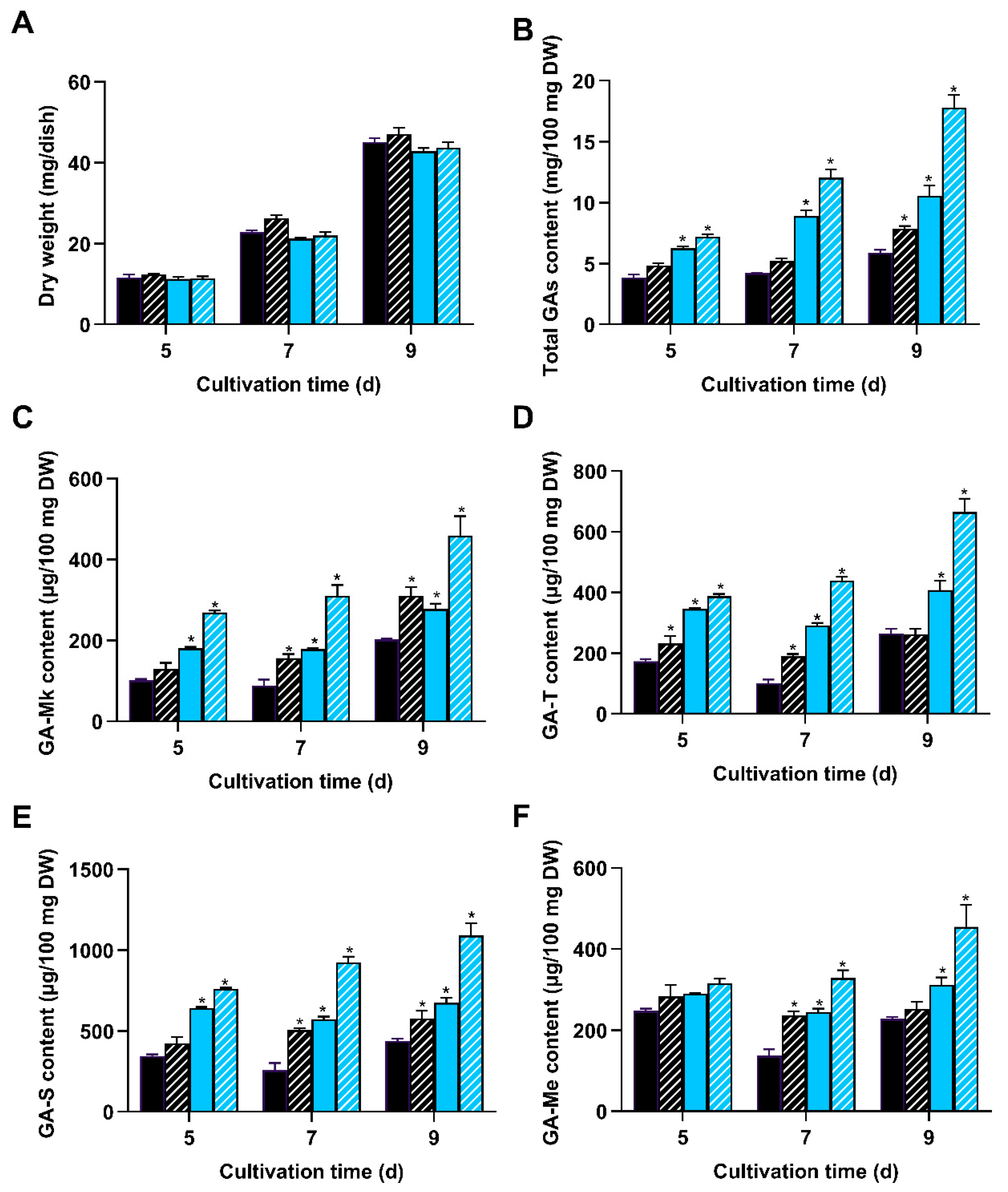
3.5. Overexpression of wc-2 Enhanced GA Content in G. lingzhi
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azi, F.; Wang, Z.; Chen, W.; Lin, D.; Xu, P. Developing Ganoderma lucidum as a next-generation cell factory for food and nutraceuticals. Trends Biotechnol. 2024, 42, 197–211. [Google Scholar] [CrossRef]
- Cor, D.; Knez, Z.; Knez Hrncic, M. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: A review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef]
- Xu, J.W.; Zhong, J.J. Genetic engineering of Ganoderma lucidum for the efficient production of ganoderic acids. Bioengineered 2015, 6, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.F.; Wahab, S.; Ahmad, F.A.; Ashraf, S.A.; Abullais, S.S.; Saad, H.H. Ganoderma lucidum: A potential pleiotropic approach of ganoderic acids in health reinforcement and factors influencing their production. Fungal Biol. Rev. 2022, 39, 100–125. [Google Scholar] [CrossRef]
- Chen, N.H.; Liu, J.W.; Zhong, J.J. Ganoderic acid T inhibits tumor invasion in vitro and in vivo through inhibition of MMP expression. Pharmacol. Rep. 2010, 62, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ren, A.; Mu, D.; Zhao, M. Current progress in the study on biosynthesis and regulation of ganoderic acids. Appl. Microbiol. Biotechnol. 2010, 88, 1243–1251. [Google Scholar] [CrossRef]
- Xu, J.W.; Xu, Y.N.; Zhong, J.J. Production of individual ganoderic acids and expression of biosynthetic genes in liquid static and shaking cultures of Ganoderma lucidum. Appl. Microbiol. Biotechnol. 2010, 85, 941–948. [Google Scholar] [CrossRef]
- Chen, S.; Xu, J.; Liu, C.; Zhu, Y.; Nelson, D.R.; Zhou, S.; Li, C.; Wang, L.; Guo, X.; Sun, Y.; et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat. Commun. 2012, 3, 913. [Google Scholar] [CrossRef]
- Sun, B.; You, H.; Xu, J.W. Enhancement of ganoderic acid production by promoting sporulation in a liquid static culture of Ganoderma species. J. Biotechnol. 2021, 328, 72–77. [Google Scholar] [CrossRef]
- Xu, J.W.; Xu, Y.N.; Zhong, J.J. Enhancement of ganoderic acid accumulation by overexpression of an N-terminally truncated 3-hydroxy-3-methylglutaryl coenzyme A reductase gene in the basidiomycete Ganoderma lucidum. Appl. Environ. Microbiol. 2012, 78, 7968–7976. [Google Scholar] [CrossRef]
- Wang, W.F.; Xiao, H.; Zhong, J.J. Biosynthesis of a ganoderic acid in Saccharomyces cerevisiae by expressing a cytochrome P450 gene from Ganoderma lucidum. Biotechnol. Bioeng. 2018, 115, 1842–1854. [Google Scholar] [CrossRef]
- Yang, C.; Li, W.; Li, C.; Zhou, Z.; Xiao, Y.; Yan, X. Metabolism of ganoderic acids by a Ganoderma lucidum cytochrome P450 and the 3-keto sterol reductase ERG27 from yeast. Phytochemistry 2018, 155, 83–92. [Google Scholar] [CrossRef]
- Yuan, W.; Jiang, C.; Wang, Q.; Fang, Y.; Wang, J.; Wang, M.; Xiao, H. Biosynthesis of mushroom-derived type II ganoderic acids by engineered yeast. Nat. Commun. 2022, 13, 7740. [Google Scholar] [CrossRef]
- Ren, A.; Shi, L.; Zhu, J.; Yu, H.S.; Jiang, A.L.; Zheng, H.H.; Zhao, M.W. Shedding light on the mechanisms underlying the environmental regulation of secondary metabolite ganoderic acid in Ganoderma lucidum using physiological and genetic methods. Fungal Genet. Biol. 2019, 128, 43–48. [Google Scholar] [CrossRef]
- Fei, Y.; Li, N.; Zhang, D.H.; Xu, J.W. Increased production of ganoderic acids by overexpression of homologous farnesyl diphosphate synthase and kinetic modeling of ganoderic acid production in Ganoderma lucidum. Microb. Cell Fact. 2019, 18, 115. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, H.J.; Xu, Y.; Wang, Z.X.; Sun, B.; Xu, J.W. Development of a 2A peptide-based multigene expression system and its application for enhanced production of ganoderic acids in Ganoderma lucidum. J. Biotechnol. 2024, 393, 109–116. [Google Scholar] [CrossRef]
- Zhang, D.H.; Li, N.; Yu, X.; Zhao, P.; Li, T.; Xu, J.W. Overexpression of the homologous lanosterol synthase gene in ganoderic acid biosynthesis in Ganoderma lingzhi. Phytochemistry 2017, 134, 46–53. [Google Scholar] [CrossRef]
- Zhou, J.S.; Ji, S.L.; Ren, M.F.; He, Y.L.; Jing, X.R.; Xu, J.W. Enhanced accumulation of individual ganoderic acids in a submerged culture of Ganoderma lucidum by the overexpression of squalene synthase gene. Biochem. Eng. J. 2014, 90, 178–183. [Google Scholar] [CrossRef]
- Li, H.J.; He, Y.L.; Zhang, D.H.; Yue, T.H.; Jiang, L.X.; Li, N.; Xu, J.W. Enhancement of ganoderic acid production by constitutively expressing Vitreoscilla hemoglobin gene in Ganoderma lucidum. J. Biotechnol. 2016, 227, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tang, Y.J. A novel three-stage light irradiation strategy in the submerged fermentation of medicinal mushroom Ganoderma lucidum for the efficient production of ganoderic acid and Ganoderma polysaccharides. Biotechnol. Prog. 2008, 24, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shi, L.; Chen, D.; Ren, A.; Gao, T.; Zhao, M. Functional analysis of the role of glutathione peroxidase (GPx) in the ROS signaling pathway, hyphal branching and the regulation of ganoderic acid biosynthesis in Ganoderma lucidum. Fungal Genet. Biol. 2015, 82, 168–180. [Google Scholar] [CrossRef]
- Liu, R.; Zhu, T.; Yang, T.; Yang, Z.; Ren, A.; Shi, L.; Zhu, J.; Yu, H.; Zhao, M. Nitric oxide regulates ganoderic acid biosynthesis by the S-nitrosylation of aconitase under heat stress in Ganoderma lucidum. Environ. Microbiol. 2021, 23, 682–695. [Google Scholar] [CrossRef]
- You, B.J.; Tien, N.; Lee, M.H.; Bao, B.Y.; Wu, Y.S.; Hu, T.C.; Lee, H.Z. Induction of apoptosis and ganoderic acid biosynthesis by cAMP signaling in Ganoderma lucidum. Sci. Rep. 2017, 7, 318. [Google Scholar] [CrossRef]
- Li, H.; Zhong, J.J. Role of calcineurin-responsive transcription factor CRZ1 in ganoderic acid biosynthesis by Ganoderma lucidum. Process Biochem. 2020, 95, 166–173. [Google Scholar] [CrossRef]
- Luo, Q.; Li, N.; Xu, J.W. A methyltransferase LaeA regulates ganoderic acid biosynthesis in Ganoderma lingzhi. Front. Microbiol. 2022, 13, 1025983. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.L.; Zhang, G.; Ren, A.; Dang, Z.H.; Shi, L.; Jiang, A.L.; Zhao, M.W. The pH-responsive transcription factor PacC regulates mycelial growth, fruiting body development, and ganoderic acid biosynthesis in Ganoderma lucidum. Mycologia 2016, 108, 1104–1113. [Google Scholar]
- Zhang, G.; Ren, A.; Shi, L.; Zhu, J.; Jiang, A.; Shi, D.; Zhao, M. Functional analysis of an APSES transcription factor (GlSwi6) involved in fungal growth, fruiting body development and ganoderic-acid biosynthesis in Ganoderma lucidum. Microbiol. Res. 2018, 207, 280–288. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, Z.; Shi, D.; Song, S.Q.; Lian, L.D.; Shi, L.; Ren, A.; Yu, H.S.; Zhao, M.W. Dual functions of AreA, a GATA transcription factor, on influencing ganoderic acid biosynthesis in Ganoderma lucidum. Environ. Microbiol. 2019, 21, 4166–4179. [Google Scholar] [CrossRef] [PubMed]
- Corrochano, L.M. Light in the fungal world: From photoreception to gene transcription and beyond. Annu. Rev. Genet. 2019, 53, 149–170. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, Y.; Guo, W.; Zhang, X.; Zheng, W.; Chen, X. Study on the effects of different light supply modes on the development and extracellular enzyme activity of Ganoderma lucidum. Agriculture 2024, 14, 835. [Google Scholar] [CrossRef]
- Poyedinok, N.L.; Mykhailova, O.B.; Shcherba, V.V.; Buchalo, A.S.; Negriyko, A.M. Light regulation of growth and biosynthetic activity of Ling Zhi or Reishi medicinal mushroom, Ganoderma lucidum (W. Curt.: Fr.) P. Karst. (Aphyllophoromycetideae), in pure culture. Int. J. Med. Mushr. 2008, 10, 369–378. [Google Scholar] [CrossRef]
- Ballario, P.; Vittorioso, P.; Magrelli, A.; Talora, C.; Cabibbo, A.; Macino, G. White collar-1, a central regulator of blue light responses in Neurospora, is a zinc finger protein. EMBO J. 1996, 15, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Fuller, K.K.; Dunlap, J.C.; Loros, J.J. Seeing the world differently: Variability in the photosensory mechanisms of two model fungi. Environ. Microbiol. 2016, 18, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Son, H.; Lee, Y.W. Effects of light on secondary metabolism and fungal development of Fusarium graminearum. J. Appl. Microbiol. 2014, 116, 380–389. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Yu, W.; Liu, Y.; Zhang, W.; Lan, J. Cloning and analysis of the Glwc-1 and Glwc-2 genes encoding putative blue light photoreceptor from Ganoderma lucidum. J. Basic Microbiol. 2017, 57, 705–711. [Google Scholar] [CrossRef]
- Zhang, W.X.; Zhong, J.J. Effect of oxygen concentration in gas phase on sporulation and individual ganoderic acids accumulation in liquid static culture of Ganoderma lucidum. J. Biosci. Bioeng. 2010, 109, 37–40. [Google Scholar] [CrossRef]
- Xu, J.W.; Zhao, W.; Xu, Y.N.; Zhong, J.J. Isolation and analysis of differentially expressed genes during asexual sporulation in liquid static culture of Ganoderma lucidum by suppression subtractive hybridization. Mol. Biol. Rep. 2012, 39, 3603–3610. [Google Scholar] [CrossRef]
- Ruger-Herreros, C.; Corrochano, L.M. Conidiation in Neurospora crassa: Vegetative reproduction by a model fungus. Int. Microbiol. 2020, 23, 97–105. [Google Scholar] [CrossRef]
- Yu, X.; Ji, S.L.; He, Y.L.; Ren, M.F.; Xu, J.W. Development of an expression plasmid and its use in genetic manipulation of Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (higher Basidiomycetes). Int. J. Med. Mushrooms 2014, 16, 161–168. [Google Scholar] [CrossRef]
- Xu, J.W.; Yue, T.H.; Yu, X.; Zhao, P.; Li, T.; Li, N. Enhanced production of individual ganoderic acids by integrating Vitreoscilla haemoglobin expression and calcium ion induction in liquid static cultures of Ganoderma lingzhi. Microb. Biotechnol. 2019, 12, 1180–1187. [Google Scholar] [CrossRef]
- Zhang, D.H.; Jiang, L.X.; Li, N.; Yu, X.; Zhao, P.; Li, T.; Xu, J.W. Overexpression of the squalene epoxidase gene alone and in combination with the 3-hydroxy-3-methylglutaryl Coenzyme A gene increases ganoderic acid production in Ganoderma lingzhi. J. Agric. Food Chem. 2017, 65, 4683–4690. [Google Scholar] [CrossRef]
- Krobanan, K.; Liang, S.W.; Chiu, H.C.; Shen, W.C. The blue-light photoreceptor Sfwc-1 gene regulates the phototropic response and fruiting-body development in the homothallic ascomycete Sordaria fimicola. Appl. Environ. Microbiol. 2019, 85, e02206-18. [Google Scholar] [CrossRef] [PubMed]
- Linden, H.; Macino, G. White collar 2, a partner in blue-light signal transduction, controlling expression of light-regulated genes in Neurospora crassa. EMBO J. 1997, 16, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Torres-Martínez, S.; Garre, V. Distinct white collar-1 genes control specific light responses in Mucor circinelloides. Mol. Microbiol. 2006, 61, 1023–1037. [Google Scholar] [CrossRef]
- Yang, T.; Guo, M.; Yang, H.; Guo, S.; Dong, C. The blue-light receptor CmWC-1 mediates fruit body development and secondary metabolism in Cordyceps militaris. Appl. Microbiol. Biotechnol. 2016, 100, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ding, R.; Liu, Y.; Li, F.; Zhang, Z.; Wang, S. GATA transcription factor WC2 regulates the biosynthesis of astaxanthin in yeast Xanthophyllomyces dendrorhous. Microb. Biotechnol. 2022, 15, 2578–2593. [Google Scholar] [CrossRef]
- Casas-Flores, S.; Rios-Momberg, M.; Bibbins, M.; Ponce-Noyola, P.; Herrera-Estrella, A. BLR-1 and BLR-2, key regulatory elements of photoconidiation and mycelial growth in Trichoderma atroviride. Microbiology 2004, 150, 3561–3569. [Google Scholar] [CrossRef]
- Castellanos, F.; Schmoll, M.; Martínez, P.; Tisch, D.; Kubicek, C.P.; Herrera-Estrella, A.; Esquivel-Naranjo, E.U. Crucial factors of the light perception machinery and their impact on growth and cellulase gene transcription in Trichoderma reesei. Fungal Genet. Biol. 2010, 47, 468–476. [Google Scholar] [CrossRef]
- Denault, D.L.; Loros, J.J.; Dunlap, J.C. WC-2 mediates WC-1-FRQ interaction within the PAS protein-linked circadian feedback loop of Neurospora. EMBO J. 2001, 20, 109–117. [Google Scholar] [CrossRef]
- Olmedo, M.; Ruger-Herreros, C.; Corrochano, L.M. Regulation by blue light of the fluffy gene encoding a major regulator of conidiation in Neurospora crassa. Genetics 2010, 184, 651–658. [Google Scholar] [CrossRef]
- Xu, S.Y.; Yu, L.; Luo, X.C.; Ying, S.H.; Feng, M.G. Co-regulatory roles of WC1 and WC2 in asexual development and photoreactivation of Beauveria bassiana. J. Fungi 2023, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.X.; Li, Y.B.; Xu, J.W.; Wang, J.L.; Miao, X.L.; Tang, Y.J.; Gu, T.; Zhong, J.J. Enhanced biosynthetic gene expressions and production of ganoderic acids in static liquid culture of Ganoderma lucidum under phenobarbital induction. Appl. Microbiol. Biotechnol. 2010, 86, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Huang, X.-M.; Wang, Z.-X.; Zhao, Y.-J.; Lv, D.-M.; Xu, J.-W. Blue Light Receptor WC-2 Regulates Ganoderic Acid Biosynthesis in Ganoderma lingzhi. J. Fungi 2025, 11, 646. https://doi.org/10.3390/jof11090646
Xu Y, Huang X-M, Wang Z-X, Zhao Y-J, Lv D-M, Xu J-W. Blue Light Receptor WC-2 Regulates Ganoderic Acid Biosynthesis in Ganoderma lingzhi. Journal of Fungi. 2025; 11(9):646. https://doi.org/10.3390/jof11090646
Chicago/Turabian StyleXu, Yan, Xiong-Min Huang, Zi-Xu Wang, Ying-Jie Zhao, Dong-Mei Lv, and Jun-Wei Xu. 2025. "Blue Light Receptor WC-2 Regulates Ganoderic Acid Biosynthesis in Ganoderma lingzhi" Journal of Fungi 11, no. 9: 646. https://doi.org/10.3390/jof11090646
APA StyleXu, Y., Huang, X.-M., Wang, Z.-X., Zhao, Y.-J., Lv, D.-M., & Xu, J.-W. (2025). Blue Light Receptor WC-2 Regulates Ganoderic Acid Biosynthesis in Ganoderma lingzhi. Journal of Fungi, 11(9), 646. https://doi.org/10.3390/jof11090646





