What Is the Prognostic Significance of Culture-Documented Breakthrough Invasive Pulmonary Aspergillosis in Patients with Hematological Malignancies? A Propensity Score-Adjusted Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Definitions
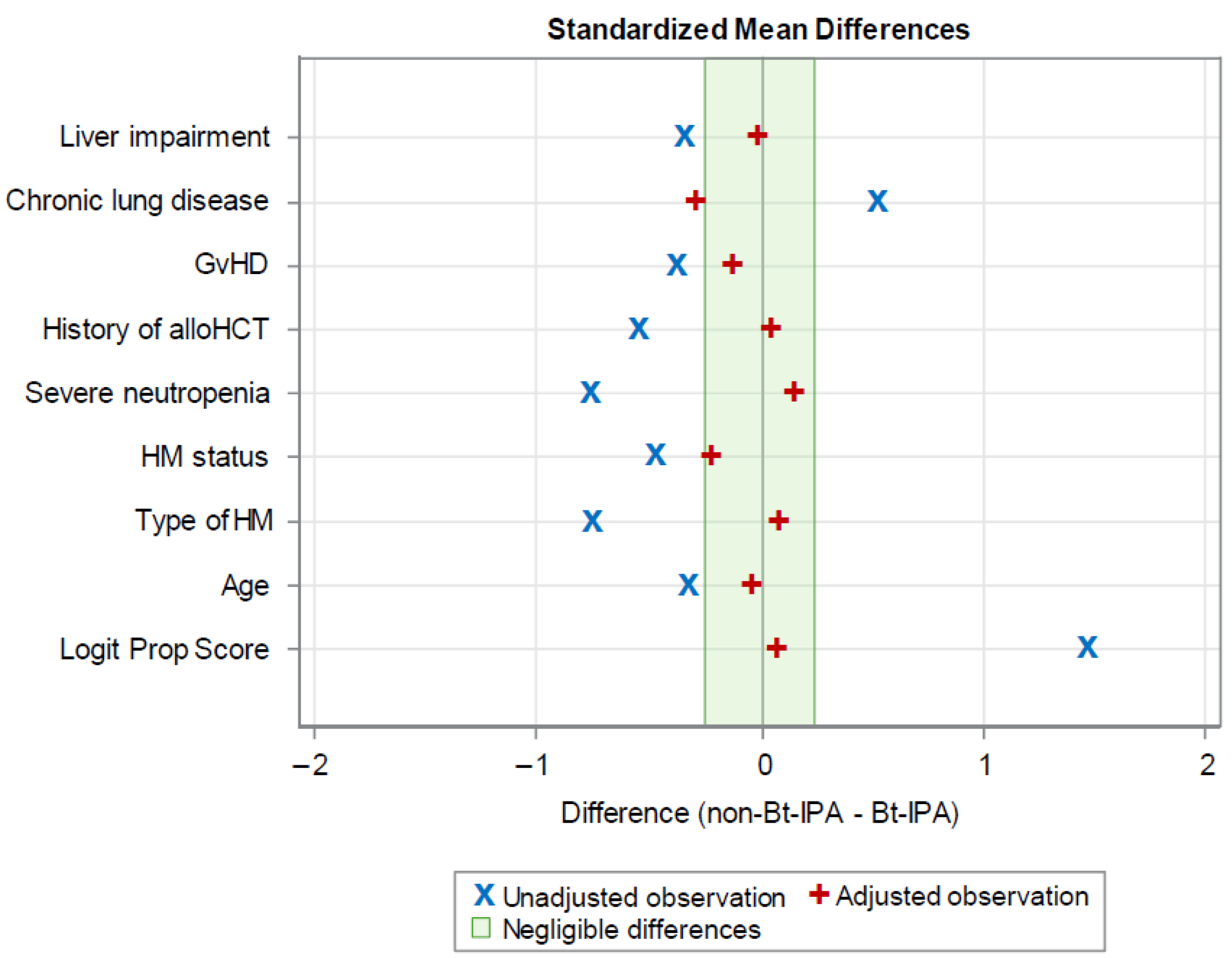
2.3. Statistical Analyses
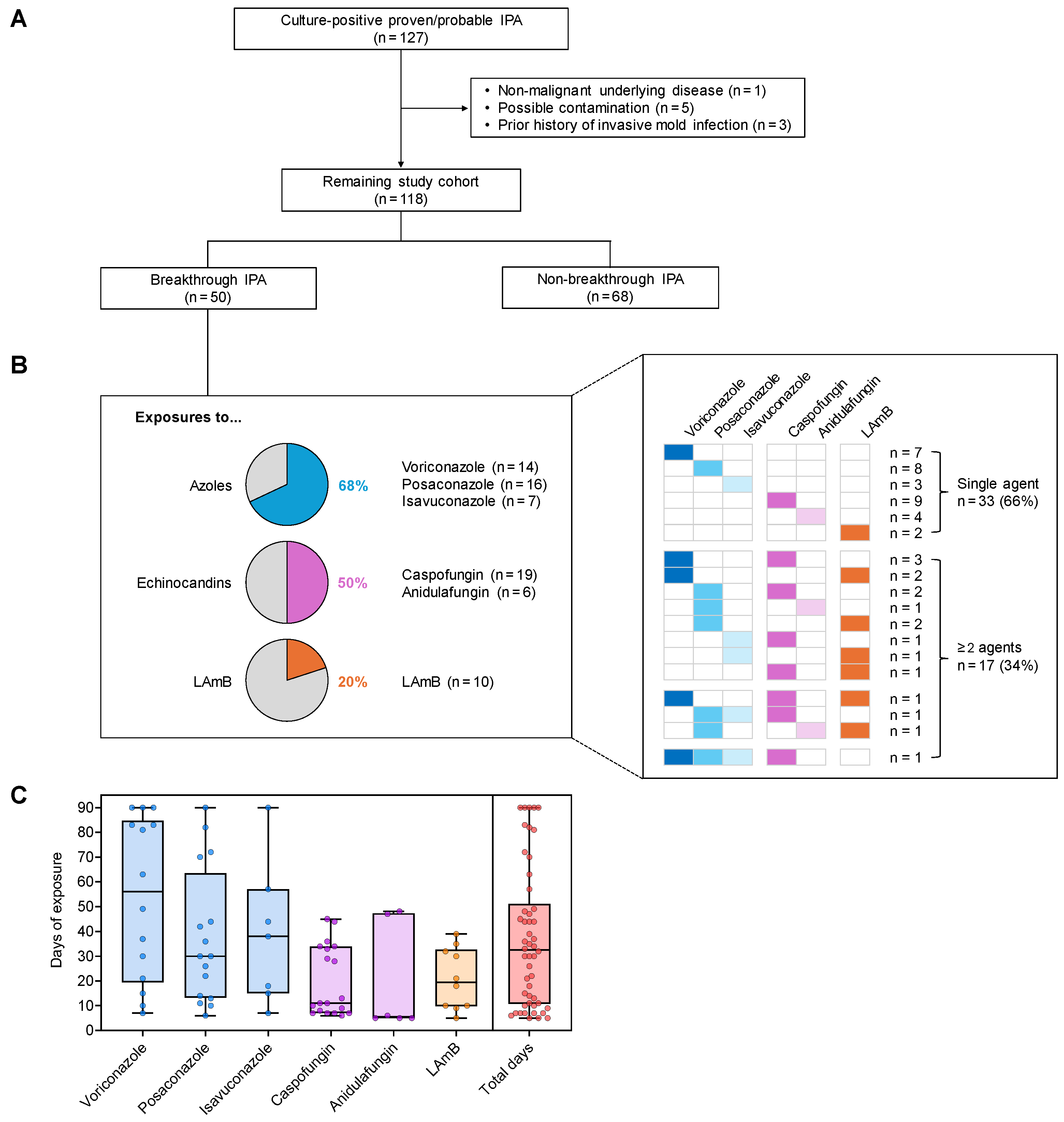
3. Results
3.1. Patient Demographics
3.2. Prior Antifungal Drug Exposure of Patients with Bt-IPA
3.3. Clinical Characteristics of Bt-IPA vs. Non-Bt-IPA
3.4. Distribution of Aspergillus Species
3.5. Therapeutic Drug Monitoring
3.6. Univariate Analysis of Factors Associated with 42-Day Mortality After IPA Diagnosis
3.7. Independent Predictors of 42-Day Mortality After IPA Diagnosis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Variable | Observations | Mean Difference | Standard Deviation | Standardized Difference | Percent Reduction | Variance Ratio |
|---|---|---|---|---|---|---|
| Logit Prop Score | Unadjusted | 2.38 | 1.63 | 1.46 | 0.95 | |
| Adjusted | 0.12 | 0.07 | 95.0 | 0.83 | ||
| Age | Unadjusted | −5.11 | 15.49 | −0.33 | 1.18 | |
| Adjusted | −0.81 | −0.05 | 84.2 | 0.89 | ||
| Type of HMs | Unadjusted | −0.36 | 0.47 | −0.76 | 0.99 | |
| Adjusted | 0.04 | 0.08 | 89.6 | 1.00 | ||
| HM status | Unadjusted | −0.18 | 0.38 | −0.47 | 0.45 | |
| Adjusted | −0.09 | −0.23 | 50.7 | 0.59 | ||
| Severe neutropenia | Unadjusted | −0.34 | 0.44 | −0.77 | 1.72 | |
| Adjusted | 0.07 | 0.15 | 80.7 | 0.92 | ||
| History of alloHCT | Unadjusted | −0.22 | 0.39 | −0.55 | 2.36 | |
| Adjusted | 0.02 | 0.04 | 92.4 | 0.94 | ||
| GvHD | Unadjusted | −0.12 | 0.32 | −0.38 | 2.67 | |
| Adjusted | −0.04 | −0.14 | 63.9 | 1.63 | ||
| Chronic lung diseases | Unadjusted | 0.14 | 0.28 | 0.51 | 0.14 | |
| Adjusted | −0.08 | −0.29 | 42.2 | 1.66 | ||
| Liver impairment | Unadjusted | −0.15 | 0.44 | −0.35 | 1.41 | |
| Adjusted | −0.01 | −0.03 | 91.7 | 1.04 |
References
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Lto, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef]
- Pagano, L.; Caira, M.; Candoni, A.; Offidani, M.; Fianchi, L.; Martino, B.; Pastore, D.; Picard, M.; Bonini, A.; Chierichini, A.; et al. The epidemiology of fungal infections in patients with hematologic malignancies: The SEIFEM-2004 study. Haematologica 2006, 91, 1068–1075. [Google Scholar] [PubMed]
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- Cadena, J.; Thompson, G.R.; Patterson, T.F. Aspergillosis: Epidemiology, diagnosis, and treatment. Infect. Dis. Clin. N. Am. 2021, 35, 415–434. [Google Scholar] [CrossRef]
- Cornely, O.A.; Maertens, J.; Winston, D.J.; Perfect, J.; Ullmann, A.J.; Walsh, T.J.; Helfgott, D.; Holowiecki, J.; Stockelberg, D.; Goh, Y.-T.; et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 2007, 356, 348–359. [Google Scholar] [CrossRef]
- Boutin, C.-A.; Durocher, F.; Beauchemin, S.; Ziegler, D.; Abou Chakra, C.N.; Dufresne, S.F. Breakthrough invasive fungal infections in patients with high-risk hematological disorders receiving voriconazole and posaconazole prophylaxis: A systematic review. Clin. Infect. Dis. 2024, 79, 151–160. [Google Scholar] [CrossRef]
- Rausch, C.R.; DiPippo, A.J.; Bose, P.; Kontoyiannis, D.P. Breakthrough fungal infections in patients with leukemia receiving isavuconazole. Clin. Infect. Dis. 2018, 67, 1610–1613. [Google Scholar] [CrossRef]
- Axell-House, D.B.; Wurster, S.; Jiang, Y.; Kyvernitakis, A.; Lewis, R.E.; Tarrand, J.J.; Raad, I.I.; Kontoyiannis, D.P. Breakthrough Mucormycosis Developing on Mucorales-active antifungals portrays a poor prognosis in patients with hematologic cancer. J. Fungi 2021, 7, 217. [Google Scholar] [CrossRef]
- Bose, P.; McCue, D.; Wurster, S.; Wiederhold, N.P.; Konopleva, M.; Kadia, T.M.; Borthakur, G.; Ravandi, F.; Masarova, L.; Takahashi, K.; et al. Isavuconazole as primary antifungal prophylaxis in patients with acute myeloid leukemia or myelodysplastic syndrome: An open-label, prospective, phase 2 study. Clin. Infect. Dis. 2021, 72, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.Z.; Jiang, Y.; Mulanovich, V.E.; Lewis, R.E.; Kontoyiannis, D.P. Effectiveness of primary anti-Aspergillus prophylaxis during remission induction chemotherapy of acute myeloid leukemia. Antimicrob. Agents Chemother. 2014, 58, 2775–2780. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Heo, S.T.; Tatara, A.M.; Jiménez-Ortigosa, C.; Jiang, Y.; Lewis, R.E.; Tarrand, J. Changes in In Vitro Susceptibility Patterns of Aspergillus to Triazoles and Correlation with Aspergillosis Outcome in a Tertiary Care Cancer Center, 1999–2015. Clin. Infect. Dis. 2017, 65, 216–225. [Google Scholar] [CrossRef]
- Cornely, O.A.; Hoenigl, M.; Lass-Flörl, C.; Chen, S.C.; Kontoyiannis, D.P.; Morrissey, C.O.; Tverdek, F.; Albert, N.D.; Verweij, P.E.; Meis, J.F.; et al. Defining breakthrough invasive fungal infection-position paper of the mycoses study group education and research consortium (MSG-ERC) and the European Confederation of Medical Mycology (ECMM). Mycoses 2019, 62, 716–729. [Google Scholar] [CrossRef]
- Tverdek, F.P.; Heo, S.T.; Aitken, S.L.; Granwehr, B.; Kontoyiannis, D.P. Real-life assessment of the safety and effectiveness of the new tablet and intravenous formulations of posaconazole in the prophylaxis of invasive fungal infections via analysis of 343 courses. Antimicrob. Agents Chemother. 2017, 61, e00188-17. [Google Scholar] [CrossRef]
- Schoemans, H.M.; Lee, S.J.; Ferrara, J.L.; Wolff, D.; Levine, J.E.; Schultz, K.R.; Shaw, B.E.; Flowers, M.E.; Ruutu, T.; Greinix, H.; et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018, 53, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.A.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Younossi, Y.; Golabi, P.; Mishra, A.; Rafiq, N.; Henry, L. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020, 69, 564–568. [Google Scholar] [CrossRef]
- Puerta-Alcalde, P.; Monzó-Gallo, P.; Aguilar-Guisado, M.; Ramos, J.C.; Laporte-Amargós, J.; Machado, M.; Martin-Davilg, P.; Franch-Sarto, M.; Sánchez-Romero, I.; Badiola, J.; et al. Breakthrough invasive fungal infection among patients with haematologic malignancies: A national, prospective, and multicenter study. J. Infect. 2023, 87, 46–53. [Google Scholar] [CrossRef]
- Nucci, M.; Perfect, J.R. When primary antifungal therapy fails. Clin. Infect. Dis. 2008, 46, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Lewis, R.E.; Kontoyiannis, D.P. Breakthrough Invasive Mold Infections in the Hematology Patient: Current Concepts and Future Directions. Clin. Infect. Dis. 2018, 67, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Selleslag, D.; Mullane, K.; Cornely, O.A.; Hope, W.; Lortholary, O.; Croos-Dabrera, R.; Lademacher, C.; Engelhardt, M.; Patterson, T.F. Impact of unresolved neutropenia in patients with neutropenia and invasive aspergillosis: A post hoc analysis of the SECURE trial. J. Antimicrob. Chemother. 2018, 73, 757–763. [Google Scholar] [CrossRef]
- Wiedermann, C.J. Hypoalbuminemia as Surrogate and Culprit of Infections. Int. J. Mol. Sci. 2021, 22, 4496. [Google Scholar] [CrossRef]
- Austermeier, S.; Pekmezović, M.; Porschitz, P.; Lee, S.; Kichik, N.; Moyes, D.L.; Ho, J.; Kotowicz, N.K.; Naglik, J.R.; Hube, B.; et al. Albumin Neutralizes Hydrophobic Toxins and Modulates Candida albicans Pathogenicity. mBio 2021, 12, e0053121. [Google Scholar] [CrossRef]
- Pikoulas, A.; Morianos, I.; Nidris, V.N.; Hamdy, R.; López-López, A.; Moran-Garrido, M.; Halabalaki, M.M.; Papadovasilaki, M.; Irene, K.; Gu, Y.; et al. Albumin orchestrates a natural host defense mechanism against mucormycosis. Res. Sq. 2024, rs.3.rs-5441197. [Google Scholar] [CrossRef]
- Vanstraelen, K.; Wauters, J.; Vercammen, I.; de Loor, H.; Maertens, J.; Lagrou, K.; Annaert, P.; Spriet, I. Impact of Hypoalbuminemia on Voriconazole Pharmacokinetics in Critically Ill Adult Patients. Antimicrob. Agents Chemother. 2014, 58, 6782–6789. [Google Scholar] [CrossRef]
- Ghez, D.; Calleja, A.; Protin, C.; Baron, M.; Ledoux, M.P.; Damaj, G.; Dupont, M.; Dreyfus, B.; Ferrant, E.; Herbaux, C.; et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood 2018, 131, 1955–1959. [Google Scholar] [CrossRef]
- Little, J.S.; Weiss, Z.F.; Hammond, S.P. Invasive fungal infections and targeted therapies in hematological malignancies. J. Fungi 2021, 7, 1058. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.R., 3rd; Boulware, D.R.; Bahr, N.C.; Clancy, C.J.; Harrison, T.S.; Kauffman, C.A.; Le, T.; Miceli, M.H.; Mylonakis, E.; Nguyen, M.H.; et al. Noninvasive Testing and Surrogate Markers in Invasive Fungal Diseases. Open Forum Infect. Dis. 2022, 9, ofac112. [Google Scholar] [CrossRef]
- Lamoth, F.; Chung, S.J.; Damonti, L.; Alexander, B.D. Changing Epidemiology of Invasive Mold Infections in Patients Receiving Azole Prophylaxis. Clin. Infect. Dis. 2017, 64, 1619–1621. [Google Scholar] [CrossRef]
- Fung, M.; Schwartz, B.S.; Doernberg, S.B.; Langelier, C.; Lo, M.; Graff, L.; Tan, M.; Logan, A.C.; Chin-Hong, P.; Babik, J.M. Breakthrough Invasive Fungal Infections on Isavuconazole Prophylaxis and Treatment: What Is Happening in the Real-World Setting? Clin. Infect. Dis. 2018, 67, 1142–1143. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Cho, S.Y.; Lee, D.G.; Choi, J.K.; Lee, H.J.; Kim, S.H.; Park, S.H.; Choi, S.-M.; Choi, J.-H.; Yoo, J.-H.; et al. Breakthrough invasive fungal diseases during voriconazole treatment for aspergillosis: A 5-year retrospective cohort study. Med. Mycol. 2017, 55, 237–245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lerolle, N.; Raffoux, E.; Socie, G.; Touratier, S.; Sauvageon, H.; Porcher, R.; Bretagne, S.; Bergeron, A.; Azoulay, E.; Molina, J.-M.; et al. Breakthrough invasive fungal disease in patients receiving posaconazole primary prophylaxis: A 4-year study. Clin. Microbiol. Infect. 2014, 20, O952–O959. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Lewis, R.E.; Kontoyiannis, D.P. Role and Interpretation of Antifungal Susceptibility Testing for the Management of Invasive Fungal Infections. J. Fungi 2020, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Hajjeh, R.A.; Warnock, D.W. Counterpoint: Invasive aspergillosis and the environment--rethinking our approach to prevention. Clin. Infect. Dis. 2001, 33, 1549–1552. [Google Scholar] [CrossRef]
- Sipsas, N.V.; Kontoyiannis, D.P. Occupation, lifestyle, diet, and invasive fungal infections. Infection 2008, 36, 515–525. [Google Scholar] [CrossRef]
- Rubin, D.B. Using Propensity Scores to Help Design Observational Studies: Application to the Tobacco Litigation. Health Serv. Outcomes Res. Methodol. 2001, 2, 169–188. [Google Scholar] [CrossRef]

| Characteristics # | All Patients | Bt-IPA | Non-Bt-IPA | p-Value |
|---|---|---|---|---|
| (N = 118) | (N = 50) | (N = 68) | ||
| Baseline | ||||
| Age (years), median (range) | 63 (22–89) | 61 (22–77) | 64 (25–89) | 0.122 |
| Gender, male, n (%) | 75 (63.6) | 35 (70.0) | 40 (58.8) | 0.213 |
| Race, n (%) | 0.359 | |||
| White | 81 (68.6) | 32 (64.0) | 49 (72.1) | |
| Hispanic | 15 (12.7) | 6 (12.0) | 9 (13.2) | |
| Asian | 10 (8.5) | 5 (10.0) | 5 (7.4) | |
| Black | 8 (6.8) | 6 (12.0) | 2 (2.9) | |
| Others | 4 (3.4) | 1 (2.0) | 3 (4.4) | |
| Type of hematological malignancy, n (%) | <0.001 | |||
| AML/ALL/MDS | 56 (47.5) | 34 (68.0) | 22 (32.4) | |
| Lymphoma/MM/CLL | 62 (52.5) | 16 (32.0) | 46 (67.7) | |
| Hematological malignancy status, n (%) | 0.017 | |||
| Active | 94 (79.7) | 45 (90.0) | 49 (72.1) | |
| Remission | 24 (20.3) | 5 (10.0) | 19 (27.9) | |
| Neutropenia (ANC <500/mm3), n (%) | 49 (41.5) | 29 (58.0) | 20 (29.4) | 0.002 |
| Severe neutropenia (ANC <100 mm3), n (%) | 38 (32.2) | 26 (52.0) | 12 (17.7) | <0.001 |
| History of allogenic HCT, n (%) | 23 (19.5) | 16 (32.0) | 7 (10.3) | 0.003 |
| History of autologous HCT, n (%) | 14 (11.9) | 2 (4.0) | 12 (17.6) | 0.063 |
| GvHD, n (%) | 13 (11.0) | 9 (18.0) | 4 (5.9) | 0.038 |
| Hypoalbuminemia <3.0 mg/dL | 54 (45.8) | 25 (50.0) | 29 (42.6) | 0.848 |
| Other comorbidities | ||||
| Diabetes mellitus, n (%) | 19 (16.1) | 6 (12.0) | 13 (19.1) | 0.299 |
| Chronic renal insufficiency, n (%) | 16 (13.6) | 6 (12.0) | 10 (14.7) | 0.671 |
| Chronic lung diseases, n (%) | 12 (10.2) | 1 (2.0) | 11 (16.2) | 0.012 |
| Liver impairment, n (%) | 32 (27.1) | 18 (36.0) | 14 (20.6) | 0.063 |
| Serum albumin <3.0 mg/dL, n (%) | 54 (45.8) | 25 (50.0) | 29 (42.7) | 0.428 |
| Previous corticosteroid use, n (%) | 29 (24.6) | 11 (22.0) | 18 (26.5) | 0.577 |
| Aspergillus species a, n (%) | 0.028 * | |||
| IPA caused by four common species b | 99/115 (86.1) | 39 (78.0) | 60/65 (92.3) | |
| A. fumigatus | 48/115 (41.7) | 20 (40.0) | 28/65 (43.1) | 0.946 |
| Non-A. fumigatus | 51/115 (44.3) | 19 (38.0) | 32/65 (49.2) | 0.486 |
| IPA caused by rare species c | 13/112 (11.6) | 10/49 (20.4) | 3/63 (4.8) | |
| Mixed Aspergillus species, n (%) | 5 (4.2) | 2 (4.0) | 3 (4.4) | >0.999 |
| Antifungal therapy of IPA, n (%) | 0.002 | |||
| Azole monotherapy | 63/115 (54.8) | 19 (38.0) | 44/65 (67.7) | |
| Liposomal amphotericin B monotherapy | 3/115 (2.5) | 0 (0) | 3/65 (4.6) | |
| Echinocandin monotherapy | 3/115 (2.5) | 1 (2.0) | 2/65 (3.1) | |
| Azole + Liposomal amphotericin B | 21/115 (17.8) | 15 (30.0) | 6/65 (9.2) | |
| Azole + Echinocandin | 15/115 (13.0) | 7 (14.0) | 8/65 (12.3) | |
| Liposomal amphotericin B + Echinocandin | 10/115 (8.7) | 8 (16.0) | 2/65 (3.1) | |
| Outcome | ||||
| 42-day mortality d, n (%) | 57/116 (49.1) | 32/49 (65.3) | 25/67 (37.3) | 0.003 |
| Characteristics # | Survived (N = 59) a | Died (N = 57) a | p-Value |
|---|---|---|---|
| Age (years), median (range) | 60 (22–82) | 64 (24–85) | 0.336 |
| Gender, male, n (%) | 36 (61.0) | 37 (64.9) | 0.664 |
| Race, n (%) | 0.669 | ||
| White | 37 (62.7) | 42 (73.7) | |
| Hispanic | 10 (17.0) | 5 (8.8) | |
| Asian | 6 (10.2) | 4 (7.0) | |
| Black | 4 (6.8) | 4 (7.0) | |
| Others | 2 (3.4) | 2 (3.5) | |
| Type of hematological malignancy, n (%) | 0.064 | ||
| AML/ALL/MDS | 23 (39.0) | 32 (56.1) | |
| Lymphoma/MM/CLL | 36 (61.0) | 25 (43.9) | |
| Hematological malignancy status, n (%) | 0.008 | ||
| Active | 41 (69.5) | 51 (89.5) | |
| Remission | 18 (30.5) | 6 (10.5) | |
| Neutropenia (ANC <500/mm3), n (%) | 16 (27.1) | 33 (57.9) | <0.001 |
| Severe neutropenia (ANC <100/mm3), n (%) | 11 (18.6) | 27 (47.4) | 0.001 |
| Neutropenia status until day 42 after IPA diagnosis, n (%) | 0.002 | ||
| Neutropenia at IPA diagnosis, no recovery | 10 (17.0) | 26 (45.6) | |
| Neutropenia at IPA diagnosis, recovery | 6 (10.2) | 7 (12.3) | |
| No neutropenia at IPA diagnosis | 43 (72.9) | 24 (42.1) | |
| History of alloHCT, n (%) | 11 (18.6) | 12 (21.1) | 0.745 |
| GvHD, n (%) | 8 (13.6) | 5 (8.8) | 0.414 |
| Serum albumin <3.0 mg/dL, n (%) | 16 (27.1) | 37 (64.9) | <0.001 |
| Previous steroids use, n (%) | 18 (30.5) | 11 (19.3) | 0.163 |
| Aspergillus species, n (%) | 0.305 | ||
| IPA caused by four common species b | 52/57 (91.2) | 45/53 (84.9) | |
| IPA caused by rare species | 5/57 (8.8) | 8/53 (15.1) | |
| Mixed Aspergillus species, n (%) | 1 (1.7) | 4 (7.0) | 0.203 |
| Breakthrough IPA, n (%) | 17 (28.8) | 32 (56.1) | 0.003 |
| Duration of antifungal exposure before breakthrough-IPA | 35 (5–90) | 31 (7–83) | 0.602 |
| Antifungal therapy of IPA, n (%) | 0.054 | ||
| Monotherapy | 40 (67.8) | 27/54 c (50.0) | |
| Combination therapy | 19 (32.2) | 27/54 c (50.0) |
| (A) Before IPTW Adjustment | |||
| Variables # | aHR | 95% CI | p-Value |
| Neutropenia (ANC <500/mm3) | 1.95 | 1.11–3.41 | 0.020 |
| Serum albumin <3.0 mg/dL | 2.52 | 1.42–4.47 | 0.002 |
| Breakthrough IPA | 1.66 | 0.97–2.83 | 0.064 |
| (B) After IPTW adjustment a | |||
| Variables # | aHR | 95% CI | p-value |
| Neutropenia (ANC <500/mm3) | 2.10 | 1.13–3.88 | 0.018 |
| Serum albumin <3.0 mg/dL | 1.84 | 1.00–3.40 | 0.052 |
| Breakthrough IPA | 1.49 | 0.81–2.72 | 0.201 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.-Y.; Wurster, S.; Matsuo, T.; Jiang, Y.; Tarrand, J.; Kontoyiannis, D.P. What Is the Prognostic Significance of Culture-Documented Breakthrough Invasive Pulmonary Aspergillosis in Patients with Hematological Malignancies? A Propensity Score-Adjusted Analysis. J. Fungi 2025, 11, 623. https://doi.org/10.3390/jof11090623
Cho S-Y, Wurster S, Matsuo T, Jiang Y, Tarrand J, Kontoyiannis DP. What Is the Prognostic Significance of Culture-Documented Breakthrough Invasive Pulmonary Aspergillosis in Patients with Hematological Malignancies? A Propensity Score-Adjusted Analysis. Journal of Fungi. 2025; 11(9):623. https://doi.org/10.3390/jof11090623
Chicago/Turabian StyleCho, Sung-Yeon, Sebastian Wurster, Takahiro Matsuo, Ying Jiang, Jeffrey Tarrand, and Dimitrios P. Kontoyiannis. 2025. "What Is the Prognostic Significance of Culture-Documented Breakthrough Invasive Pulmonary Aspergillosis in Patients with Hematological Malignancies? A Propensity Score-Adjusted Analysis" Journal of Fungi 11, no. 9: 623. https://doi.org/10.3390/jof11090623
APA StyleCho, S.-Y., Wurster, S., Matsuo, T., Jiang, Y., Tarrand, J., & Kontoyiannis, D. P. (2025). What Is the Prognostic Significance of Culture-Documented Breakthrough Invasive Pulmonary Aspergillosis in Patients with Hematological Malignancies? A Propensity Score-Adjusted Analysis. Journal of Fungi, 11(9), 623. https://doi.org/10.3390/jof11090623







